
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (6): 588-596.DOI: 10.15541/jim20150521
• Orginal Article • Previous Articles Next Articles
ZHAI Li-Li1,2, ZHANG Jiang1,2, LI Xuan-Ke1,2, CONG Ye1,2, DONG Zhi-Jun1,2, YUAN Guan-Ming1,2
Received:2015-10-26
Revised:2015-12-22
Published:2016-06-20
Online:2016-05-19
About author:ZHAI Li-Li. E-mail: happy123zhai@163.com
Supported by:CLC Number:
ZHAI Li-Li, ZHANG Jiang, LI Xuan-Ke, CONG Ye, DONG Zhi-Jun, YUAN Guan-Ming. F127 Template on Pore Structure and Electrochemical Performances of Mesoporous SnO2[J]. Journal of Inorganic Materials, 2016, 31(6): 588-596.
| Sample | D(110)/nm |
|---|---|
| SnO2 | 3.98 |
| 3F-SnO2 | 4.39 |
| 4.5F-SnO2 | 4.06 |
| 6F-SnO2 | 3.84 |
| 7.5F-SnO2 | 3.66 |
| 9F-SnO2 | 3.59 |
Table 1 Crystalline sizes of mesoporous SnO2 synthesized with different F127 additive amounts
| Sample | D(110)/nm |
|---|---|
| SnO2 | 3.98 |
| 3F-SnO2 | 4.39 |
| 4.5F-SnO2 | 4.06 |
| 6F-SnO2 | 3.84 |
| 7.5F-SnO2 | 3.66 |
| 9F-SnO2 | 3.59 |
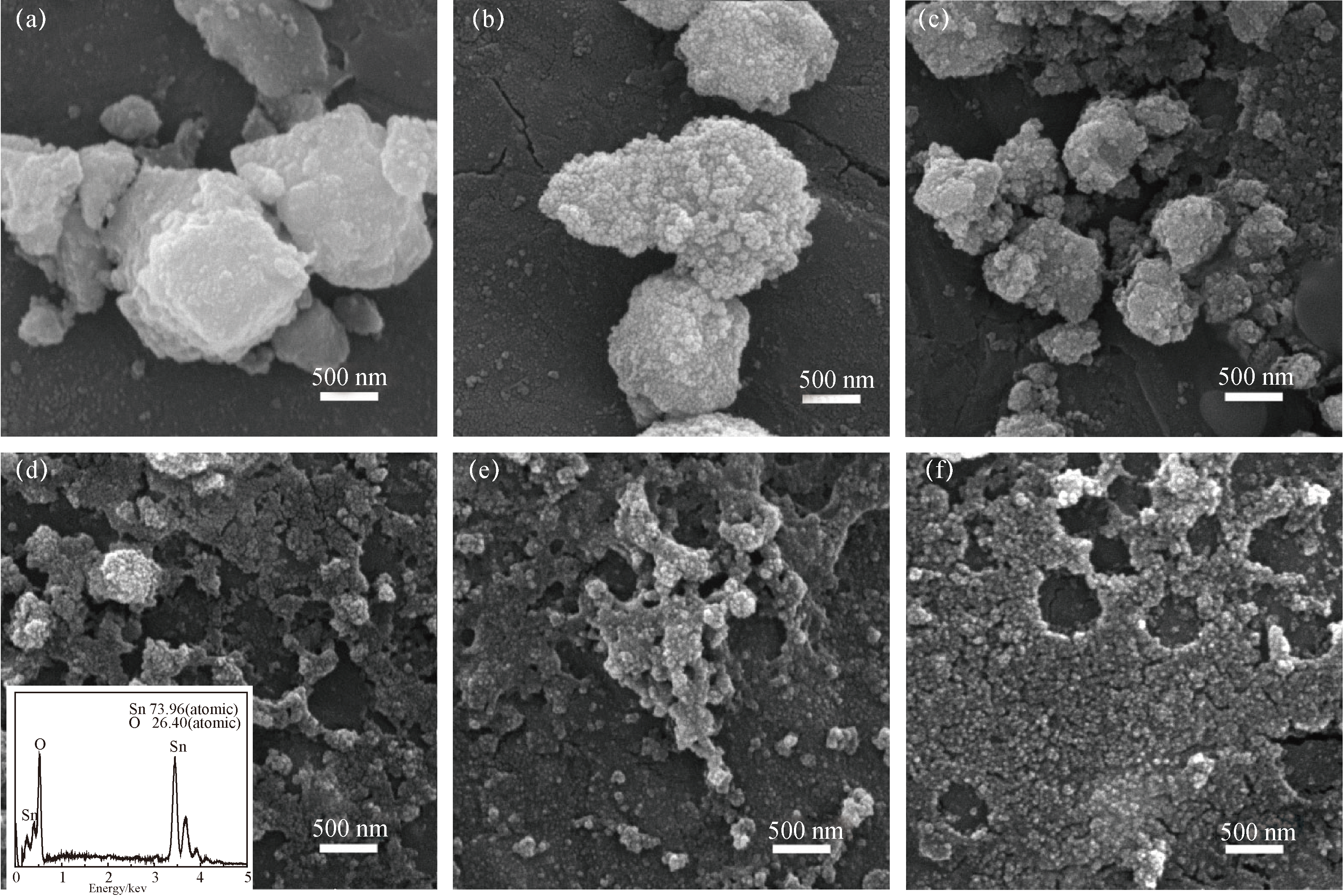
Fig. 2 SEM images of SnO2 synthesized with different F127 additive amounts. (a) SnO2; (b) 3F-SnO2; (c) 4.5F-SnO2; (d) 6F-SnO2; (e) 7.5F-SnO2; (f) 9F-SnO2

Fig. 4 N2 adsorption/desorption isotherms and pore size distribution curves (inset) of mesoporous SnO2 synthesized with different F127 additive amounts. (a) SnO2; (b) 3F-SnO2; (c) 6F-SnO2; (d) 9F-SnO2
| Samples | BET/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| SnO2 | 94 | 0.097 | 4.10 |
| 3F-SnO2 | 104 | 0.110 | 4.25 |
| 4.5F-SnO2 | 110 | 0.122 | 4.42 |
| 6F-SnO2 | 124 | 0.153 | 4.94 |
| 7.5F-SnO2 | 126 | 0.152 | 4.86 |
| 9F-SnO2 | 138 | 0.179 | 5.19 |
Table 2 Pore structural parameters of mesoporous SnO2 synthesized with different F127 additive amounts
| Samples | BET/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| SnO2 | 94 | 0.097 | 4.10 |
| 3F-SnO2 | 104 | 0.110 | 4.25 |
| 4.5F-SnO2 | 110 | 0.122 | 4.42 |
| 6F-SnO2 | 124 | 0.153 | 4.94 |
| 7.5F-SnO2 | 126 | 0.152 | 4.86 |
| 9F-SnO2 | 138 | 0.179 | 5.19 |
