
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (10): 1065-1072.DOI: 10.15541/jim20220020
• RESEARCH ARTICLE • Previous Articles Next Articles
CHEN Yaling1,2( ), SHU Song1,2, WANG Shaoxin1,2, LI Jianjun1,2(
), SHU Song1,2, WANG Shaoxin1,2, LI Jianjun1,2( )
)
Received:2022-01-13
Revised:2022-04-14
Published:2022-10-20
Online:2022-04-26
Contact:
LI Jianjun, professor. E-mail: jjli@scu.edu.cnAbout author:CHEN Yaling (1997-), female, Master candidate. E-mail: chenyaling@stu.scu.edu.cn
Supported by:CLC Number:
CHEN Yaling, SHU Song, WANG Shaoxin, LI Jianjun. Mn-HAP SCR Catalyst: Preparation and Sulfur Resistance[J]. Journal of Inorganic Materials, 2022, 37(10): 1065-1072.
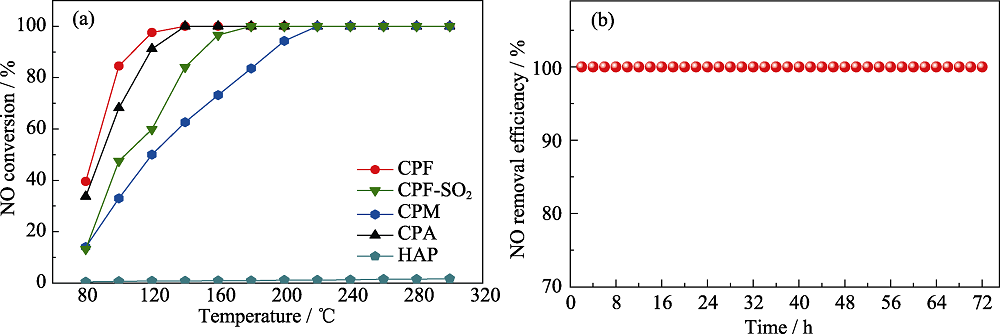
Fig. 3 (a) SCR activity of CPF, CPM, CPA, and HAP, and sulfur resistance test of CPF and (b) stability test of CPF Colorful figures are available on website
| Catalyst | Preparation method | NO conversion/% | Temperature and condition | Ref. |
|---|---|---|---|---|
| Mn-HAP | Co-preparation | 62.60 | 140 ℃, 200×10-6 SO2, 2 h | This research |
| NB-C-P | Hydrothermal | 45.00 | 150 ℃, 250×10-6 SO2, 2 h | [ |
| MnO2 | Oxalic acid co-precipitation | 17.65 | 150 ℃, 200×10-6 SO2, 2 h | [ |
| MnCrOx | Hydrothermal redox reaction | 37.46 | 150 ℃, 50×10-6 SO2, 2 h | [ |
Table 1 Activity comparison of different catalysts
| Catalyst | Preparation method | NO conversion/% | Temperature and condition | Ref. |
|---|---|---|---|---|
| Mn-HAP | Co-preparation | 62.60 | 140 ℃, 200×10-6 SO2, 2 h | This research |
| NB-C-P | Hydrothermal | 45.00 | 150 ℃, 250×10-6 SO2, 2 h | [ |
| MnO2 | Oxalic acid co-precipitation | 17.65 | 150 ℃, 200×10-6 SO2, 2 h | [ |
| MnCrOx | Hydrothermal redox reaction | 37.46 | 150 ℃, 50×10-6 SO2, 2 h | [ |
| Sample | Specific area/ (m2·g-1) | Pore volume/ (cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| CPF | 153.6 | 0.5500 | 13.49 |
| CPM | 117.3 | 0.4600 | 14.11 |
| CPA | 133.2 | 0.4300 | 11.57 |
Table 2 Physical parameters of fresh catalysts and toxic catalysts
| Sample | Specific area/ (m2·g-1) | Pore volume/ (cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| CPF | 153.6 | 0.5500 | 13.49 |
| CPM | 117.3 | 0.4600 | 14.11 |
| CPA | 133.2 | 0.4300 | 11.57 |
| Sample | O | Mn | ||||
|---|---|---|---|---|---|---|
| Olatt/O | Oads/O | Oads/Olatt | Mn2+/Mn | Mn3+/Mn | Mn4+/Mn | |
| CPF | 0.9050 | 0.0950 | 0.1050 | 0.06510 | 0.1670 | 0.7300 |
| CPM | 0.9210 | 0.0790 | 0.0860 | 0.10000 | 0.5900 | 0.3100 |
| CPA | 0.8760 | 0.1230 | 0.1410 | 0.13500 | 0.2240 | 0.6410 |
Table 3 XPS results of Mn and O on the surface of fresh catalysts and toxic catalysts
