
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (1): 39-46.DOI: 10.15541/jim20240296
Special Issue: 【能源环境】燃料电池(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
LIU Lei1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), WANG Li4, WANG Yan5, ZHANG Guofang1, GUAN Lili1,2
), WANG Li4, WANG Yan5, ZHANG Guofang1, GUAN Lili1,2
Received:2024-06-18
Revised:2024-08-23
Published:2025-01-20
Online:2024-09-02
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:LIU Lei (2000-), male, Master candidate. E-mail: 1371281920@qq.com
Supported by:CLC Number:
LIU Lei, GUO Ruihua, WANG Li, WANG Yan, ZHANG Guofang, GUAN Lili. Oxygen Reduction Reaction on Pt3Co High-index Facets by Density Functional Theory[J]. Journal of Inorganic Materials, 2025, 40(1): 39-46.
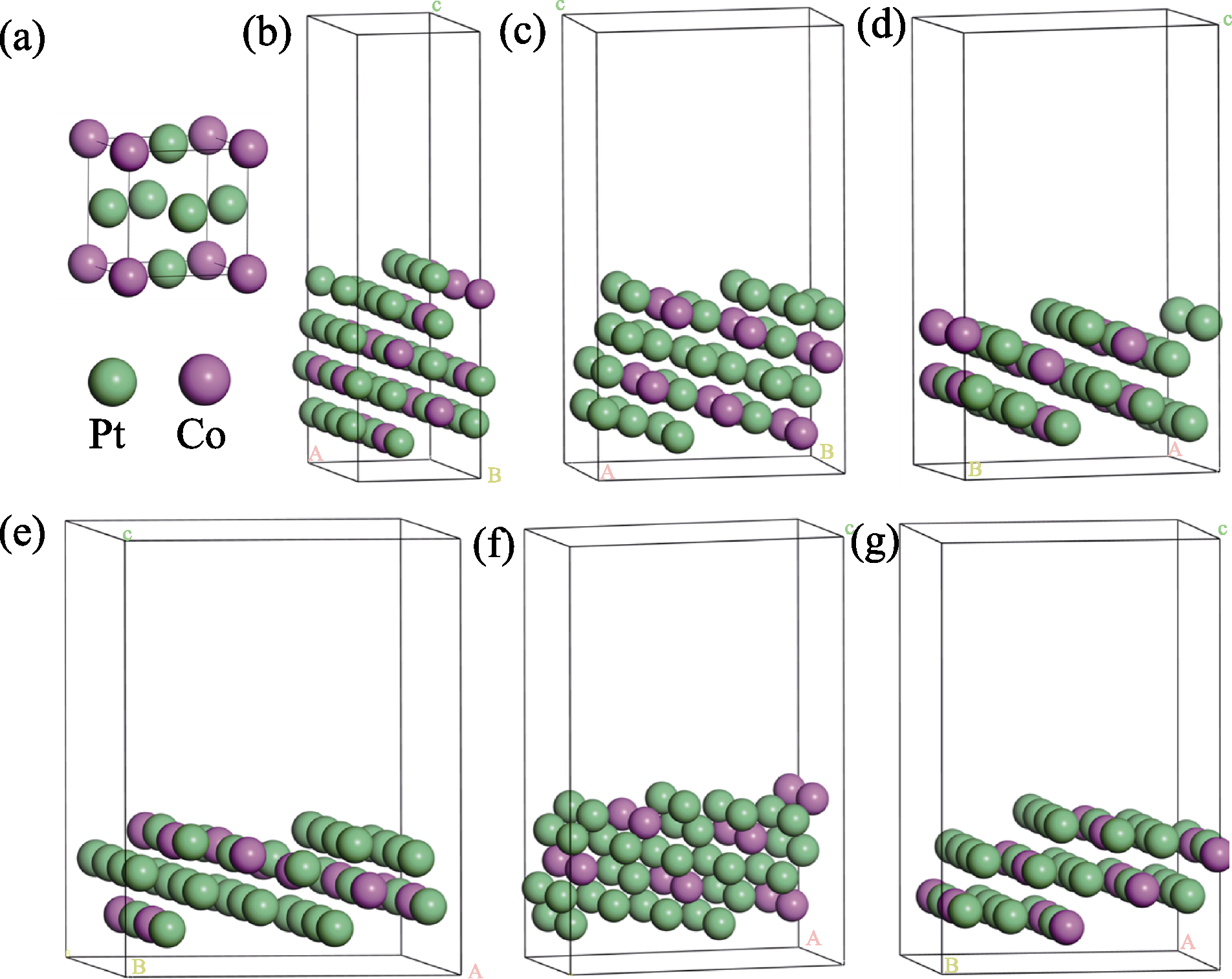
Fig. 2 Surface models of Pt3Co unitcell and six Pt3Co HIFs (a) Pt3Co unitcell; (b) Pt3Co(211); (c) Pt3Co(310); (d) Pt3Co(331); (e) Pt3Co(511); (f) Pt3Co(320); (g) Pt3Co(332)
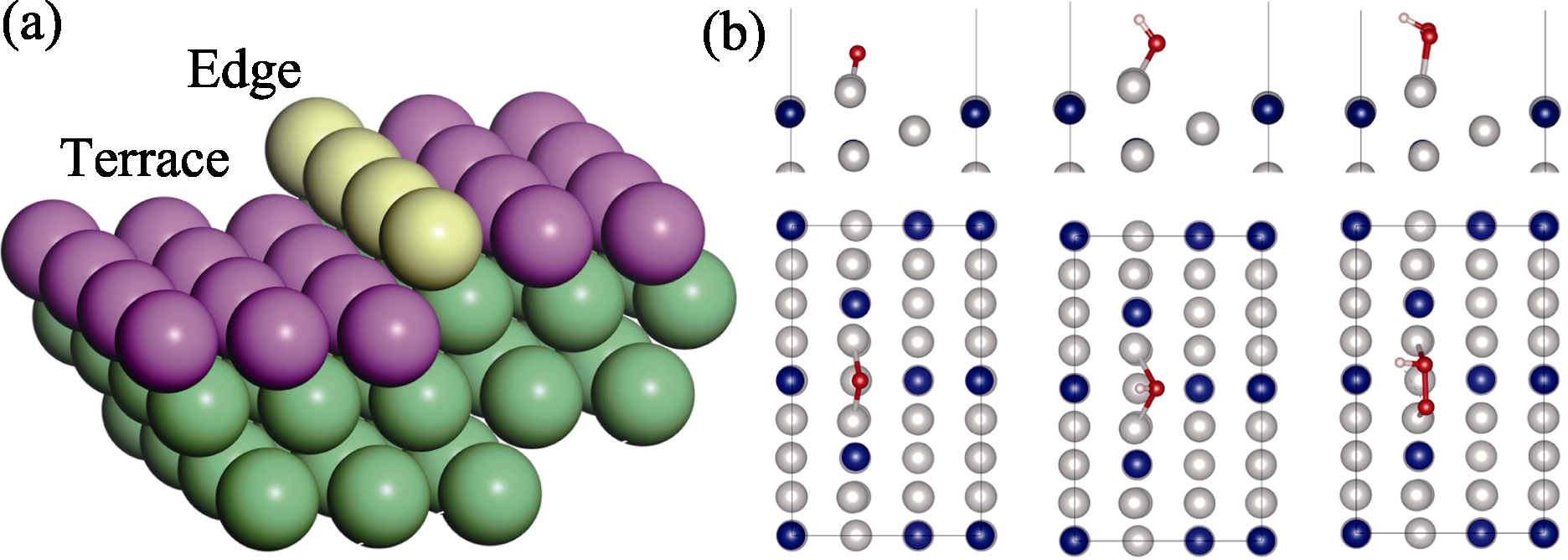
Fig. 3 (a) Schematic representation of HIFs terrace and edge positions, and (b) optimal adsorption configurations of *O, *OH, and *OOH at Pt3Co(211) edge
| HIFs | Site | *O | *OH | *OOH | |||
|---|---|---|---|---|---|---|---|
| BE/eV | BD/nm | BE/eV | BD/nm | BE/eV | BD/nm | ||
| Pt3Co(211) | Edge | 3.043 | 0.195 | 0.903 | 0.213 | 3.643 | 0.217 |
| Terrace | 2.334 | 0.206 | 0.936 | 0.200 | 3.658 | 0.206 | |
| Pt3Co(310) | Edge | 1.505 | 0.179 | 0.341 | 0.195 | 3.484 | 0.196 |
| Terrace | 1.405 | 0.197 | 0.674 | 0.213 | 3.828 | 0.212 | |
| Pt3Co(331) | Edge | 1.175 | 0.194 | 0.620 | 0.214 | 3.499 | 0.216 |
| Terrace | 1.512 | 0.208 | 0.838 | 0.200 | 3.702 | 0.221 | |