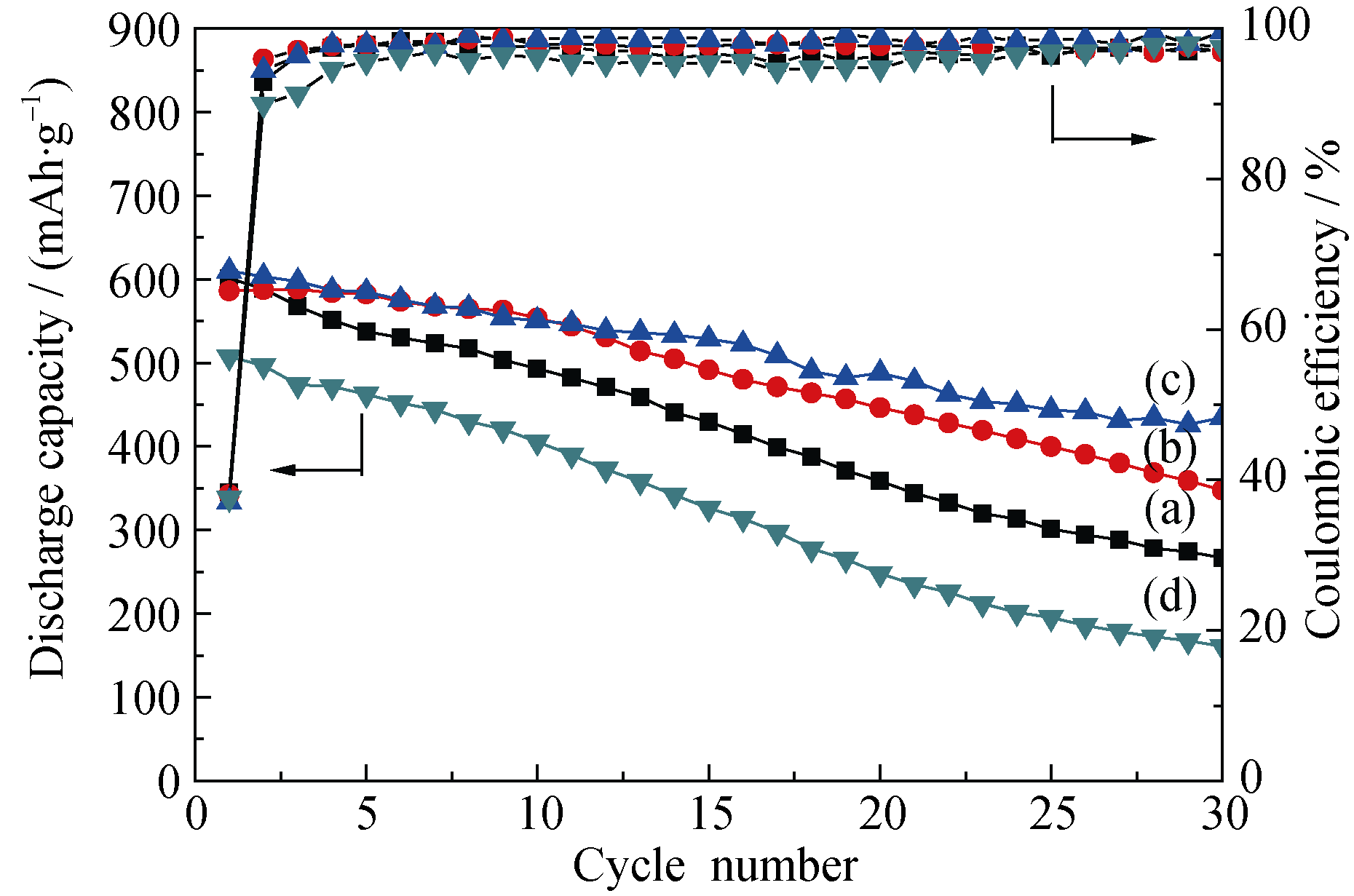
Fig. 6 Cycling performances and coulombic efficiency of mesoporous SnO2 synthesized with different F127 additive amounts. (a) SnO2; (b) 3F-SnO2; (c) 6F-SnO2; (d) 9F-SnO2
| [1] | DAHN J R, ZHENG T, LIU Y, et al.Mechanisms for lithium insertion in carbonaceous materials.Science, 1995, 270(5236): 590-593. |
| [2] | IDOTA Y, KUBOTA T, MATSUFUJI A, et al.Tin-based amorphous oxide: a high-capacity lithium-ion-storage material.Science, 1997, 276(5317): 1395-1397. |
| [3] | COURTNEY I A, DAHN J R.Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites.Journal of the Electrochemical Society, 1997, 144(6): 2045-2052. |
| [4] | FAN J, WANG T, YU C Z, et al.Ordered nanostructured tin-based oxides/carbon composite as the negative-electrode material for lithium-ion batteries.Advanced Materials, 2004, 16(16): 1432-1436. |
| [5] | WANG J Z, DU N, ZHANG H, et al.Large-scale synthesis of SnO2 nanotube arrays as high-performance anode materials of Li-ion batteries.The Journal of Physical Chemistry, 2011, 115(22): 11302-11305. |
| [6] | YIN X M, LI C C, ZHANG M, et al.One-step synthesis of hierarchical SnO2 hollow nanostructures via self-assembly for high power lithium-ion batteries.The Journal of Physical Chemistry, 2010, 114(17): 8084-8088. |
| [7] | WANG C, ZHOU Y, GE M Y, et al.Large-scale synthesis of SnO2 nanosheets with high lithium-ion storage capacity.Journal of the American Chemical Society, 2009, 132(1): 46-47. |
| [8] | ZHANG X, JIANG B, GUO J X, et al.Large and stable reversible lithium-ion storages from mesoporous SnO2 nanosheets with ultralong lifespan over 1000 cycle.Journal of Power Sources, 2014, 268: 365-371. |
| [9] | SHIVA K, KIRAN M S R N, RAMAMURTY U, et al. A broad pore size distribution mesoporous SnO2 as anode for lithium-ion batteries.J. Solid State Electrochem., 2012, 16(11): 3643-3649. |
| [10] | LIU X W, ZHONG X W, YANG Z Z, et al.Gram-scale synthesis of graphene-mesoporous SnO2 composite as anode for lithium-ion batteries.Electrochimica Acta, 2015, 152(10): 178-182. |