| Sample | O | Mn | ||||
|---|---|---|---|---|---|---|
| Olatt/O | Oads/O | Oads/Olatt | Mn2+/Mn | Mn3+/Mn | Mn4+/Mn | |
| CPF | 0.9050 | 0.0950 | 0.1050 | 0.06510 | 0.1670 | 0.7300 |
| CPM | 0.9210 | 0.0790 | 0.0860 | 0.10000 | 0.5900 | 0.3100 |
| CPA | 0.8760 | 0.1230 | 0.1410 | 0.13500 | 0.2240 | 0.6410 |
| [1] | 刘福东, 单文坡, 石晓燕, 等. 用于NH3选择性催化还原NO的非钒基催化剂研究进展. 催化学报, 2011, 32(7): 1113-1128. |
| [2] |
HUANG X B, WANG P, TAO J Z, XI, et al. CeO2 modified Mn-Fe-O composites and their catalytic performance for NH3-SCR of NO. Journal of Inorganic Materials, 2020, 35(5): 573-580.
DOI URL |
| [3] |
CHEN J, ZHENG Y Y, ZHANG Y B, et al. Preparation of MnO2/MWCNTs catalysts by a redox method and their activity in low-temperature SCR. Journal of Inorganic Materials, 2016, 31(12): 1347-1354.
DOI URL |
| [4] | 谭青, 冯雅晨. 我国烟气脱硝行业现状与前景及SCR脱硝催化剂的研究进展. 化工进展, 2011, 30(S1): 709-713. |
| [5] |
LI J, CHANG H, MA L, et al. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts: a review. Catalysis Today, 2011, 175(1): 147-156.
DOI URL |
| [6] |
PUTLURU S S R, SCHILL L, JENSEN A D, et al. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation- promising for selective catalytic reduction of NO with NH3 at low temperatures. Applied Catalysis B: Environmental, 2015, 165: 628-635.
DOI URL |
| [7] | LEE S, CHOI S, MIN C, et al. Study of variation of internal target volume between 4DCT and slow-ct in respiratory patterns using respiratory motion phantom. Med. Phys., 2014, 41: 180. |
| [8] | 沈伯雄, 郭宾彬, 史展亮, 等. CeO2/ACF的低温SCR烟气脱硝性能研究. 燃料化学学报, 2007(1): 125-128. |
| [9] |
IBRAHIM M, LABAKI M, GIRAUDON J-M, et al. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: a review. Journal of Hazardous Materials, 2020, 383: 121139.
DOI URL |
| [10] |
SILVESTER L, LAMONIER J F, VANNIER R N, et al. Structural, textural and acid-base properties of carbonate-containing hydroxyapatites. Journal of Materials Chemistry A, 2014, 2(29): 11073-11090.
DOI URL |
| [11] |
KUMAR P A, REDDY M P, JU L K, et al. Novel silver loaded hydroxyapatite catalyst for the selective catalytic reduction of NOx by propene. Catalysis Letters, 2008, 126(1): 78.
DOI URL |
| [12] |
BOUKHA Z, GONZALEZ-PRIOR J, DE RIVAS B, et al. Synthesis, characterisation and behaviour of Co/hydroxyapatite catalysts in the oxidation of 1,2-dichloroethane. Applied Catalysis B-Environmental, 2016, 190: 125-136.
DOI URL |
| [13] |
FAN Z, SHI J W, GAO C, et al. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: the promoting effect of Gd on the catalytic performance and sulfur resistance. Chemical Engineering Journal, 2018, 348: 820-830.