| Pt3Co(511) | Edge | 1.291 | 0.192 | 0.524 | 0.212 | 3.633 | 0.215 |
| Terrace | 1.335 | 0.197 | 0.599 | 0.213 | 3.789 | 0.214 | |
| Pt3Co(320) | Edge | 1.204 | 0.179 | 0.105 | 0.195 | 3.218 | 0.193 |
| Terrace | 1.638 | 0.181 | 0.356 | 0.197 | 3.473 | 0.199 | |
| Pt3Co(332) | Edge | 1.418 | 0.194 | 0.576 | 0.215 | 3.419 | 0.214 |
| Terrace | 1.618 | 0.201 | 0.723 | 0.215 | 3.778 | 0.212 | |
Table 1 BE and BD of different reaction intermediates at different positions of six Pt3Co HIFs
| HIFs | Site | *O | *OH | *OOH | |||
|---|---|---|---|---|---|---|---|
| BE/eV | BD/nm | BE/eV | BD/nm | BE/eV | BD/nm | ||
| Pt3Co(211) | Edge | 3.043 | 0.195 | 0.903 | 0.213 | 3.643 | 0.217 |
| Terrace | 2.334 | 0.206 | 0.936 | 0.200 | 3.658 | 0.206 | |
| Pt3Co(310) | Edge | 1.505 | 0.179 | 0.341 | 0.195 | 3.484 | 0.196 |
| Terrace | 1.405 | 0.197 | 0.674 | 0.213 | 3.828 | 0.212 | |
| Pt3Co(331) | Edge | 1.175 | 0.194 | 0.620 | 0.214 | 3.499 | 0.216 |
| Terrace | 1.512 | 0.208 | 0.838 | 0.200 | 3.702 | 0.221 | |
| Pt3Co(511) | Edge | 1.291 | 0.192 | 0.524 | 0.212 | 3.633 | 0.215 |
| Terrace | 1.335 | 0.197 | 0.599 | 0.213 | 3.789 | 0.214 | |
| Pt3Co(320) | Edge | 1.204 | 0.179 | 0.105 | 0.195 | 3.218 | 0.193 |
| Terrace | 1.638 | 0.181 | 0.356 | 0.197 | 3.473 | 0.199 | |
| Pt3Co(332) | Edge | 1.418 | 0.194 | 0.576 | 0.215 | 3.419 | 0.214 |
| Terrace | 1.618 | 0.201 | 0.723 | 0.215 | 3.778 | 0.212 | |
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| Bader charge transfer number (edge)/e | 0.199 | 0.206 | 0.187 | 0.173 | 0.214 | 0.182 |
| Bader charge transfer number (terrace)/e | 0.185 | 0.149 | 0.194 | 0.138 | 0.170 | 0.203 |
| εd (edge)/eV | -1.502 | -1.560 | -1.683 | -1.746 | -1.530 | -1.671 |