| [11] | YANG Z L, ZHAO S J, JIANG W, et al.Carbon-supported SnO2 nanowire arrays with enhanced lithium storage properties.Electrochimica Acta, 2015, 158: 321-326. |
| [12] | WU P, DU N, ZHANG H, et al.Carbon-coated SnO2 nanotubes template-engaged synthesis and their application in lithium-ion batteries.Nanoscale, 2011, 3(2): 746-750. |
| [13] | YANG Q, HU W B.Amorphous SnO2-C composite fibers and their electrochemical performance.Journal of Inorganic Materials, 2015, 30(8): 861-888. |
| [14] | ZHANG C F, PENG X, GUO Z P, et al.Carbon-coated SnO2/graphene nanosheets as highly reversible anode materials for lithium ion batteries.Carbon, 2012, 50(5): 1897-1903. |
| [15] | YU Z J, WANG Y L, DENG H G, et al.Synthesis and electrochemical performance of SnO2/graphene anode material for lithium ion batteries.Journal of Inorganic Materials, 2013, 28(5): 515-520. |
| [16] | LI Y D, LU X, WANG H K, et al.Growth of ultrafine SnO2 nanoparticles within multiwall carbon nanotube networks: non-solution synthesis and excellent electrochemical properties as anodes for lithium ion batteries.Electrochimica Acta, 2015, 178: 778-785. |
| [17] | LIU Y F, HU Z H, XU K, et al.Surface modification and performance of activated carbon electrode material.Acta Phys. -Chim. Sin., 2008, 24(7): 1143-1148. |
| [18] | KIM H, CHO J.Hard templating synthesis of mesoporous and nanowire SnO2 lithium battery anode materials.Journal of Materials Chemistry, 2008, 18(7): 771-775. |
| [19] | SONG H H, YANG S B, CHEN X H.The effect on high charge/discharge rate performance of the lithium ion battery.Chinese Journal of Power Sources, 2009, 33(6): 443-448. |
| [20] | WANG Y, SAKAMOTO J, KOSTOV S, et al.Structural aspects of electrochemically lithiated SnO: nuclear magnetic resonance and X-ray absorption studies.Journal of Power Sources, 2000, 89(2): 232-236. |
| [21] | LOU X W, LI C M, ARCHER L A.Designed synthesis of coaxial SnO2@carbon hollow spheres for highly reversible lithium storage.Advanced Materials, 2009, 21(24): 2536-2539. |