DOI URL |
| [14] |
ROBLES-AGUILA M J, REYES-AVENDANO J A, MENDOZA M E. Structural analysis of metal-doped (Mn, Fe, Co, Ni, Cu, Zn) calcium hydroxyapatite synthetized by a Sol-Gel microwave- assisted method. Ceramics International, 2017, 43(15): 12705-12709.
DOI URL |
| [15] |
CAMPISI S, GALLONI M G, BOSSOLA F, et al. Comparative performance of copper and iron functionalized hydroxyapatite catalysts in NH3-SCR. Catalysis Communications, 2019, 123: 79-85.
DOI URL |
| [16] |
GARCIABORDEJE E, PINILLA J, LAZARO M, et al. Role of sulphates on the mechanism of NH3-SCR of NO at low temperatures over presulphated vanadium supported on carbon-coated monoliths: 1. Journal of Catalysis, 2005, 233(1): 166-175.
DOI URL |
| [17] |
WANG Y, LI X, ZHAN L, et al. Effect of SO2 on activated carbon honeycomb supported CeO2-MnOx catalyst for NO removal at low-temperature. Industrial & Engineering Chemistry Research, 2015, 54(8): 2274-2278.
DOI URL |
| [18] |
ZHANG P, CHEN T, ZOU X, et al. V2O5/hematite catalyst for low temperature selective catalytic reduction of NOx with NH3. Chinese Journal of Catalysis, 2014, 35(1): 99-107.
DOI URL |
| [19] |
CHEN H, XIA Y, HUANG H, et al. Highly dispersed surface active species of Mn/Ce/TiW catalysts for high performance at low temperature NH3-SCR. Chemical Engineering Journal, 2017, 330: 1195-1202.
DOI URL |
| [20] |
FANG X, LIU Y, CHEN L, et al. Influence of surface active groups on SO2 resistance of birnessite for low-temperature NH3-SCR. Chemical Engineering Journal, 2020, 399: 125798.
DOI URL |
| [21] |
LI Y, LI Y, WANG P, et al. Low-temperature selective catalytic reduction of NOx with NH3 over MnFeOx nanorods. Chemical Engineering Journal, 2017, 330: 213-222.
DOI URL |
| [22] |
LIU Y, GUO R, DUAN C, et al. A highly effective urchin-like MnCrOxcatalyst for the selective catalytic reduction of NOx with NH3. Fuel, 2020, 271: 117667.
DOI URL |
| [23] | XIAO X, SHENG Z, YANG L, et al. Low-temperature selective catalytic reduction of NOx with NH3 over a manganese and cerium oxide/graphene composite prepared by a hydrothermal method. Catalysis Science & Technology, 2016, 6(5): 1507-1514. |
| [24] |
GAO F, TANG X, YI H, et al. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chemical Engineering Journal, 2017, 317: 20-31.
DOI URL |
| [25] |
HUANG Z, ZHU Z, LIU Z. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Applied Catalysis B: Environmental, 2002, 39(4): 361-368.
DOI URL |
| [26] |
FANG X, LIU Y, CEN W, et al. Birnessite as a highly efficient catalyst for low-temperature NH3-SCR: the vital role of surface oxygen vacancies: 33. Industrial & Engineering Chemistry Research, 2020, 59(33): 14606-14615.
DOI URL |
| [27] |
WANG J, ZHANG P, LI J, et al. Room-temperature oxidation of formaldehyde by layered manganese oxide: effect of water. Environmental Science & Technology, 2015, 49(20): 12372-12379.
DOI URL |
| [28] | PANGILINAN C D C, KURNIAWAN W, HINODE H. Effect of MnOx/TiO2 oxidation state on ozone concentration in a nonthermal plasma-driven catalysis reactor. Ozone: Science & Engineering, 2016, 38(2): 156-162. |
| [29] |
PARK M Y, KIM Y J, CHOI S M, et al. Synthesis and characterization of hydroxyapatite using ammonium hydroxide and ethylenediaminetetraacetic acid: synthesis and characterization of hydroxyapatite. Bulletin of the Korean Chemical Society, 2015, 36(7): 1806-1811.