| εd (terrace)/eV | -1.729 | -1.667 | -1.820 | -1.831 | -1.707 | -1.836 |
Table 2 Bader charge transfer number and εd of Pt atoms at different positions of six Pt3Co HIFs
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| Bader charge transfer number (edge)/e | 0.199 | 0.206 | 0.187 | 0.173 | 0.214 | 0.182 |
| Bader charge transfer number (terrace)/e | 0.185 | 0.149 | 0.194 | 0.138 | 0.170 | 0.203 |
| εd (edge)/eV | -1.502 | -1.560 | -1.683 | -1.746 | -1.530 | -1.671 |
| εd (terrace)/eV | -1.729 | -1.667 | -1.820 | -1.831 | -1.707 | -1.836 |
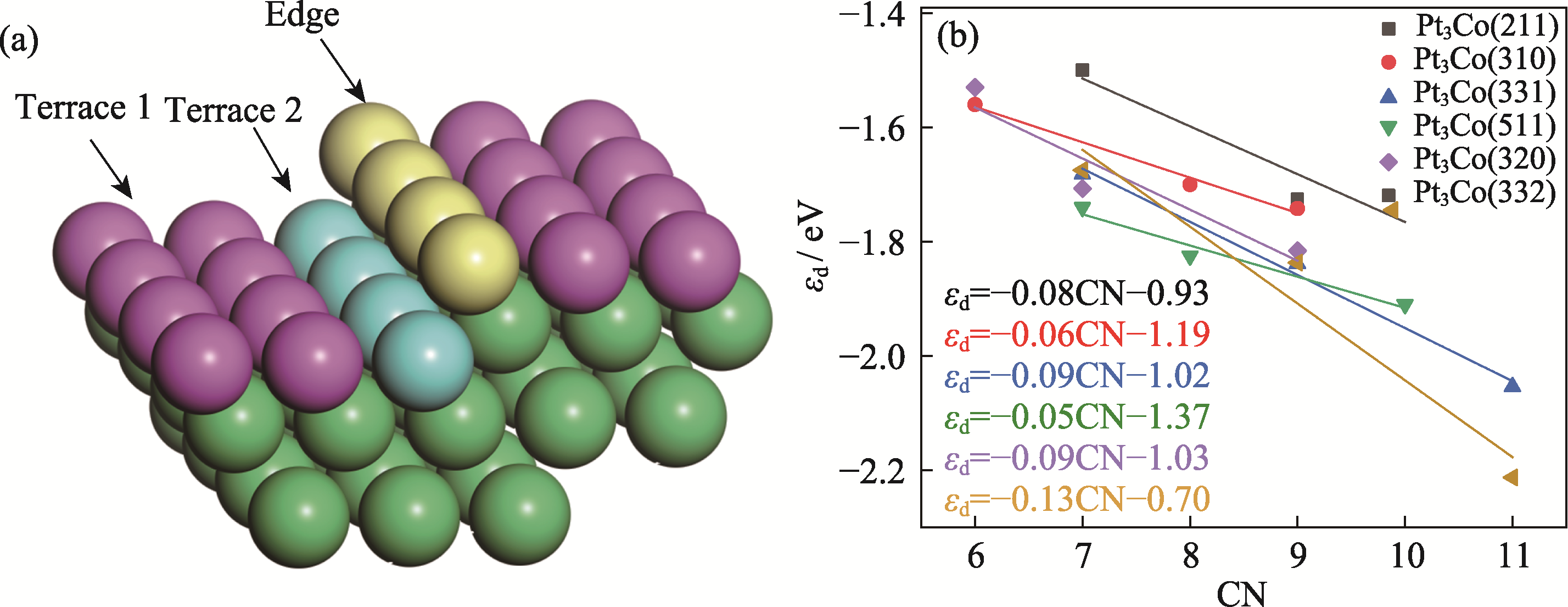
Fig. 4 (a) Schematic representation of the positions of surface terrace and edge identified on the basis of CN, and (b) relationships of εd and CN for the six HIFs adsorbed atoms Colorful figures are available on website
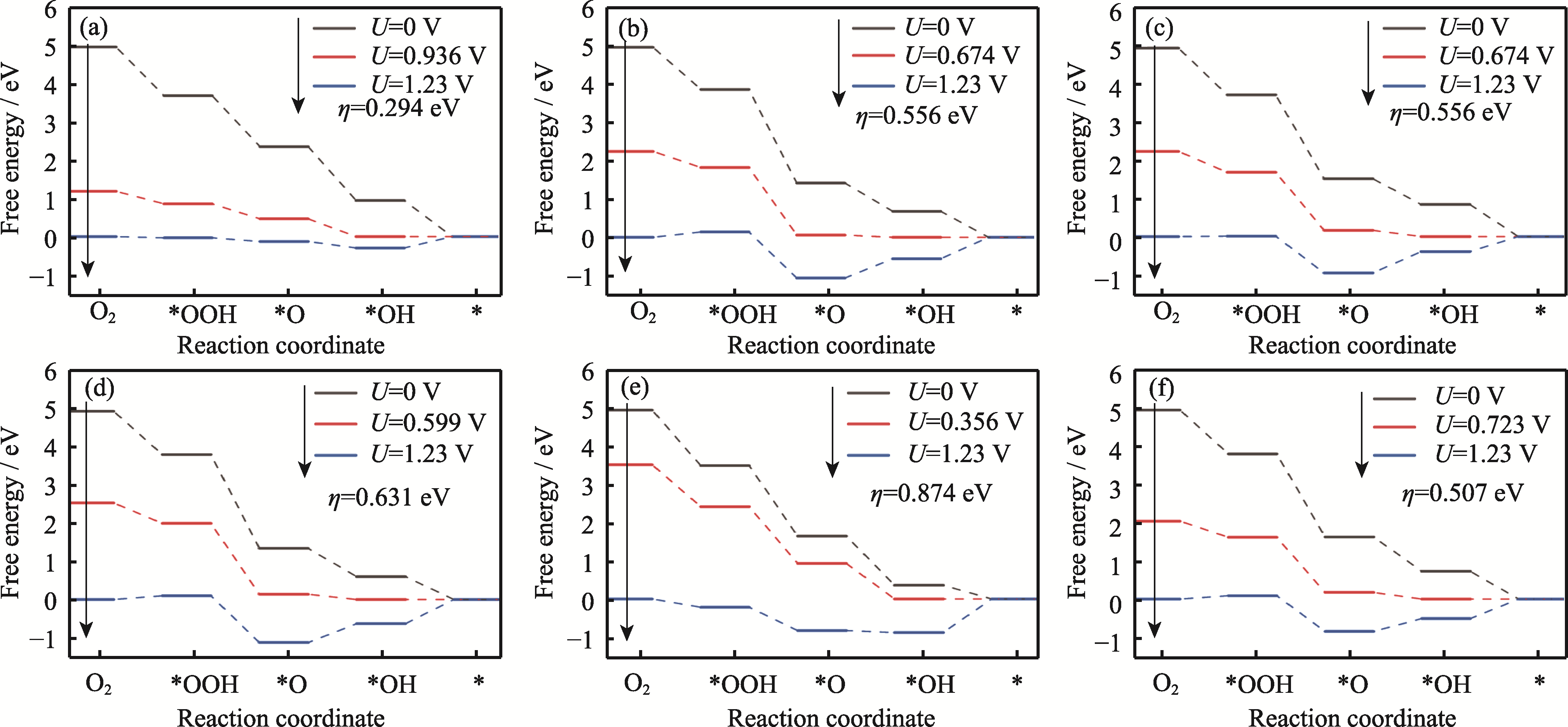
Fig. 5 Plots of ORR free energy at terrace for Pt3Co HIFs catalysts (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| ηORR (edge)/eV | 0.630 | 0.889 | 0.675 | 0.706 | 1.125 | 0.654 |
| ηORR (terrace)/eV | 0.294 | 0.556 | 0.556 | 0.631 | 0.874 | 0.507 |
Table 3 Bader charge transfer number and εd of Pt atoms at different positions of six Pt3Co HIFs
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| ηORR (edge)/eV | 0.630 | 0.889 | 0.675 | 0.706 | 1.125 | 0.654 |
| ηORR (terrace)/eV | 0.294 | 0.556 | 0.556 | 0.631 | 0.874 | 0.507 |
| HIFs | Microfact notation | k-point | Number of atoms |
|---|---|---|---|
| Pt3Co(211) | n(111)×(100) | 3×2×1 | 48 |
| Pt3Co(310) | n(100)×(110) | 3×2×1 | 48 |
| Pt3Co(331) | n(110)×(111) | 2×2×1 | 48 |
| Pt3Co(511) | n(100)×(111) | 2×2×1 | 48 |
| Pt3Co(320) | n(110)×(100) | 3×2×1 | 48 |
| Pt3Co(332) | n(111)×(110) | 2×2×1 | 48 |
