无机材料学报 ›› 2025, Vol. 40 ›› Issue (1): 39-46.DOI: 10.15541/jim20240296 CSTR: 32189.14.10.15541/jim20240296
所属专题: 【能源环境】燃料电池(202506)
刘磊1,2,3( ), 郭瑞华1,2,3(
), 郭瑞华1,2,3( ), 王丽4, 王艳5, 张国芳1, 关丽丽1,2
), 王丽4, 王艳5, 张国芳1, 关丽丽1,2
收稿日期:2024-06-18
修回日期:2024-08-23
出版日期:2025-01-20
网络出版日期:2024-09-02
通讯作者:
郭瑞华, 教授. E-mail: grh7810@163.com作者简介:刘磊(2000-), 男, 硕士研究生. E-mail: 1371281920@qq.com
基金资助:
LIU Lei1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), WANG Li4, WANG Yan5, ZHANG Guofang1, GUAN Lili1,2
), WANG Li4, WANG Yan5, ZHANG Guofang1, GUAN Lili1,2
Received:2024-06-18
Revised:2024-08-23
Published:2025-01-20
Online:2024-09-02
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:LIU Lei (2000-), male, Master candidate. E-mail: 1371281920@qq.com
Supported by:摘要:
Pt3Co催化剂是Pt基合金中氧还原反应(ORR)活性最高的催化剂, 合成Pt3Co高指数晶面(HIFs)是一种提高其催化性能的有效策略, 但拥有最高ORR活性的HIFs尚未明确, 并且目前缺乏对Pt3Co HIFs ORR的系统研究。本研究构建了六种不同Pt3Co HIFs, 通过从头算分子动力学(AIMD)计算证明了其稳定性, 通过密度泛函理论(DFT)计算了六种Pt3Co HIFs的ORR过程中间物*O、*OH、*OOH的结合能(BE), 通过d带中心(εd)、Bader电荷及配位数(CN)解释了其在台阶与边缘位点BE不同的原因。同时分析了吸附原子CN与εd的关系, 通过ORR自由能台阶图分析了ORR过程中的过电位(η), 发现η大小主要与*OH结合能(BE-*OH)有关, 其中η最小的晶面为Pt3Co(211), 其在台阶处的η达到了0.294 eV。本工作为高ORR活性HIFs催化剂研发提供了一定的理论依据。
中图分类号:
刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46.
LIU Lei, GUO Ruihua, WANG Li, WANG Yan, ZHANG Guofang, GUAN Lili. Oxygen Reduction Reaction on Pt3Co High-index Facets by Density Functional Theory[J]. Journal of Inorganic Materials, 2025, 40(1): 39-46.
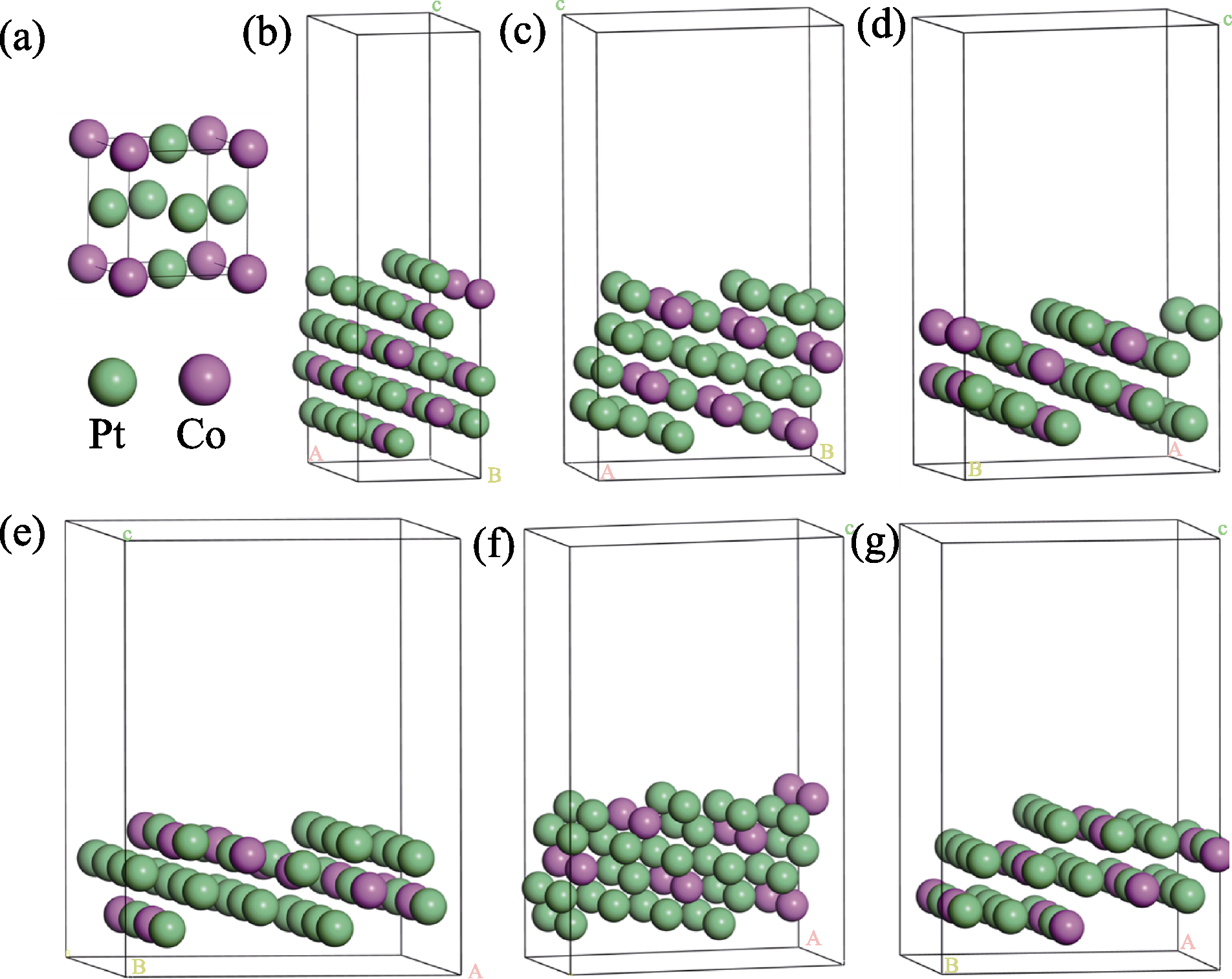
图2 Pt3Co原胞及六个Pt3Co HIFs的表面模型
Fig. 2 Surface models of Pt3Co unitcell and six Pt3Co HIFs (a) Pt3Co unitcell; (b) Pt3Co(211); (c) Pt3Co(310); (d) Pt3Co(331); (e) Pt3Co(511); (f) Pt3Co(320); (g) Pt3Co(332)
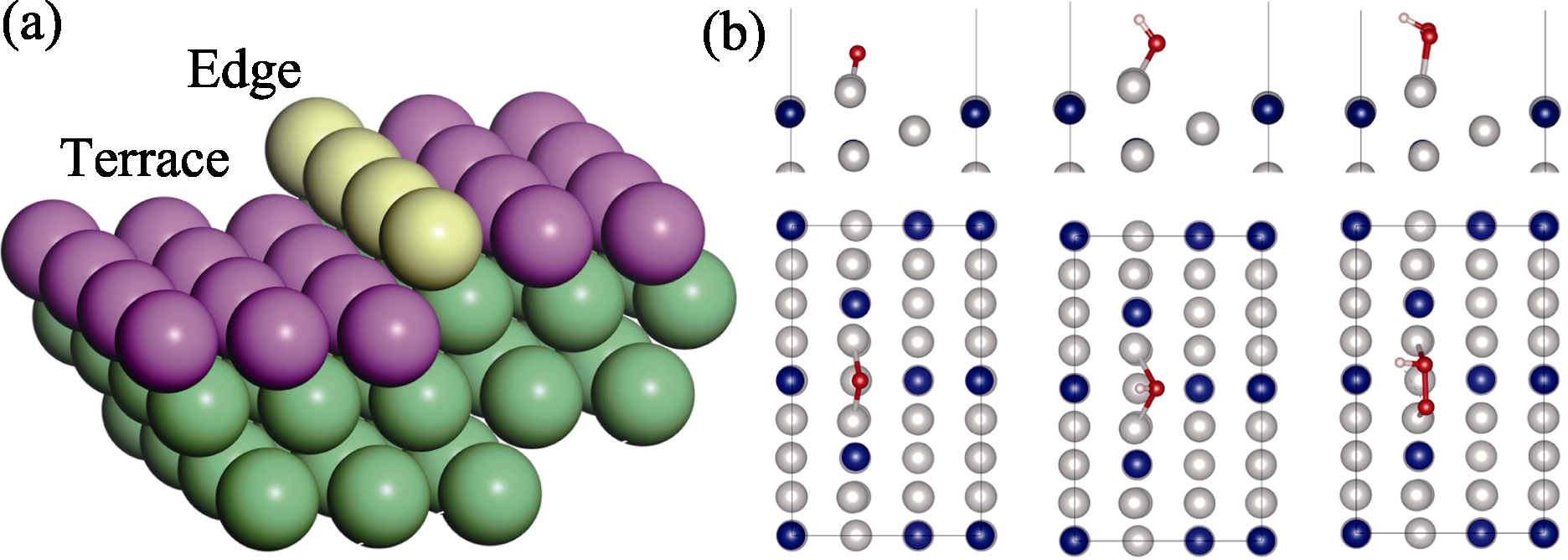