| [22] | ZHANG Y L, LIU Y, LIU M L.Nano-structured columnar tin oxide thin film electrode for lithium ion batteries.Chemistry of Materials, 2006, 18(19): 4643-4646. |
| [23] | WANG J H, LI B, WU H Y, et al.Synthesis of mesoporous SnO2 and its application in lithium ion battery.Acta Phys. -Chim. Sin., 2008, 24(4): 681-685. |
| [24] | LIU B, CAO M H, ZHAO X Y, et al.Facile synthesis of ultrafine carbon-coated SnO2 nanoparticles for high-performance reversible lithium storage.Journal of Power Sources, 2013, 243: 54-59. |
| [25] | ZHANG Y X, ZHANG X J.The influence of the template agent on the order mesoporous carbon channel structure.Journal of Beijing University of Chemical Technology(Natural Science), 2010, 37(5): 83-87. |
| [26] | LI Z P, ZHAO R H, GUO F, et al.Preparation and characterization of ordered mesoporous alumina with high specific surface area with F127 as template.Chemical Journal of Chinese Universities, 2008, 29(1): 13-17. |
| [27] | COURTNEY I A, MCKINNON W R, Dahn J R.On the aggregation of tin in SnO composite glasses caused by the reversible reaction with lithium.Journal of the Electrochemical Society, 1999, 146(1): 59-68. |
| [1] | TAN Bowen, GENG Shuanglong, ZHANG Kai, ZHENG Bailin. Composition-gradient Design of Silicon Electrodes to Mitigate Mechanochemical Coupling Degradation [J]. Journal of Inorganic Materials, 2025, 40(7): 772-780. |
| [2] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [3] | LIU Pengdong, WANG Zhen, LIU Yongfeng, WEN Guangwu. Research Progress on the Application of Silicon Slurry in Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(9): 992-1004. |
| [4] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [5] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [6] | SU Nana, HAN Jingru, GUO Yinhao, WANG Chenyu, SHI Wenhua, WU Liang, HU Zhiyi, LIU Jing, LI Yu, SU Baolian. ZIF-8-derived Three-dimensional Silicon-carbon Network Composite for High-performance Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(9): 1016-1022. |