Table S1 Microfact notation, k-point, number of atoms of six HIFs
| HIFs | Microfact notation | k-point | Number of atoms |
|---|---|---|---|
| Pt3Co(211) | n(111)×(100) | 3×2×1 | 48 |
| Pt3Co(310) | n(100)×(110) | 3×2×1 | 48 |
| Pt3Co(331) | n(110)×(111) | 2×2×1 | 48 |
| Pt3Co(511) | n(100)×(111) | 2×2×1 | 48 |
| Pt3Co(320) | n(110)×(100) | 3×2×1 | 48 |
| Pt3Co(332) | n(111)×(110) | 2×2×1 | 48 |
| HIFs | Site | BE-*OH/eV | εd/eV |
|---|---|---|---|
| Pt3Co(211) | Edge | 0.903 | -1.502 |
| Terrace | 0.936 | -1.729 | |
| Pt3Co(310) | Edge | 0.341 | -1.746 |
| Terrace | 0.674 | -1.831 | |
| Pt3Co(331) | Edge | 0.620 | -1.560 |
| Terrace | 0.838 | -1.667 | |
| Pt3Co(511) | Edge | 0.524 | -1.530 |
| Terrace | 0.599 | -1.707 | |
| Pt3Co(320) | Edge | 0.105 | -1.683 |
| Terrace | 0.356 | -1.820 | |
| Pt3Co(332) | Edge | 0.576 | -1.671 |
| Terrace | 0.723 | -1.836 |
Table S2 BE-*OH and εd at different positions of six HIFs
| HIFs | Site | BE-*OH/eV | εd/eV |
|---|---|---|---|
| Pt3Co(211) | Edge | 0.903 | -1.502 |
| Terrace | 0.936 | -1.729 | |
| Pt3Co(310) | Edge | 0.341 | -1.746 |
| Terrace | 0.674 | -1.831 | |
| Pt3Co(331) | Edge | 0.620 | -1.560 |
| Terrace | 0.838 | -1.667 | |
| Pt3Co(511) | Edge | 0.524 | -1.530 |
| Terrace | 0.599 | -1.707 | |
| Pt3Co(320) | Edge | 0.105 | -1.683 |
| Terrace | 0.356 | -1.820 | |
| Pt3Co(332) | Edge | 0.576 | -1.671 |
| Terrace | 0.723 | -1.836 |
Fig. S1 Temperature and energy balance curves during AIMD simulations of six Pt3Co HIFs (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
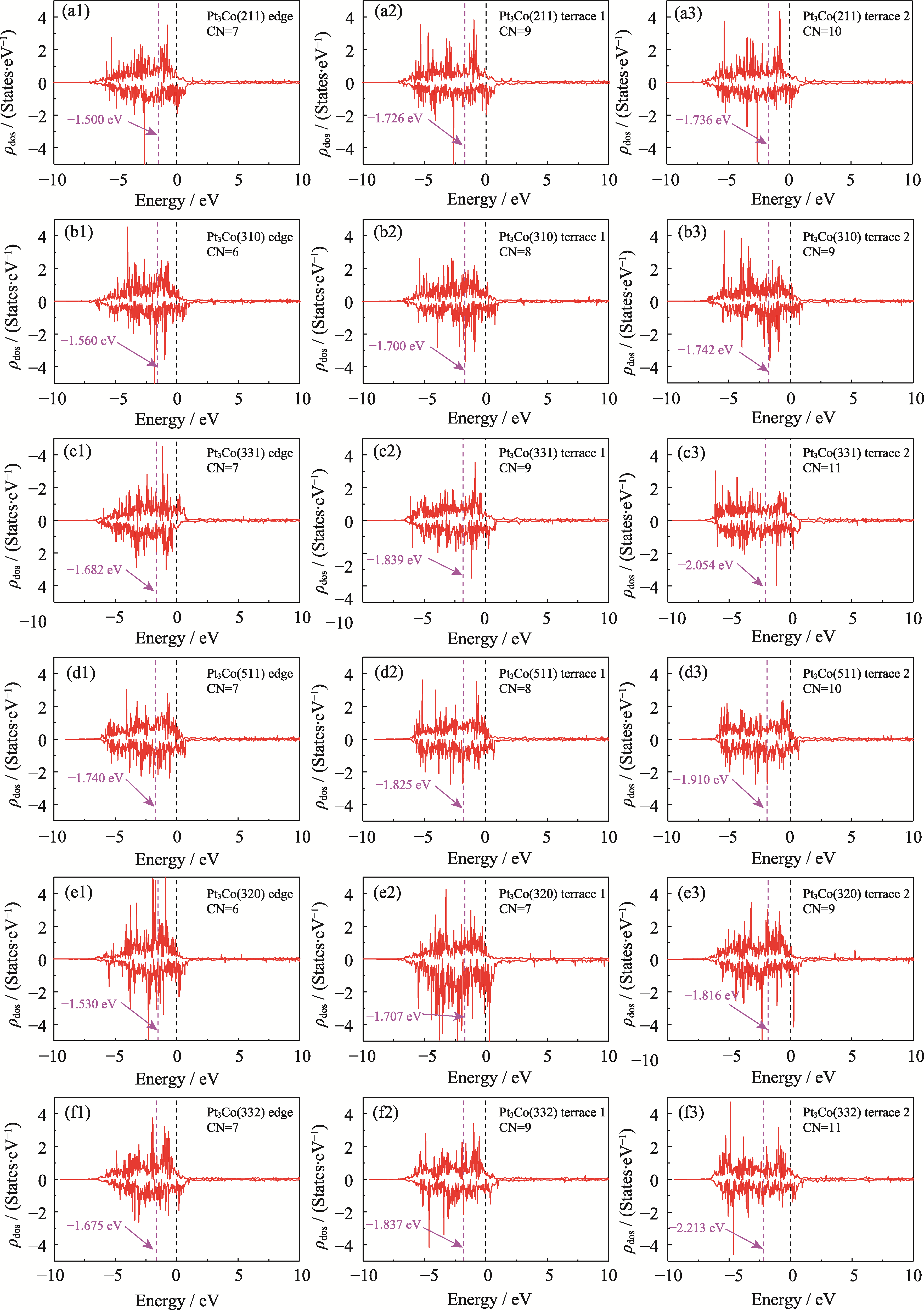
Fig. S8 ρdos and εd for the six HIFs at edge, terrace 1, and terrace 2 (a1-a3) Pt3Co(211); (b1-b3) Pt3Co(310); (c1-c3) Pt3Co(331); (d1-d3) Pt3Co(511); (e1-e3) Pt3Co(320); (f1-f3) Pt3Co(332)
