无机材料学报 ›› 2021, Vol. 36 ›› Issue (4): 339-346.DOI: 10.15541/jim20200611 CSTR: 32189.14.10.15541/jim20200611
孙鲁超1( ), 任孝旻1,2, 杜铁锋1, 罗颐秀1, 张洁1, 王京阳1(
), 任孝旻1,2, 杜铁锋1, 罗颐秀1, 张洁1, 王京阳1( )
)
收稿日期:2020-10-27
修回日期:2020-12-14
出版日期:2021-04-20
网络出版日期:2020-12-10
通讯作者:
王京阳, 研究员. E-mail: jywang@imr.ac.cn
作者简介:孙鲁超(1984-), 男, 副研究员. E-mail: lcsun@imr.ac.cn
基金资助:
SUN Luchao1( ), REN Xiaomin1,2, DU Tiefeng1, LUO Yixiu1, ZHANG Jie1, WANG Jingyang1(
), REN Xiaomin1,2, DU Tiefeng1, LUO Yixiu1, ZHANG Jie1, WANG Jingyang1( )
)
Received:2020-10-27
Revised:2020-12-14
Published:2021-04-20
Online:2020-12-10
Contact:
WANG Jingyang, professor. E-mail: jywang@imr.ac.cn
About author:SUN Luchao(1984-), male, associate professor. E-mail: lcsun@imr.ac.cn
Supported by:摘要:
环境障涂层是先进航空发动机高温结构部件用碳化硅纤维增强碳化硅(SiCf/SiC)陶瓷基复合材料的关键防护。稀土硅酸盐具有低热膨胀系数、优良的抗水氧/CMAS腐蚀性能以及与硅基陶瓷良好的化学相容性, 是目前国际公认的优选环境障涂层体系。常规含单一稀土元素的稀土硅酸盐环境障涂层材料, 存在热应力失配、高温相转变和耐腐蚀性能差等问题, 尚无法完全满足极端燃气环境中的长寿命服役要求。本综述介绍了为解决稀土硅酸盐环境障涂层的关键性能局限, 利用高熵化合物设计方法, 针对稀土硅酸盐热学性能(热膨胀系数和热导率)调控、耐CMAS腐蚀性能提升和相结构稳定性优化方面获得的新进展。这些研究进展为稀土硅酸盐材料的创新设计提供了新思路, 为其作为下一代环境障涂层的性能突破提供了支撑。
中图分类号:
孙鲁超, 任孝旻, 杜铁锋, 罗颐秀, 张洁, 王京阳. 高熵化设计: 稀土硅酸盐材料关键性能优化新策略[J]. 无机材料学报, 2021, 36(4): 339-346.
SUN Luchao, REN Xiaomin, DU Tiefeng, LUO Yixiu, ZHANG Jie, WANG Jingyang. High Entropy Engineering: New Strategy for the Critical Property Optimizations of Rare Earth Silicates[J]. Journal of Inorganic Materials, 2021, 36(4): 339-346.