DOI URL |
| [30] |
LEE T, BAI H. Metal sulfate poisoning effects over MnFe/TiO2 for selective catalytic reduction of NO by NH3 at low-temperature. Industrial & Engineering Chemistry Research, 2018, 57(14): 4848-4858.
DOI URL |
| [31] |
FANG N, GUO J, SHU S, et al. Effect of calcination temperature on low-temperature NH3-SCR activity and the resistance of SO2 with or without H2O over Fe-Mn-Zr catalyst. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 277-288.
DOI URL |
| [32] |
GAO F, TANG X, YI H, et al. Novel Co- or Ni-Mn binary oxide catalysts with hydroxyl groups for NH3-SCR of NOx at low temperature. Applied Surface Science, 2018, 443: 103-113.
DOI URL |
| [33] |
JI J, LU X, CHEN C, et al. Potassium-modulated δ-MnO2 as robust catalysts for formaldehyde oxidation at room temperature. Applied Catalysis B: Environmental, 2020, 260: 118210.
DOI URL |
| [34] |
KAPTEIJN F, SINGOREDJO L, ANDREINI A, et al. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Applied Catalysis B:Environmental, 1994, 3(2): 173-189.
DOI URL |
| [35] |
BONINGARI T, ETTIREDDY P R, SOMOGYVARI A, et al. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. Journal of Catalysis, 2015, 325: 145-155.
DOI URL |
| [36] | LIU Z M, ZHU J Z, LI J H, et al. Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOx with NH3. ACS Applied Materials & Interfaces, 2014, 6(16): 14500-14508. |
| [37] |
LIU F. Structure-activity relationship of iron titanate catalysts in the selective catalytic reduction of NOx with NH3. The Journal of Physical Chemistry C, 2010, 114(40): 16929-16936.
DOI URL |
| [38] |
JARRIGE J, VERVISCH P. Plasma-enhanced catalysis of propane and isopropyl alcohol at ambient temperature on a MnO2-based catalyst. Applied Catalysis B: Environmental, 2009, 90(1): 74-82.
DOI URL |
| [39] | 杨超, 刘小青, 黄碧纯, 等. Zr改性MnOx/MWCNTs催化剂的结构特征与低温SCR活性. 物理化学学报, 2014, 30(10): 1895-1902. |
| [40] | 于国峰, 韦彦斐, 金瑞奔, 等. Mn-Ce-Co/TiO2催化剂低温脱硝活性研究. 环境科学学报, 2012, 32(07): 1743-1749. |
| [41] |
WANG X, ZHENG Y, XU Z, et al. Amorphous MnO2 supported on carbon nanotubes as a superior catalyst for low temperature NO reduction with NH3. RSC Advances, 2013, 3(29): 11539-11542.
DOI URL |
| [42] |
WANG L, HUANG B, SU Y, et al. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: catalytic activity and characterization. Chemical Engineering Journal, 2012, 192: 232-241.
DOI URL |
| [43] |
JIN R, LIU Y, WU Z, et al. Low-temperature selective catalytic reduction of NO with NH3 over Mn-Ce oxides supported on TiO2 and Al2O3: a comparative study. Chemosphere, 2010, 78(9): 1160-1166.
DOI URL |
| [44] | 王淑勤, 李金梦, 杜志辉. Co共掺TiO2催化剂选择性催化还原脱硝性能研究. 环境污染与防治, 2021, 43(4): 475-480. |
| [45] |
KIJLSTRA W S, BRANDS D S, SMIT H I, et al. Mechanism of the selective catalytic reduction of NO by NH3 over MnOx/Al2O3 reactivity of adsorbed NH3 and NO complexes. Journal of Catalysis, 1997, 171(1): 219-230.
DOI URL |
| [46] |
PEÑA D A, UPHADE B S, SMIRNIOTIS P G. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: I. evaluation and characterization of first row transition metals. Journal of Catalysis, 2004, 221(2): 421-431.