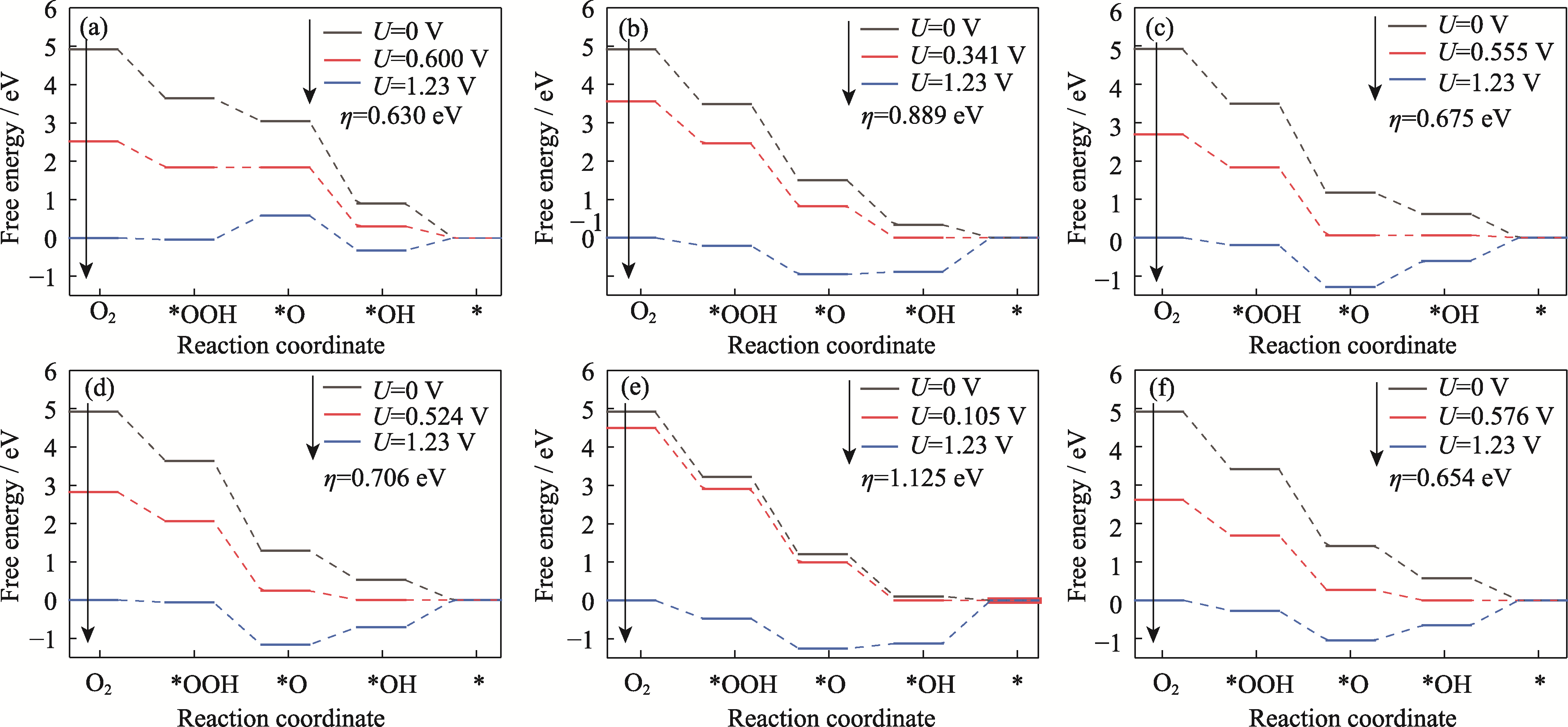
Fig. S9 Plots of ORR free energy at edge for Pt3Co HIFs catalysts (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
| [1] | XIA B Y, WU H B, WANG X, et al. Highly concave platinum nanoframes with high-index facets and enhanced electrocatalytic properties. Angewandte Chemie International Edition, 2013, 52(47): 12337. |
| [2] | WANG Y, MA S, LI Q, et al. Hollow platinum nanospheres and nanotubes templated by shear flow-induced lipid vesicles and tubules and their applications on hydrogen evolution. ACS Sustainable Chemistry & Engineering, 2016, 4(7): 3773. |
| [3] | SUBBARAMAN R, TRIPKOVIC D, STRMCNIK D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science, 2011, 334(6060): 1256. |
| [4] | LIU M, XIAO X, LI Q, et al. Recent progress of electrocatalysts for oxygen reduction in fuel cells. Journal of Colloid and Interface Science, 2022, 607: 791. |
| [5] | WANG X X, SWIHART M T, WU G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nature Catalysis, 2019, 2: 578. |
| [6] | ZENG Z, KÜSPERT S, BALAGHI S E, et al. Ultrahigh mass activity Pt entities consisting of Pt single atoms, clusters, and nanoparticles for improved hydrogen evolution reaction. Small, 2023, 19(29): 2205885. |
| [7] | GUO R, AN N, AN S, et al. One-step preparation of nitrogen- doped platinum-based catalysts for electrocatalytic oxidation of ethanol. Catalysts, 2021, 11(11): 1264. |
| [8] | DU L, PRABHAKARAN V, XIE X, et al. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: stability challenges and material solutions. Advanced Materials, 2021, 33(6): 1908232. |