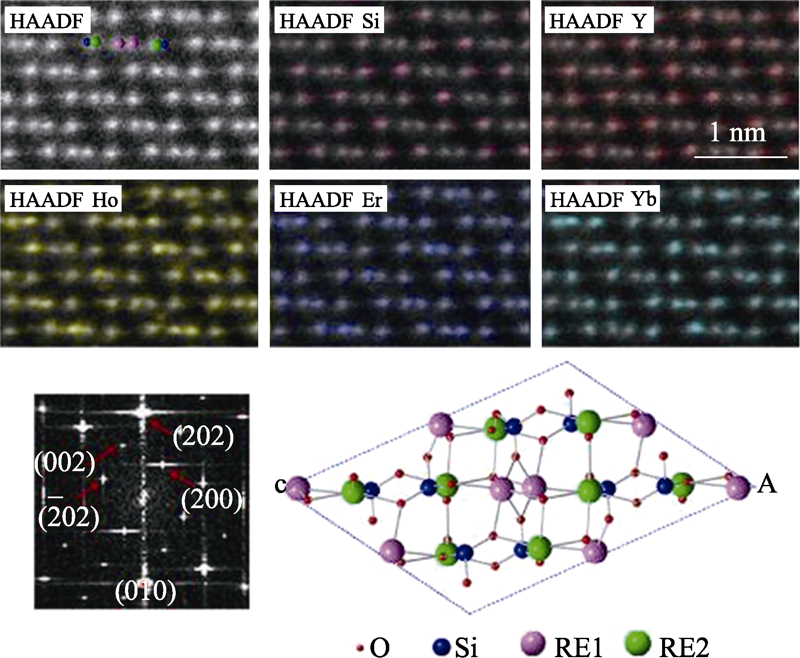
图1 高熵稀土单硅酸盐(Y1/4Ho1/4Er1/4Yb1/4)2SiO5的HAADF- STEM照片及其原子尺度元素分布图[40]
Fig. 1 HAADF-STEM image of high entropy (Y1/4Ho1/4Er1/4Yb1/4)2- SiO5, EDS mapping of uniform spatial distributions for each element[40]
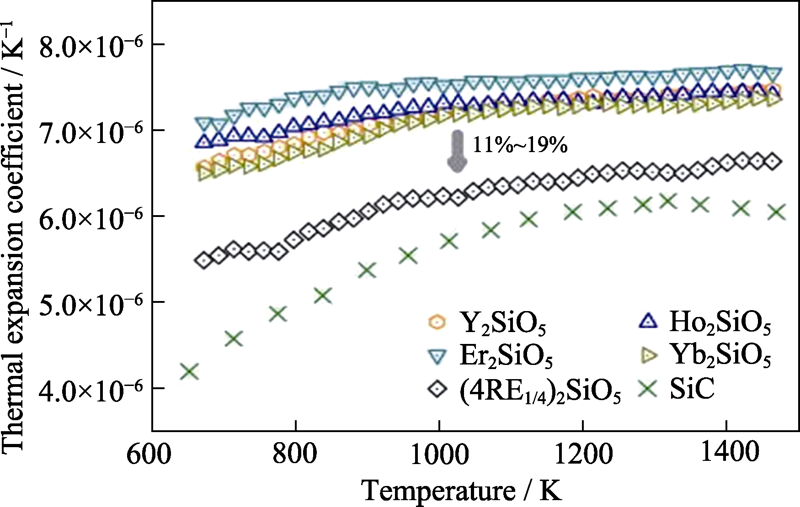
图2 高熵稀土单硅酸盐(Y1/4Ho1/4Er1/4Yb1/4)2SiO5的热膨胀系数随温度变化关系[40]
Fig. 2 Temperature dependent thermal expansion coefficient of high entropy (Y1/4Ho1/4Er1/4Yb1/4)2SiO5[40]
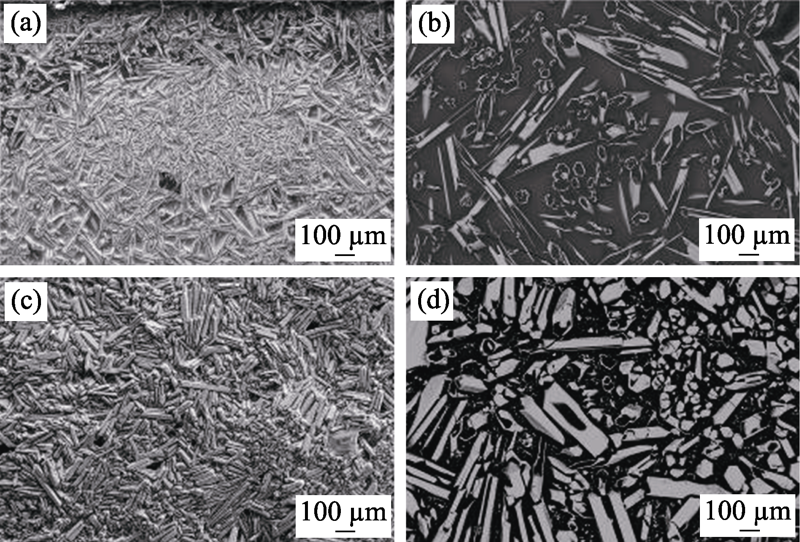
图3 高熵稀土双硅酸盐(Er1/4Tm1/4Yb1/4Lu1/4)2Si2O7在1500 ℃高温CMAS腐蚀(a~b)4 h和(c~d)50 h后样品表面形貌[48]
Fig. 3 Surface observations of high entropy (Er1/4Tm1/4Yb1/4Lu1/4)2Si2O7 after CMAS corrosion at 1500 ℃ for 4 h (a-b) and 50 h (c-d) [48]
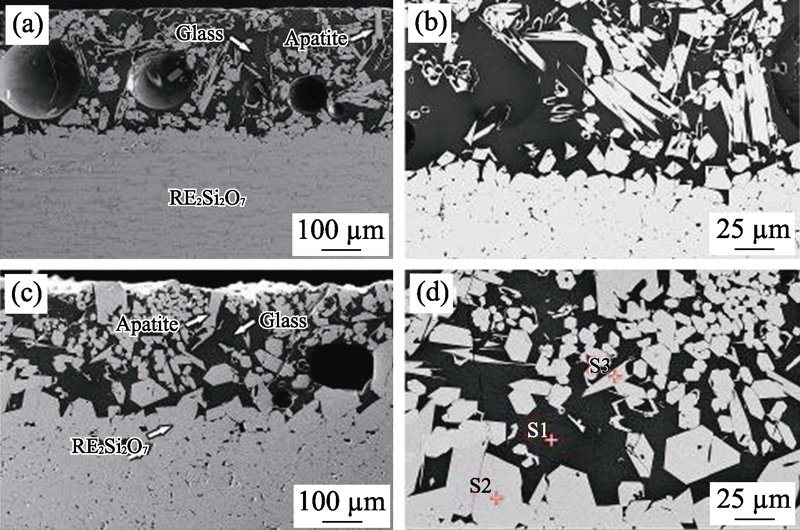
图4 高熵稀土双硅酸盐(Er1/4Tm1/4Yb1/4Lu1/4)2Si2O7在1500 ℃下CMAS腐蚀(a~b)4 h和(c~d)50 h截面形貌[48]
Fig. 4 Observations of the reaction front in the cross-sections of high entropy (Er1/4Tm1/4Yb1/4Lu1/4)2Si2O7 after CMAS corrosion at 1500 ℃ for 4 h (a,b) and 50 h (c,d)[48]
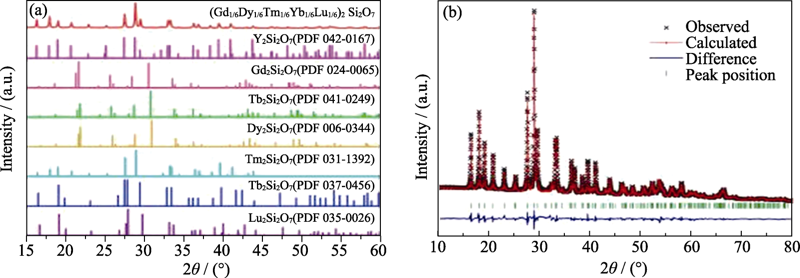
图5 (a)高熵稀土双硅酸盐(Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2 Si2O7及RE2Si2O7 (RE = Y, Gd, Tb, Dy, Tm, Yb和Lu)的XRD图谱和(b)高熵稀土双硅酸盐(Gd1/6 Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2Si2O7的 XRD图谱Rietveld精修结果[50]