图3 (a)HIFs台阶和边缘位置示意图, 以及(b)*O、*OH、*OOH在Pt3Co(211)边缘处的最佳吸附构型
Fig. 3 (a) Schematic representation of HIFs terrace and edge positions, and (b) optimal adsorption configurations of *O, *OH, and *OOH at Pt3Co(211) edge
| HIFs | Site | *O | *OH | *OOH | |||
|---|---|---|---|---|---|---|---|
| BE/eV | BD/nm | BE/eV | BD/nm | BE/eV | BD/nm | ||
| Pt3Co(211) | Edge | 3.043 | 0.195 | 0.903 | 0.213 | 3.643 | 0.217 |
| Terrace | 2.334 | 0.206 | 0.936 | 0.200 | 3.658 | 0.206 | |
| Pt3Co(310) | Edge | 1.505 | 0.179 | 0.341 | 0.195 | 3.484 | 0.196 |
| Terrace | 1.405 | 0.197 | 0.674 | 0.213 | 3.828 | 0.212 | |
| Pt3Co(331) | Edge | 1.175 | 0.194 | 0.620 | 0.214 | 3.499 | 0.216 |
| Terrace | 1.512 | 0.208 | 0.838 | 0.200 | 3.702 | 0.221 | |
| Pt3Co(511) | Edge | 1.291 | 0.192 | 0.524 | 0.212 | 3.633 | 0.215 |
| Terrace | 1.335 | 0.197 | 0.599 | 0.213 | 3.789 | 0.214 | |
| Pt3Co(320) | Edge | 1.204 | 0.179 | 0.105 | 0.195 | 3.218 | 0.193 |
| Terrace | 1.638 | 0.181 | 0.356 | 0.197 | 3.473 | 0.199 | |
| Pt3Co(332) | Edge | 1.418 | 0.194 | 0.576 | 0.215 | 3.419 | 0.214 |
| Terrace | 1.618 | 0.201 | 0.723 | 0.215 | 3.778 | 0.212 | |
表1 不同反应中间物在六个HIFs不同位置的BE与BD
Table 1 BE and BD of different reaction intermediates at different positions of six Pt3Co HIFs
| HIFs | Site | *O | *OH | *OOH | |||
|---|---|---|---|---|---|---|---|
| BE/eV | BD/nm | BE/eV | BD/nm | BE/eV | BD/nm | ||
| Pt3Co(211) | Edge | 3.043 | 0.195 | 0.903 | 0.213 | 3.643 | 0.217 |
| Terrace | 2.334 | 0.206 | 0.936 | 0.200 | 3.658 | 0.206 | |
| Pt3Co(310) | Edge | 1.505 | 0.179 | 0.341 | 0.195 | 3.484 | 0.196 |
| Terrace | 1.405 | 0.197 | 0.674 | 0.213 | 3.828 | 0.212 | |
| Pt3Co(331) | Edge | 1.175 | 0.194 | 0.620 | 0.214 | 3.499 | 0.216 |