| [9] | BANHAM D, CHOI J Y, KISHIMOTO T, et al. Integrating PGM- free catalysts into catalyst layers and proton exchange membrane fuel cell devices. Advanced Materials, 2019, 31(31): 1804846. |
| [10] | HE Y, WU G. PGM-free oxygen-reduction catalyst development for proton-exchange membrane fuel cells: challenges, solutions, and promises. Accounts of Materials Research, 2022, 3(2): 224. |
| [11] | YAO Y, GUO R, AN S, et al. In-situ loaded Pt-Co high index facets catalysts: preparation and electrocatalytic performance. Journal of Inorganic Materials, 2023, 38(1): 71. |
| [12] |
TIAN N, ZHOU Z Y, SUN S G, et al. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science, 2007, 316(5825): 732.
PMID |
| [13] |
VAN SANTEN R A. Complementary structure sensitive and insensitive catalytic relationships. Accounts of Chemical Research, 2009, 42(1): 57.
DOI PMID |
| [14] | SUN L, WANG Q, MA M, et al. Etching-assisted synthesis of single atom Ni-tailored Pt nanocatalyst enclosed by high-index facets for active and stable oxygen reduction catalysis. Nano Energy, 2022, 103: 107800. |
| [15] |
STAMENKOVIC V R, MUN B S, ARENZ M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature Materials, 2007, 6(3): 241.
DOI PMID |
| [16] | WANG X X, HWANG S, PAN Y T, et al. Ordered Pt3Co intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction. Nano Letters, 2018, 18(7): 4163. |
| [17] | XU W C, ZHANG Z M, YANG C H, et al. Promotion mechanism of PtCo intermetallic ordered alloys in oxygen reduction reaction and its application in fuel cells. Electrochemistry Communications, 2023, 152: 107516. |
| [18] |
ZHANG C, CHEN Z, YANG H, et al. Surface-structure tailoring of dendritic PtCo nanowires for efficient oxygen reduction reaction. Journal of Colloid and Interface Science, 2023, 652: 1597.