| [7] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [8] | ZHU Hezhen, WANG Xuanpeng, HAN Kang, YANG Chen, WAN Ruizhe, WU Liming, MAI Liqiang. Enhanced Lithium Storage Stability Mechanism of Ultra-high Nickel LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2 Cathode Materials [J]. Journal of Inorganic Materials, 2022, 37(9): 1030-1036. |
| [9] | FENG Kun, ZHU Yong, ZHANG Kaiqiang, CHEN Zhang, LIU Yu, GAO Yanfeng. Boehmite Nanosheets-coated Separator with Enhanced Performance for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(9): 1009-1015. |
| [10] | CHEN Ying, LUAN Weiling, CHEN Haofeng, ZHU Xuanchen. Multi-scale Failure Behavior of Cathode in Lithium-ion Batteries Based on Stress Field [J]. Journal of Inorganic Materials, 2022, 37(8): 918-924. |
| [11] | WANG Yutong, ZHANG Feifan, XU Naicai, WANG Chunxia, CUI Lishan, HUANG Guoyong. Research Progress of LiTi2(PO4)3 Anode for Aqueous Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(5): 481-492. |
| [12] | LI Kunru, HU Xinghui, ZHANG Zhengfu, GUO Yuzhong, HUANG Ruian. Three-dimensional Porous Biogenic Si/C Composite for High Performance Lithium-ion Battery Anode Derived from Equisetum Fluviatile [J]. Journal of Inorganic Materials, 2021, 36(9): 929-935. |
| [13] | WANG Ying, ZHANG Wenlong, XING Yanfeng, CAO suqun, DAI Xinyi, LI Jingze. Performance of Amorphous Lithium Phosphate Coated Lithium Titanate Electrodes in Extended Working Range of 0.01-3.00 V [J]. Journal of Inorganic Materials, 2021, 36(9): 999-1005. |
| [14] | ZHANG Junmin, CHEN Xiaowu, LIAO Chunjin, GUO Feiyu, YANG Jinshan, ZHANG Xiangyu, DONG Shaoming. Optimizing Microstructure and Properties of SiCf/SiC Composites Prepared by Reactive Melt Infiltration [J]. Journal of Inorganic Materials, 2021, 36(10): 1103-1110. |
| [15] | CHENG Fu-Qiang,JI Tian-Tian,XUE Min,MENG Zi-Hui,WU Yu-Kai. Thiohydroxy-functionalized Mesoporous Materials: Preparation and its Adsorption to Cr6+ [J]. Journal of Inorganic Materials, 2020, 35(2): 193-198. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||