| Terrace | 1.512 | 0.208 | 0.838 | 0.200 | 3.702 | 0.221 | |
| Pt3Co(511) | Edge | 1.291 | 0.192 | 0.524 | 0.212 | 3.633 | 0.215 |
| Terrace | 1.335 | 0.197 | 0.599 | 0.213 | 3.789 | 0.214 | |
| Pt3Co(320) | Edge | 1.204 | 0.179 | 0.105 | 0.195 | 3.218 | 0.193 |
| Terrace | 1.638 | 0.181 | 0.356 | 0.197 | 3.473 | 0.199 | |
| Pt3Co(332) | Edge | 1.418 | 0.194 | 0.576 | 0.215 | 3.419 | 0.214 |
| Terrace | 1.618 | 0.201 | 0.723 | 0.215 | 3.778 | 0.212 | |
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| Bader charge transfer number (edge)/e | 0.199 | 0.206 | 0.187 | 0.173 | 0.214 | 0.182 |
| Bader charge transfer number (terrace)/e | 0.185 | 0.149 | 0.194 | 0.138 | 0.170 | 0.203 |
| εd (edge)/eV | -1.502 | -1.560 | -1.683 | -1.746 | -1.530 | -1.671 |
| εd (terrace)/eV | -1.729 | -1.667 | -1.820 | -1.831 | -1.707 | -1.836 |
表2 六个Pt3Co HIFs不同位置Pt原子的Bader电荷转移数和εd
Table 2 Bader charge transfer number and εd of Pt atoms at different positions of six Pt3Co HIFs
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| Bader charge transfer number (edge)/e | 0.199 | 0.206 | 0.187 | 0.173 | 0.214 | 0.182 |
| Bader charge transfer number (terrace)/e | 0.185 | 0.149 | 0.194 | 0.138 | 0.170 | 0.203 |
| εd (edge)/eV | -1.502 | -1.560 | -1.683 | -1.746 | -1.530 | -1.671 |
| εd (terrace)/eV | -1.729 | -1.667 | -1.820 | -1.831 | -1.707 | -1.836 |
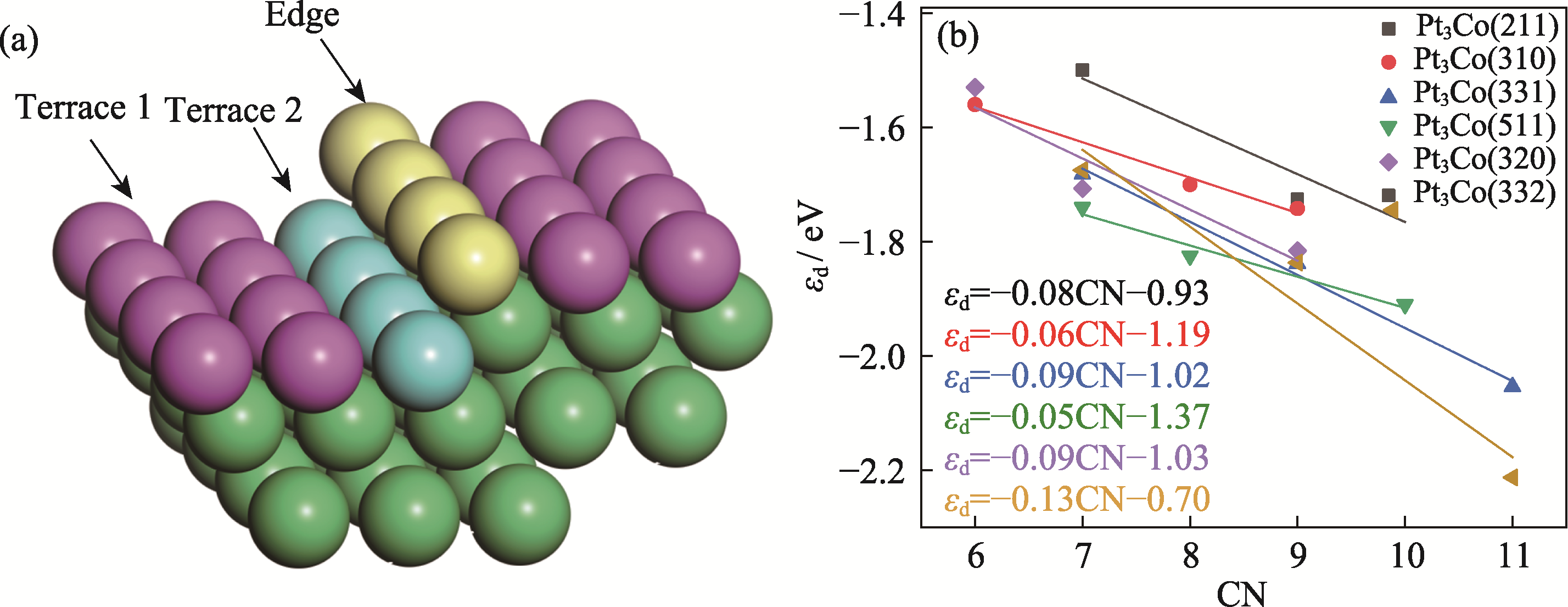
图4 (a)依据CN标识的表面台阶和边缘位置示意图, 以及(b)六个HIFs吸附原子εd和CN的关系
Fig. 4 (a) Schematic representation of the positions of surface terrace and edge identified on the basis of CN, and (b) relationships of εd and CN for the six HIFs adsorbed atoms Colorful figures are available on website
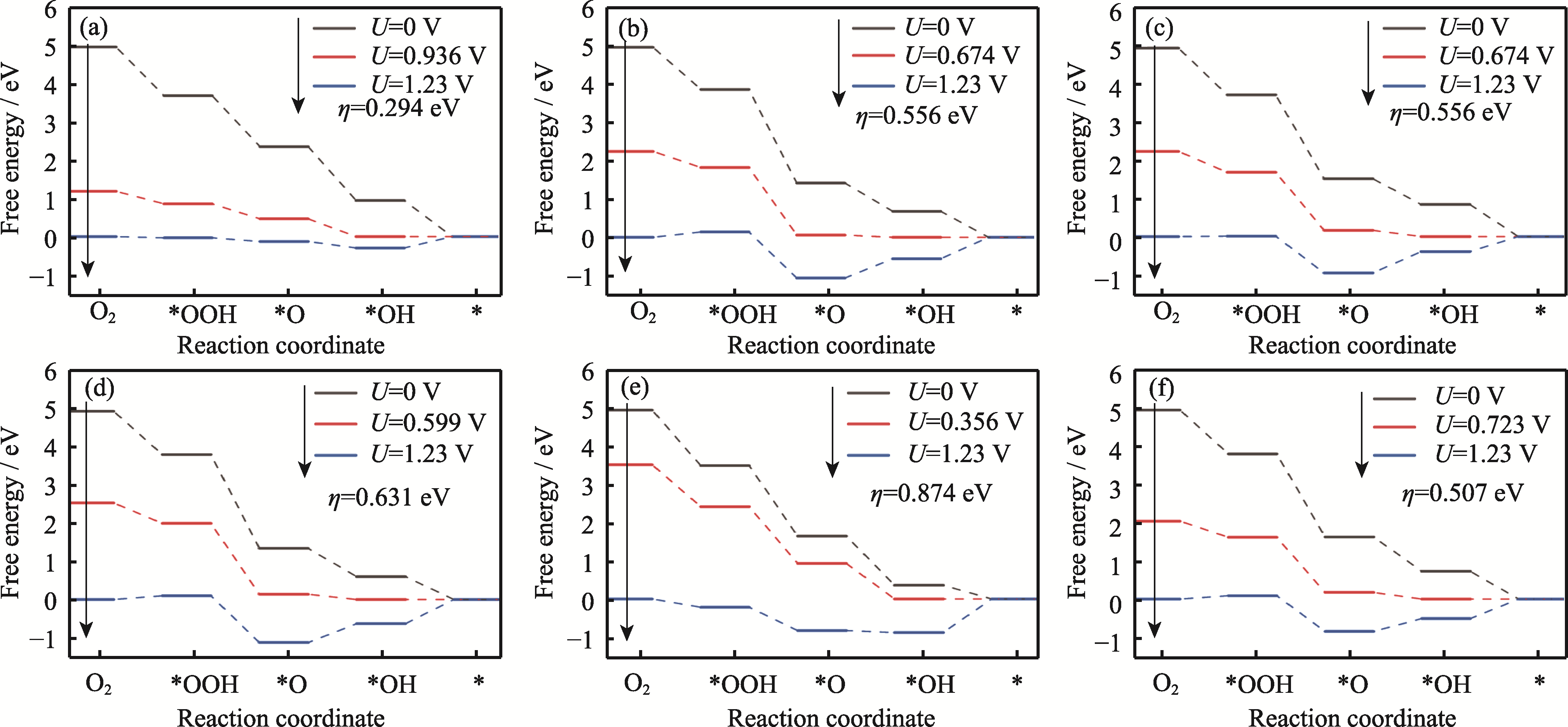
图5 Pt3Co HIFs催化剂在台阶处ORR自由能变化图