DOI PMID |
| [19] | TETTEH E B, GYAN-BARIMAH C, LEE H Y, et al. Strained Pt(221) facet in a PtCo@Pt-rich catalyst boosts oxygen reduction and hydrogen evolution activity. ACS Applied Materials & Interfaces, 2022, 14(22): 25246. |
| [20] | DONG J C, SU M, BRIEGA-MARTOS V, et al. Direct in situ Raman spectroscopic evidence of oxygen reduction reaction intermediates at high-index Pt(hkl) surfaces. Journal of the American Chemical Society, 2020, 142(2): 715. |
| [21] |
HUANG L, LIU M, LIN H, et al. Shape regulation of high-index facet nanoparticles by dealloying. Science, 2019, 365(6458): 1159.
DOI PMID |
| [22] | KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 1996, 6(1): 15. |
| [23] |
PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77(18): 3865.
DOI PMID |
| [24] | GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Journal of Chemical Physics, 2010, 132(15): 154104. |
| [25] | KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 1999, 59(3): 1758. |
| [26] | LI K, LI Y, WANG Y, et al. The oxygen reduction reaction on Pt(111) and Pt(100) surfaces substituted by subsurface Cu: a theoretical perspective. Journal of Materials Chemistry A, 2015, 3(21): 11444. |
| [27] | ZHU Y, WANG Z. Tuning the N coordination environment of Ir single-atom-catalyst for highly efficient ORR and OER: a computational study. Catalysis Letters, 2024, 154(5): 2464. |
| [28] | ZHANG X, LIU X, WU D, et al. Self-assembly intermetallic PtCu3 core with high-index faceted Pt shell for high-efficiency oxygen reduction. Nano Letters, 2024, 24(10): 3213. |
| [29] | KAN D, WANG D, ZHANG X, et al. Rational design of bifunctional ORR/OER catalysts based on Pt/Pd-doped Nb2CT2 MXene by first-principles calculations. Journal of Materials Chemistry A, 2020, 8(6): 3097. |
| [30] | NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. Journal of Physical Chemistry B, 2004, 108(46): 17886. |
| [31] | HAMMER B, NØRSKOV J K. Electronic factors determining the reactivity of metal surfaces. Surface Science, 1995, 343(3): 211. |
| [32] | MAVRIKAKIS M, HAMMER B, NØRSKOV J K. Effect of strain on the reactivity of metal surfaces. Physical Review Letters, 1998, 81(13): 2819. |
| [33] |
KITCHIN J R, NØRSKOV J K, BARTEAU M A, et al. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. Journal of Chemical Physics, 2004, 120(21): 10240.
PMID |
| [34] | TIAN N, ZHOU Z Y, SUN S G. Platinum metal catalysts of high- index surfaces: from single-crystal planes to electrochemically shape-controlled nanoparticles. Journal of Physical Chemistry C, 2008, 112(50): 19801. |
| [35] | VAN HOVE M A, SOMORJAI G A. A new microfacet notation for high-Miller-index surfaces of cubic materials with terrace, step and kink structures. Surface Science, 1980, 92(2/3): 489. |
| [36] | LANG B, JOYNER R W, SOMORJAI G A. Low energy electron diffraction studies of chemisorbed gases on stepped surfaces of platinum. Surface Science, 1972, 30(2): 454. |
| [37] |
SHENG T, LIN W F, SUN S G. Elucidation of the surface structure-selectivity relationship in ethanol electro-oxidation over platinum by density functional theory. Physical Chemistry Chemical Physics, 2016, 18(23): 15501.
DOI PMID |
| [38] | ZHANG B W, SHENG T, WANG Y X, et al. Platinum-cobalt bimetallic nanoparticles with Pt skin for electro-oxidation of ethanol. ACS Catalysis, 2017, 7(1): 892. |
| [39] | WANG C, CAI D, LIU B, et al. Ethanol-sensing performance of tin dioxide octahedral nanocrystals with exposed high-energy {111} and {332} facets. Journal of Materials Chemistry A, 2014, 2(27): 10623. |
| [40] | BARICUATRO J H, KIM Y G, TSANG C F, et al. Reprint of “Selective conversion of CO into ethanol on Cu(511) surface reconstructed from Cu(pc): operando studies by electrochemical scanning tunneling microscopy, mass spectrometry, quartz crystal nanobalance, and infrared spectroscopy”. Journal of Electroanalytical Chemistry, 2020, 875: 114757. |
| [41] | WEI L, MAO Y J, LIU F, et al. Concave cubic Pt-Sm alloy nanocrystals with high-index facets and enhanced electrocatalytic ethanol oxidation. ACS Applied Energy Materials, 2019, 2(10): 7204. |
| [42] | BOWKER M, KING D A. Oxygen diffusion on tungsten single crystal surfaces: secondary electron emission studies. Surface Science, 1980, 94(2/3): 564. |
| [43] | XUE F, GUO X, MIN B, et al. Unconventional high-index facet of iridium boosts oxygen evolution reaction: how the facet matters. ACS Catalysis, 2021, 11(13): 8239. |
| [44] | MA Y, BALBUENA P B. Pt surface segregation in bimetallic Pt3M alloys: a density functional theory study. Surface Science, 2008, 602(1): 107. |
| [45] | ZENG X M, HUANG R, SHAO G F, et al. High-index-faceted platinum nanoparticles: insights into structural and thermal stabilities and shape evolution from atomistic simulations. Journal of Materials Chemistry A, 2014, 2(29): 11480. |
| [46] | WANG H, AN W, LIU X, et al. Oxygen reduction reaction on Pt(111), Pt(221), and Ni/Au1Pt3(221) surfaces: probing scaling relationships of reaction energetics and interfacial composition. Chemical Engineering Science, 2018, 184: 239. |
| [47] |
ZHU Q, SAIDI W A, YANG J C. Step-edge directed metal oxidation. Journal of Physical Chemistry Letters, 2016, 7(13): 2530.