Fig. 5 (a) XRD patterns of (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2 Si2O7, along with the standard XRD patterns of RE2Si2O7 (RE = Y, Gd, Tb, Dy, Tm, Yb and Lu) and (b) Rietveld refinement of XRD pattern for (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2Si2O7[50]
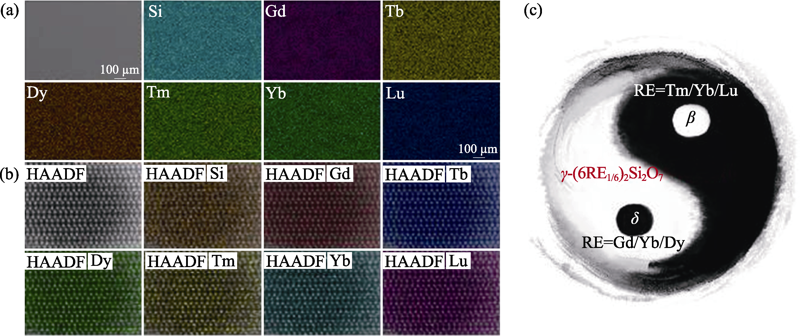
图6 高熵稀土双硅酸盐(Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2Si2O7的(a) SEM照片和各元素分布的面扫描能谱分析; (b) HAADF-STEM及原子尺度元素分布; (c)稀土双硅酸盐全新相稳定模式示意图[50]
Fig. 6 (a) SEM image of (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2Si2O7 surface with EDS mappings of Si, Gd, Tb, Dy, Tm, Yb and Lu, (b) STEM high angle annular dark field (HAADF) image and corresponding selected compositional EDS maps of high entropy (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2Si2O7, and (c) schematic diagram of the phase formation of (6RE1/6)2Si2O7[50]
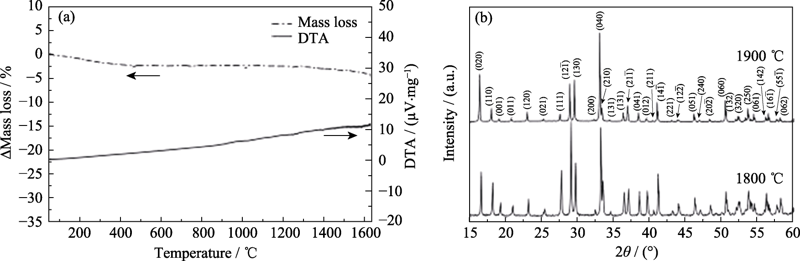
图7 高熵稀土双硅酸盐(Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2 Si2O7的(a)热重-差热分析曲线和(b)1800及1900 ℃热处理2 h后样品的XRD图谱[50]
Fig. 7 (a)TG/DTA curves of (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/4Lu1/6)2 Si2O7 and (b) XRD patterns of specimens after being heat-treated at 1800 and 1900 ℃ for 2 h[50]
| [1] |
TSAI M H, YEH J W. High-entropy alloys: a critical review. Materials Research Letters, 2014,2(3):107-123.