Fig. 5 Plots of ORR free energy at terrace for Pt3Co HIFs catalysts (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| ηORR (edge)/eV | 0.630 | 0.889 | 0.675 | 0.706 | 1.125 | 0.654 |
| ηORR (terrace)/eV | 0.294 | 0.556 | 0.556 | 0.631 | 0.874 | 0.507 |
表3 六个Pt3Co HIFs在不同位置的ηORR
Table 3 Bader charge transfer number and εd of Pt atoms at different positions of six Pt3Co HIFs
| HIFs | Pt3Co(211) | Pt3Co(310) | Pt3Co(331) | Pt3Co(511) | Pt3Co(320) | Pt3Co(332) |
|---|---|---|---|---|---|---|
| ηORR (edge)/eV | 0.630 | 0.889 | 0.675 | 0.706 | 1.125 | 0.654 |
| ηORR (terrace)/eV | 0.294 | 0.556 | 0.556 | 0.631 | 0.874 | 0.507 |
| HIFs | Microfact notation | k-point | Number of atoms |
|---|---|---|---|
| Pt3Co(211) | n(111)×(100) | 3×2×1 | 48 |
| Pt3Co(310) | n(100)×(110) | 3×2×1 | 48 |
| Pt3Co(331) | n(110)×(111) | 2×2×1 | 48 |
| Pt3Co(511) | n(100)×(111) | 2×2×1 | 48 |
| Pt3Co(320) | n(110)×(100) | 3×2×1 | 48 |
| Pt3Co(332) | n(111)×(110) | 2×2×1 | 48 |
表S1 六个HIFs的微面标记方式、k点、原子数
Table S1 Microfact notation, k-point, number of atoms of six HIFs
| HIFs | Microfact notation | k-point | Number of atoms |
|---|---|---|---|
| Pt3Co(211) | n(111)×(100) | 3×2×1 | 48 |
| Pt3Co(310) | n(100)×(110) | 3×2×1 | 48 |
| Pt3Co(331) | n(110)×(111) | 2×2×1 | 48 |
| Pt3Co(511) | n(100)×(111) | 2×2×1 | 48 |
| Pt3Co(320) | n(110)×(100) | 3×2×1 | 48 |
| Pt3Co(332) | n(111)×(110) | 2×2×1 | 48 |
| HIFs | Site | BE-*OH/eV | εd/eV |
|---|---|---|---|
| Pt3Co(211) | Edge | 0.903 | -1.502 |
| Terrace | 0.936 | -1.729 | |
| Pt3Co(310) | Edge | 0.341 | -1.746 |
| Terrace | 0.674 | -1.831 | |
| Pt3Co(331) | Edge | 0.620 | -1.560 |
| Terrace | 0.838 | -1.667 | |
| Pt3Co(511) | Edge | 0.524 | -1.530 |
| Terrace | 0.599 | -1.707 | |
| Pt3Co(320) | Edge | 0.105 | -1.683 |