DOI PMID |
| [48] | LIU S, HUANG S. The role of interface charge transfer on Pt based catalysts for water splitting. International Journal of Hydrogen Energy, 2018, 43(32): 15225. |
| [49] | RAJKAMAL A, KIM H. Theoretical verification on adsorptive removal of caffeine by carbon and nitrogen-based surfaces: role of charge transfer, π electron occupancy, and temperature. Chemosphere, 2023, 339: 139667. |
| [50] | YAN T, HOU H, WU C, et al. Unraveling the molecular mechanism for enhanced gas adsorption in mixed-metal MOFs via solid-state NMR spectroscopy. Proceedings of the National Academy of Sciences, 2024, 121(6):e2312959121. |
| [51] | NØRSKOV J K, STUDT F, ABILD-PEDERSEN F, et al. Fundamental concepts in heterogeneous catalysis. Angewandte Chemie International Edition, 2015, 54(36): 10404. |
| [52] | STEPHENS I E L, BONDARENKO A S, GRØNBJERG U, et al. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy & Environmental Science, 2012, 5(5): 6744. |
| [53] |
GREELEY J, STEPHENS I E L, BONDARENKO A S, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nature Chemistry, 2009, 1(7): 552.
DOI PMID |
| [54] | CALLE-VALLEJO F, TYMOCZKO J, COLIC V, et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science, 2015, 350(6257): 185. |
| [55] | CALLE-VALLEJO F, LOFFREDA D, KOPER M T M, et al. Introducing structural sensitivity into adsorption-energy scaling relations by means of coordination numbers. Nature Chemistry, 2015, 7(5): 403. |
| [56] | YUE J, DU Z, SHAO M.Mechanisms of enhanced electrocatalytic activity for oxygen reduction reaction on high-index platinum n(111)-(111) surfaces. Journal of Physical Chemistry Letters, 2015, 6(17): 3346. |
| [1] | LI Xueru, MA Zhejie, GUO Yujie, LI Ping. Influence of Support Characteristics on Coverage of Ionomer and Oxygen Reduction Performance for Pt/C Catalysts [J]. Journal of Inorganic Materials, 2025, 40(12): 1395-1404. |
| [2] | LI Honglan, ZHANG Junmiao, SONG Erhong, YANG Xinglin. Mo/S Co-doped Graphene for Ammonia Synthesis: a Density Functional Theory Study [J]. Journal of Inorganic Materials, 2024, 39(5): 561-568. |
| [3] | WU Guangyu, SHU Song, ZHANG Hongwei, LI Jianjun. Enhanced Styrene Adsorption by Grafted Lactone-based Activated Carbon [J]. Journal of Inorganic Materials, 2024, 39(4): 390-398. |
| [4] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| [5] | YANG Daihui, SUN Tian, TIAN Hexin, SHI Xiaofei, MA Dongwei. Iron-nitrogen-codoped Mesoporous Carbon: Facile Synthesis and Catalytic Performance of Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2023, 38(11): 1309-1315. |
| [6] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [7] | WANG Peng, JIN Zunlong, CHEN Ningguang, LIU Yonghao. Theoretical Investigation of Mo Doped α-MnO2 Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(5): 541-546. |
| [8] | JIANG Lili, XU Shuaishuai, XIA Baokai, CHEN Sheng, ZHU Junwu. Defect Engineering of Graphene Hybrid Catalysts for Oxygen Reduction Reactions [J]. Journal of Inorganic Materials, 2022, 37(2): 215-222. |
| [9] | WU Jing, YU Libing, LIU Shuaishuai, HUANG Qiuyan, JIANG Shanshan, ANTON Matveev, WANG Lianli, SONG Erhong, XIAO Beibei. NiN4/Cr Embedded Graphene for Electrochemical Nitrogen Fixation [J]. Journal of Inorganic Materials, 2022, 37(10): 1141-1148. |
| [10] | LIU Ziruo, LIU Wei, HAO Ce, HU Jinwen, SHI Yantao. Honeycomb-like Carbon-supported Fe Single Atom Catalyst: Preparation and Electrocatalytic Performance in Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(9): 943-949. |
| [11] | HAO Ce, LIU Ziruo, LIU Wei, SHI Yantao. Research Progress of Carbon-supported Metal Single Atom Catalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(8): 820-834. |
| [12] | ZHU Yong, GU Jun, YU Tao, HE Haitong, YAO Rui. Synthesis and Property of Platinum-cobalt Alloy Nano Catalyst [J]. Journal of Inorganic Materials, 2021, 36(3): 299-305. |
| [13] | ZHANG Ruihong, WEI Xin, LU Zhanhui, AI Yuejie. Training Model for Predicting Adsorption Energy of Metal Ions Based on Machine Learning [J]. Journal of Inorganic Materials, 2021, 36(11): 1178-1184. |
| [14] | HE Junlong, SONG Erhong, WANG Lianjun, JIANG Wan. DFT Calculation of NO Adsorption on Cr Doped Graphene [J]. Journal of Inorganic Materials, 2021, 36(10): 1047-1052. |
| [15] | DING Sheng, NING Kai, YUAN Binxia, PAN Weiguo, YIN Shibin, LIU Jianfeng. Durability of Fe-N/C Catalysts with Different Nanostructures for Electrochemical Oxygen Reduction in Alkaline Solution [J]. Journal of Inorganic Materials, 2020, 35(8): 953-958. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||