DOI URL |
| [2] |
MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Materialia, 2017,122:448-511.
DOI URL |
| [3] |
YEH J W, CHEN S K, LIN S J, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Advanced Engineering Materials, 2004,6(5):299-303.
DOI URL |
| [4] |
HUANG P K, YEH J W, SHUN T T, et al. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Advanced Engineering Materials, 2004,6(1/2):74-78.
DOI URL |
| [5] | TONG C J, CHEN Y L, CHEN S K, et al. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metallurgical and Materials Transactions A, 2005,36(4):881-893. |
| [6] | ZHANG Y, ZUO T T, TANG Z, et al. Microstructures and properties of high-entropy alloys. Progress in Materials Science, 2014,61:1-93. |
| [7] | MURTY B S, YEH J W, RANGANATHAN S. High-entropy Alloys. London: Elsevier, 2014. |
| [8] | ZHANG Y, ZUO T T, CHENG Y Q, et al. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Scientific Reports, 2013,3:1-7. |
| [9] | CHUANG M H, TSAI M H, WANG W R, et al. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high entropy alloys. Acta Materialia, 2011,59(16):6308-6317. |
| [10] | JIANG S C, HU T, GILD J, et al. A new class of high-entropy perovskite oxides. Scripta Materialia, 2018,142:116-120. |
| [11] | TSAI M H. Physical properties of high entropy alloys. Entropy, 2013,15:5338-5345. |
| [12] | 顾俊峰, 邹冀, 张帆, 等. 高熵陶瓷材料研究进展. 中国材料进展, 2019,38(9):855-865. |
| [13] | YEH J W. Recent progress in high-entropy alloys. Annales De Chimie-Science des Materiaux, 2006,31:633-648. |
| [14] | MIRACLE D B. High-entropy alloys: a current evaluation of founding ideas and core effects and exploring “nonlinear alloys”. JOM, 2017,69(11):2130-2136. |
| [15] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nature Communications, 2015,6(1):8485. |
| [16] | CHELLALI M R, SARKAR A, NANDAM S H, et al. On the homogeneity of high entropy oxides: an investigation at the atomic scale. Scripta Materialia, 2019,166:58-63. |
| [17] | DJENADIC R, SARKAR A, CLEMENS O, et al. Multicomponent equiatomic rare earth oxides. Materials Research Letters, 2017,5(2):102-109. |
| [18] | DUPUY A D, WANG X, SCHOENUNG J M. Entropic phase transformation in nanocrystalline high entropy oxides. Materials Research Letters, 2019,7(2):60-67. |
| [19] |
GILD J, ZHANG Y Y, HARRINGTON T, et al. High-entropy metal diborides: a new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Scientific Reports, 2016,6:37946.
URL PMID |
| [20] | YAN X L, CONSTANTIN L, LU Y F, et al. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. Journal of the American Ceramic Society, 2018,101(10):4486-4491. |
| [21] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. Journal of Materials Science & Technology, 2019,35(8):1700-1705. |
| [22] | CASTLE E, CSANADI T, GRASSO S, et al. Processing and properties of high-entropy ultra-high temperature carbides. Scientific Reports, 2018,8(1):8609. |
| [23] |
SARKER P, HARRINGTON T, TOHER C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nature Communications, 2018,9(1):4980.