| Terrace | 0.356 | -1.820 | |
| Pt3Co(332) | Edge | 0.576 | -1.671 |
| Terrace | 0.723 | -1.836 |
表S2 六个HIFs不同位置的BE-*OH与εd
Table S2 BE-*OH and εd at different positions of six HIFs
| HIFs | Site | BE-*OH/eV | εd/eV |
|---|---|---|---|
| Pt3Co(211) | Edge | 0.903 | -1.502 |
| Terrace | 0.936 | -1.729 | |
| Pt3Co(310) | Edge | 0.341 | -1.746 |
| Terrace | 0.674 | -1.831 | |
| Pt3Co(331) | Edge | 0.620 | -1.560 |
| Terrace | 0.838 | -1.667 | |
| Pt3Co(511) | Edge | 0.524 | -1.530 |
| Terrace | 0.599 | -1.707 | |
| Pt3Co(320) | Edge | 0.105 | -1.683 |
| Terrace | 0.356 | -1.820 | |
| Pt3Co(332) | Edge | 0.576 | -1.671 |
| Terrace | 0.723 | -1.836 |
图S1 AIMD模拟过程中六个Pt3Co HIFs的温度、能量平衡曲线
Fig. S1 Temperature and energy balance curves during AIMD simulations of six Pt3Co HIFs (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
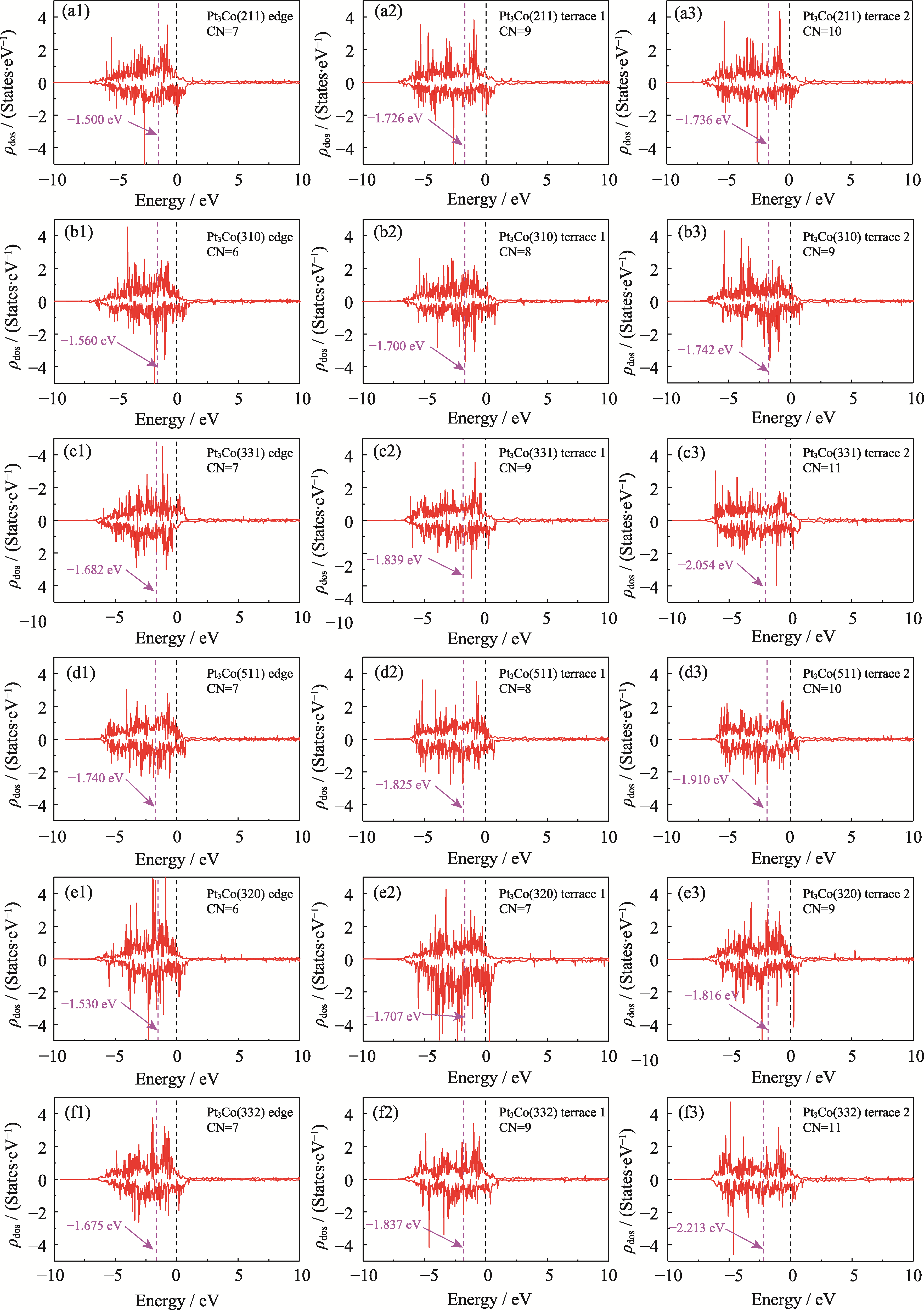
图S8 六个HIFs在边缘、台阶1、台阶2处的ρdos和εd
Fig. S8 ρdos and εd for the six HIFs at edge, terrace 1, and terrace 2 (a1-a3) Pt3Co(211); (b1-b3) Pt3Co(310); (c1-c3) Pt3Co(331); (d1-d3) Pt3Co(511); (e1-e3) Pt3Co(320); (f1-f3) Pt3Co(332)
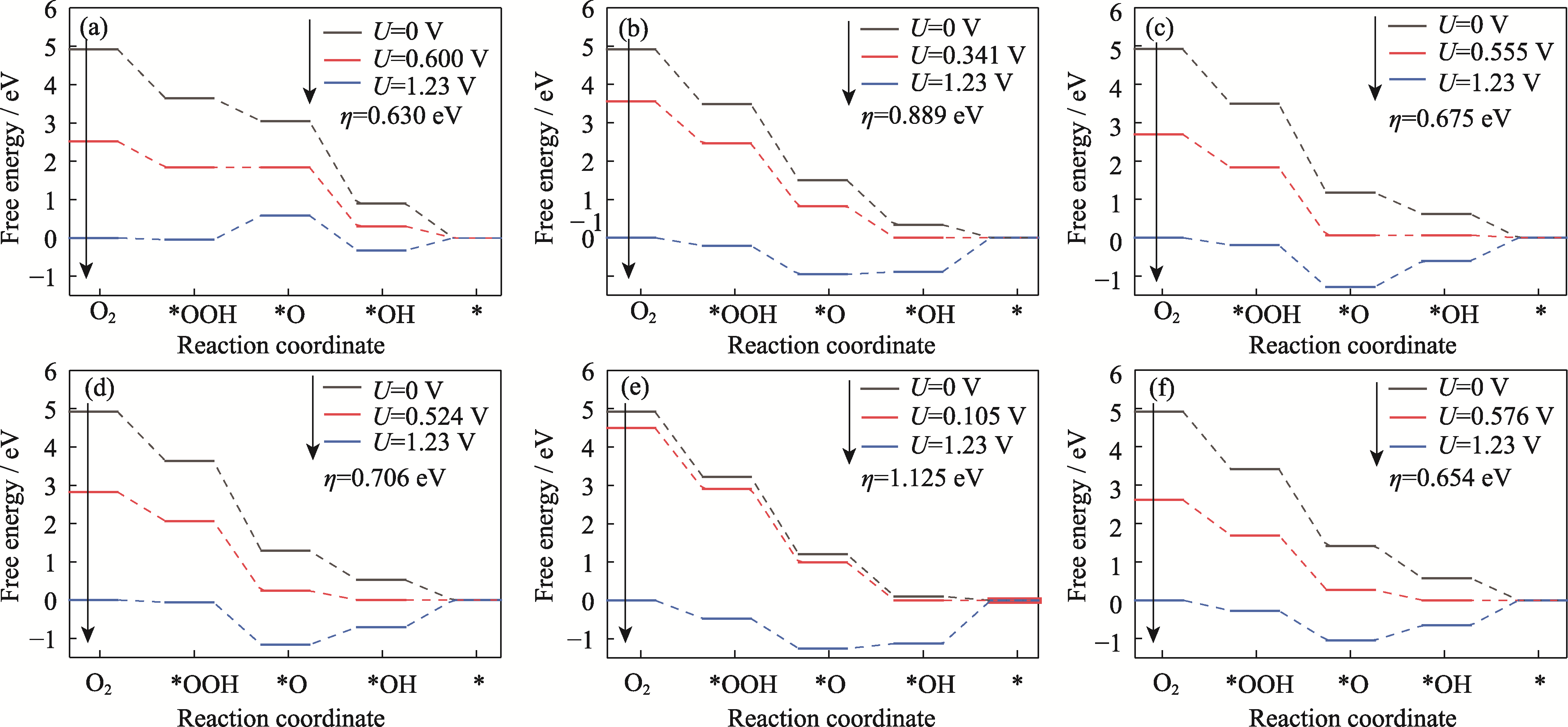
图S9 Pt3Co HIFs催化剂在边缘处的ORR自由能变化图
Fig. S9 Plots of ORR free energy at edge for Pt3Co HIFs catalysts (a) Pt3Co(211); (b) Pt3Co(310); (c) Pt3Co(331); (d) Pt3Co(511); (e) Pt3Co(320); (f) Pt3Co(332)
| [1] | XIA B Y, WU H B, WANG X, et al. Highly concave platinum nanoframes with high-index facets and enhanced electrocatalytic properties. Angewandte Chemie International Edition, 2013, 52(47): 12337. |
| [2] | WANG Y, MA S, LI Q, et al. Hollow platinum nanospheres and nanotubes templated by shear flow-induced lipid vesicles and tubules and their applications on hydrogen evolution. ACS Sustainable Chemistry & Engineering, 2016, 4(7): 3773. |