DOI URL |
| [1] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [2] | LI Chengyu, DING Ziyou, HAN Yingchao. In vitro Antibacterial and Osteogenic Properties of Manganese Doped Nano Hydroxyapatite [J]. Journal of Inorganic Materials, 2024, 39(3): 313-320. |
| [3] | LIU Yan, ZHANG Yufan, WANG Ximan, LI Ting, MA Wenting, YANG Fuwei, CHEN Liang, ZHAO Dongyue, YAN Xiaoqin. Consolidation of Fragile Weathered Bone Relics Using Hydroxyapatite Material as Consolidant [J]. Journal of Inorganic Materials, 2023, 38(11): 1345-1354. |
| [4] | ZHU Yutong, TAN Peijie, LIN Hai, ZHU Xiangdong, ZHANG Xingdong. Injectable Hyaluronan/Hydroxyapatite Composite: Preparation, Physicochemical Property and Biocompatibility [J]. Journal of Inorganic Materials, 2021, 36(9): 981-990. |
| [5] | LIN Ziyang, CHANG Yuchen, WU Zhangfan, BAO Rong, LIN Wenqing, WANG Deping. Different Simulated Body Fluid on Mineralization of Borosilicate Bioactive Glass-based Bone Cement [J]. Journal of Inorganic Materials, 2021, 36(7): 745-752. |
| [6] | WU Zhongcao, HUAN Zhiguang, ZHU Yufang, WU Chengtie. 3D Printing and Characterization of Microsphere Hydroxyapatite Scaffolds [J]. Journal of Inorganic Materials, 2021, 36(6): 601-607. |
| [7] | WU Yonghao, LI Xiangfeng, ZHU Xiangdong, ZHANG Xingdong. Construction of Hydroxyapatite Nanoceramics with High Mechanical Strength and Efficiency in Promoting the Spreading and Viability of Osteoblasts [J]. Journal of Inorganic Materials, 2021, 36(5): 552-560. |
| [8] | SONG Keke, HUANG Hao, LU Mengjie, YANG Anchun, WENG Jie, DUAN Ke. Hydrothermal Preparation and Characterization of Zn, Si, Mg, Fe Doped Hydroxyapatite [J]. Journal of Inorganic Materials, 2021, 36(10): 1091-1096. |
| [9] | SHAO Yueting, ZHU Yingjie, DONG Liying, CAI Anyong. Nanocomposite “Xuan Paper” Made from Ultralong Hydroxyapatite Nanowires and Cellulose Fibers and Its Anti-mildew Properties [J]. Journal of Inorganic Materials, 2021, 36(1): 107-112. |
| [10] | SUN Tuanwei,ZHU Yingjie. One-step Solvothermal Synthesis of Strontium-doped Ultralong Hydroxyapatite Nanowires [J]. Journal of Inorganic Materials, 2020, 35(6): 724-728. |
| [11] | LIU Ziyang, GENG Zhen, LI Zhaoyang. Preparing Biomedical CaCO3/HA Composite with Oyster Shell [J]. Journal of Inorganic Materials, 2020, 35(5): 601-607. |
| [12] | DAI Zhao,WANG Ming,WANG Shuang,LI Jing,CHEN Xiang,WANG Da-Lin,ZHU Ying-Chun. Zirconia Reinforced Trace Element Co-doped Hydroxyapatite Coating [J]. Journal of Inorganic Materials, 2020, 35(2): 179-186. |
| [13] | FU Ya-Kang,WENG Jie,LIU Yao-Wen,ZHANG Ke-Hong. hBMP-2 Contained Composite Coatings on Titanium Mesh Surface: Preparation and hBMP-2 Release [J]. Journal of Inorganic Materials, 2020, 35(2): 173-178. |
| [14] | ZHOU Zihang, WANG Qun, GE Xiang, LI Zhaoyang. Strontium Doped Hydroxyapatite Nanoparticles: Synthesis, Characterization and Simulation [J]. Journal of Inorganic Materials, 2020, 35(11): 1283-1289. |
| [15] | XIAO Wen-Qian,ZHANG Jing,LI Ke-Jiang,ZOU Xin-Yu,CAI Yu-Dong,LI Bo,LIU Xue,LIAO Xiao-Ling. Litchi-like Superparamagnetic Hydroxyapatite Microspheres with Hierarchically Mesoporous Microspheres [J]. Journal of Inorganic Materials, 2019, 34(9): 925-932. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||