| [3] | SUBBARAMAN R, TRIPKOVIC D, STRMCNIK D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science, 2011, 334(6060): 1256. |
| [4] | LIU M, XIAO X, LI Q, et al. Recent progress of electrocatalysts for oxygen reduction in fuel cells. Journal of Colloid and Interface Science, 2022, 607: 791. |
| [5] | WANG X X, SWIHART M T, WU G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nature Catalysis, 2019, 2: 578. |
| [6] | ZENG Z, KÜSPERT S, BALAGHI S E, et al. Ultrahigh mass activity Pt entities consisting of Pt single atoms, clusters, and nanoparticles for improved hydrogen evolution reaction. Small, 2023, 19(29): 2205885. |
| [7] | GUO R, AN N, AN S, et al. One-step preparation of nitrogen- doped platinum-based catalysts for electrocatalytic oxidation of ethanol. Catalysts, 2021, 11(11): 1264. |
| [8] | DU L, PRABHAKARAN V, XIE X, et al. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: stability challenges and material solutions. Advanced Materials, 2021, 33(6): 1908232. |
| [9] | BANHAM D, CHOI J Y, KISHIMOTO T, et al. Integrating PGM- free catalysts into catalyst layers and proton exchange membrane fuel cell devices. Advanced Materials, 2019, 31(31): 1804846. |
| [10] | HE Y, WU G. PGM-free oxygen-reduction catalyst development for proton-exchange membrane fuel cells: challenges, solutions, and promises. Accounts of Materials Research, 2022, 3(2): 224. |
| [11] | YAO Y, GUO R, AN S, et al. In-situ loaded Pt-Co high index facets catalysts: preparation and electrocatalytic performance. Journal of Inorganic Materials, 2023, 38(1): 71. |
| [12] |
TIAN N, ZHOU Z Y, SUN S G, et al. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science, 2007, 316(5825): 732.
PMID |
| [13] |
VAN SANTEN R A. Complementary structure sensitive and insensitive catalytic relationships. Accounts of Chemical Research, 2009, 42(1): 57.
DOI PMID |
| [14] | SUN L, WANG Q, MA M, et al. Etching-assisted synthesis of single atom Ni-tailored Pt nanocatalyst enclosed by high-index facets for active and stable oxygen reduction catalysis. Nano Energy, 2022, 103: 107800. |
| [15] |
STAMENKOVIC V R, MUN B S, ARENZ M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature Materials, 2007, 6(3): 241.
DOI PMID |
| [16] | WANG X X, HWANG S, PAN Y T, et al. Ordered Pt3Co intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction. Nano Letters, 2018, 18(7): 4163. |
| [17] | XU W C, ZHANG Z M, YANG C H, et al. Promotion mechanism of PtCo intermetallic ordered alloys in oxygen reduction reaction and its application in fuel cells. Electrochemistry Communications, 2023, 152: 107516. |
| [18] |
ZHANG C, CHEN Z, YANG H, et al. Surface-structure tailoring of dendritic PtCo nanowires for efficient oxygen reduction reaction. Journal of Colloid and Interface Science, 2023, 652: 1597.
DOI PMID |
| [19] | TETTEH E B, GYAN-BARIMAH C, LEE H Y, et al. Strained Pt(221) facet in a PtCo@Pt-rich catalyst boosts oxygen reduction and hydrogen evolution activity. ACS Applied Materials & Interfaces, 2022, 14(22): 25246. |
| [20] | DONG J C, SU M, BRIEGA-MARTOS V, et al. Direct in situ Raman spectroscopic evidence of oxygen reduction reaction intermediates at high-index Pt(hkl) surfaces. Journal of the American Chemical Society, 2020, 142(2): 715. |
| [21] |
HUANG L, LIU M, LIN H, et al. Shape regulation of high-index facet nanoparticles by dealloying. Science, 2019, 365(6458): 1159.
DOI PMID |
| [22] | KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science, 1996, 6(1): 15. |
| [23] |
PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77(18): 3865.
DOI PMID |
| [24] | GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Journal of Chemical Physics, 2010, 132(15): 154104. |
| [25] | KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 1999, 59(3): 1758. |
| [26] | LI K, LI Y, WANG Y, et al. The oxygen reduction reaction on Pt(111) and Pt(100) surfaces substituted by subsurface Cu: a theoretical perspective. Journal of Materials Chemistry A, 2015, 3(21): 11444. |
| [27] | ZHU Y, WANG Z. Tuning the N coordination environment of Ir single-atom-catalyst for highly efficient ORR and OER: a computational study. Catalysis Letters, 2024, 154(5): 2464. |
| [28] | ZHANG X, LIU X, WU D, et al. Self-assembly intermetallic PtCu3 core with high-index faceted Pt shell for high-efficiency oxygen reduction. Nano Letters, 2024, 24(10): 3213. |
| [29] | KAN D, WANG D, ZHANG X, et al. Rational design of bifunctional ORR/OER catalysts based on Pt/Pd-doped Nb2CT2 MXene by first-principles calculations. Journal of Materials Chemistry A, 2020, 8(6): 3097. |
| [30] | NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. Journal of Physical Chemistry B, 2004, 108(46): 17886. |
| [31] | HAMMER B, NØRSKOV J K. Electronic factors determining the reactivity of metal surfaces. Surface Science, 1995, 343(3): 211. |
| [32] | MAVRIKAKIS M, HAMMER B, NØRSKOV J K. Effect of strain on the reactivity of metal surfaces. Physical Review Letters, 1998, 81(13): 2819. |
| [33] |
KITCHIN J R, NØRSKOV J K, BARTEAU M A, et al. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. Journal of Chemical Physics, 2004, 120(21): 10240.
PMID |
| [34] | TIAN N, ZHOU Z Y, SUN S G. Platinum metal catalysts of high- index surfaces: from single-crystal planes to electrochemically shape-controlled nanoparticles. Journal of Physical Chemistry C, 2008, 112(50): 19801. |
| [35] | VAN HOVE M A, SOMORJAI G A. A new microfacet notation for high-Miller-index surfaces of cubic materials with terrace, step and kink structures. Surface Science, 1980, 92(2/3): 489. |
| [36] | LANG B, JOYNER R W, SOMORJAI G A. Low energy electron diffraction studies of chemisorbed gases on stepped surfaces of platinum. Surface Science, 1972, 30(2): 454. |
| [37] |
SHENG T, LIN W F, SUN S G. Elucidation of the surface structure-selectivity relationship in ethanol electro-oxidation over platinum by density functional theory. Physical Chemistry Chemical Physics, 2016, 18(23): 15501.
DOI PMID |
| [38] | ZHANG B W, SHENG T, WANG Y X, et al. Platinum-cobalt bimetallic nanoparticles with Pt skin for electro-oxidation of ethanol. ACS Catalysis, 2017, 7(1): 892. |
| [39] | WANG C, CAI D, LIU B, et al. Ethanol-sensing performance of tin dioxide octahedral nanocrystals with exposed high-energy {111} and {332} facets. Journal of Materials Chemistry A, 2014, 2(27): 10623. |
| [40] | BARICUATRO J H, KIM Y G, TSANG C F, et al. Reprint of “Selective conversion of CO into ethanol on Cu(511) surface reconstructed from Cu(pc): operando studies by electrochemical scanning tunneling microscopy, mass spectrometry, quartz crystal nanobalance, and infrared spectroscopy”. Journal of Electroanalytical Chemistry, 2020, 875: 114757. |
| [41] | WEI L, MAO Y J, LIU F, et al. Concave cubic Pt-Sm alloy nanocrystals with high-index facets and enhanced electrocatalytic ethanol oxidation. ACS Applied Energy Materials, 2019, 2(10): 7204. |
| [42] | BOWKER M, KING D A. Oxygen diffusion on tungsten single crystal surfaces: secondary electron emission studies. Surface Science, 1980, 94(2/3): 564. |
| [43] | XUE F, GUO X, MIN B, et al. Unconventional high-index facet of iridium boosts oxygen evolution reaction: how the facet matters. ACS Catalysis, 2021, 11(13): 8239. |
| [44] | MA Y, BALBUENA P B. Pt surface segregation in bimetallic Pt3M alloys: a density functional theory study. Surface Science, 2008, 602(1): 107. |
| [45] | ZENG X M, HUANG R, SHAO G F, et al. High-index-faceted platinum nanoparticles: insights into structural and thermal stabilities and shape evolution from atomistic simulations. Journal of Materials Chemistry A, 2014, 2(29): 11480. |
| [46] | WANG H, AN W, LIU X, et al. Oxygen reduction reaction on Pt(111), Pt(221), and Ni/Au1Pt3(221) surfaces: probing scaling relationships of reaction energetics and interfacial composition. Chemical Engineering Science, 2018, 184: 239. |
| [47] |
ZHU Q, SAIDI W A, YANG J C. Step-edge directed metal oxidation. Journal of Physical Chemistry Letters, 2016, 7(13): 2530.
DOI PMID |
| [48] | LIU S, HUANG S. The role of interface charge transfer on Pt based catalysts for water splitting. International Journal of Hydrogen Energy, 2018, 43(32): 15225. |
| [49] | RAJKAMAL A, KIM H. Theoretical verification on adsorptive removal of caffeine by carbon and nitrogen-based surfaces: role of charge transfer, π electron occupancy, and temperature. Chemosphere, 2023, 339: 139667. |
| [50] | YAN T, HOU H, WU C, et al. Unraveling the molecular mechanism for enhanced gas adsorption in mixed-metal MOFs via solid-state NMR spectroscopy. Proceedings of the National Academy of Sciences, 2024, 121(6):e2312959121. |
| [51] | NØRSKOV J K, STUDT F, ABILD-PEDERSEN F, et al. Fundamental concepts in heterogeneous catalysis. Angewandte Chemie International Edition, 2015, 54(36): 10404. |
| [52] | STEPHENS I E L, BONDARENKO A S, GRØNBJERG U, et al. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy & Environmental Science, 2012, 5(5): 6744. |
| [53] |
GREELEY J, STEPHENS I E L, BONDARENKO A S, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nature Chemistry, 2009, 1(7): 552.
DOI PMID |
| [54] | CALLE-VALLEJO F, TYMOCZKO J, COLIC V, et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science, 2015, 350(6257): 185. |
| [55] | CALLE-VALLEJO F, LOFFREDA D, KOPER M T M, et al. Introducing structural sensitivity into adsorption-energy scaling relations by means of coordination numbers. Nature Chemistry, 2015, 7(5): 403. |
| [56] | YUE J, DU Z, SHAO M.Mechanisms of enhanced electrocatalytic activity for oxygen reduction reaction on high-index platinum n(111)-(111) surfaces. Journal of Physical Chemistry Letters, 2015, 6(17): 3346. |
| [1] | 李薛茹, 马哲杰, 郭宇杰, 李平. 载体特性对Pt/C催化剂上离聚物覆盖度及氧还原性能的影响[J]. 无机材料学报, 2025, 40(12): 1395-1404. |
| [2] | 李红兰, 张俊苗, 宋二红, 杨兴林. Mo/S共掺杂的石墨烯用于合成氨: 密度泛函理论研究[J]. 无机材料学报, 2024, 39(5): 561-568. |
| [3] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [4] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [5] | 杨代辉, 孙甜, 田合鑫, 史晓斐, 马东伟. 铁氮共掺杂介孔碳材料的简易制备及其氧还原反应催化性能[J]. 无机材料学报, 2023, 38(11): 1309-1315. |
| [6] | 姚仪帅, 郭瑞华, 安胜利, 张捷宇, 周国治, 张国芳, 黄雅荣, 潘高飞. 原位负载Pt-Co高指数晶面催化剂的制备及其电催化性能[J]. 无机材料学报, 2023, 38(1): 71-78. |
| [7] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [8] | 王鹏, 靳遵龙, 陈宁光, 刘勇豪. Mo掺杂α-MnO2电催化析氧反应的理论研究[J]. 无机材料学报, 2022, 37(5): 541-546. |
| [9] | 蒋丽丽, 徐帅帅, 夏宝凯, 陈胜, 朱俊武. 缺陷调控石墨烯复合催化剂在氧还原反应中的作用[J]. 无机材料学报, 2022, 37(2): 215-222. |
| [10] | 刘自若, 刘炜, 郝策, 胡金文, 史彦涛. 蜂窝状碳负载铁基单原子催化剂的制备及ORR催化性能研究[J]. 无机材料学报, 2021, 36(9): 943-949. |
| [11] | 郝策, 刘自若, 刘炜, 史彦涛. 用于氧还原反应的碳基负载金属单原子催化剂研究进展[J]. 无机材料学报, 2021, 36(8): 820-834. |
| [12] | 李友兵, 秦彦卿, 陈科, 陈露, 张霄, 丁浩明, 李勉, 张一鸣, 都时禹, 柴之芳, 黄庆. 熔盐法合成纳米层状Sc2SnC MAX相[J]. 无机材料学报, 2021, 36(7): 773-778. |
| [13] | 朱勇, 顾军, 于涛, 何海佟, 姚睿. 铂钴合金纳米电催化剂的制备及性能研究[J]. 无机材料学报, 2021, 36(3): 299-305. |
| [14] | 张瑞鸿, 魏鑫, 卢占会, 艾玥洁. 基于机器学习训练金属离子吸附能预测模型的研究[J]. 无机材料学报, 2021, 36(11): 1178-1184. |
| [15] | 何俊龙, 宋二红, 王连军, 江莞. DFT方法研究一氧化氮在铬掺杂石墨烯上的吸附行为[J]. 无机材料学报, 2021, 36(10): 1047-1052. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||