URL PMID |
| [24] | YE B L, WEN T Q, NGUYEN M C, et al. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high- entropy ceramics. Acta Materialia, 2019,170:15-23. |
| [25] | HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Materialia, 2019,166:271-280. |
| [26] | YE B L, WEN T Q, HUANG K H, et al. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high- entropy ceramic. Journal of the American Ceramic Society, 2019,102(7):4344-4352. |
| [27] | WANG K, CHEN L, XU C G, et al. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. Journal of Materials Science & Technology, 2020,39:99-105. |
| [28] | ZHANG W, CHEN L, XU C G, et al. Densification, microstructure and mechanical properties of multicomponent (TiZrHfNbTaMo)C ceramic prepared by pressureless sintering. Journal of Materials Science & Technology, 2021,72:23-28. |
| [29] | JIN T, SANG X H, UNOCIC R R, et al. Mechanochemical- assisted synthesis of high-entropy metal nitride via a soft urea strategy. Advanced Materials, 2018,30(23):1707512. |
| [30] |
GILD J, BRAUN J, KAUFMANN K, et al. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. Journal of Materiomics, 2019,5(3):337-343.
DOI URL |
| [31] | ZHAO Z F, XIANG H M, DAI F Z, et al. (TiZrHf)P2O7: an equimolar multicomponent or high entropy ceramic with good thermal stability and low thermal conductivity. Journal of Materials Science & Technology, 2019,35(10):2227-2231. |
| [32] | LIU Y C, JIA D C, ZHOU Y, et al. Zn0.1Ca0.1Sr0.4Ba0.4ZrO3: a non-equimolar multicomponent perovskite ceramic with low thermal conductivity. Journal of the European Ceramic Society, 2020,40:6272-6277. |
| [33] | ZHU D M. Advanced Environmental Barrier Coatings for SiC/SiC Ceramic Matrix Composite Turbine Components. Engineered Ceramics: Current Status and Future Prospects, Hoboken, New Jersey: John Wiley & Sons, Inc, 2016. |
| [34] | LEE K N, FOX D S, BANSAL N P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. Journal of the European Ceramic Society, 2005,25(10):1705-1715. |
| [35] | 田志林. 几种稀土硅酸盐陶瓷的预测、制备和性能研究. 北京: 中国科学院大学博士学位论文, 2016. |
| [36] | LUO Y X, SUN L C, WANG J M, et al. Tunable thermal properties in yttrium silicates switched by anharmonicity of low-frequency phonons. Journal of the European Ceramic Society, 2018,38:2043-2052. |
| [37] | POERSCHKE D L, HASS D D, EUSTIS S, et al. Stability and CMAS resistance of ytterbium-silicate/hafnate EBCs/TBC for SiC composites. Journal of the American Ceramic Society, 2015,98(1):278-286. |
| [38] | DONG Y, REN K, LU Y H, et al. High-entropy environmental barrier coating for the ceramic matrix composites. Journal of the European Ceramic Society, 2019,39:2574-2579. |
| [39] | CHEN H, XIANG H M, DAI F Z, et al. High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion. Journal of Materials Science & Technology, 2020,36:134-139. |
| [40] | REN X M, TIAN Z L, ZHANG J, et al. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: a perspective multifunctional thermal and environmental barrier coating material. Scripta Materialia, 2019,168:47-50. |
| [41] | RIDLEY M, GASKINS J, HOPKINS P, et al. Tailoring thermal properties of multi-component rare earth monosilicates. Acta Materialia, 2020,195:698-707. |
| [42] | TURCER L R, SENGUPTA A, PADTURE N P. Low thermal conductivity in high-entropy rare-earth pyrosilicate solid-solutions for thermal environmental barrier coatings. Scripta Materialia, 2021,191:40-45. |
| [43] | POERSCHKE D L, JACKSON R W, LEVI C G. Silicate deposit degradation of engineered coatings in gas turbines: progress toward models and materials solutions. Annual Review of Materials Research, 2017,47:297-330. |
| [44] | LIU J, ZHANG L T, LIU Q M, et al. Calcium-magnesium- aluminosilicate corrosion behaviors of rare-earth disilicates at 1400 ℃. Journal of the European Ceramic Society, 2013,33:3419-3428. |
| [45] | TIAN Z L, REN X M, LEI Y M, et al. Corrosion of RE2Si2O7 (RE=Y, Yb, and Lu) environmental barrier coating materials by molten calcium-magnesium-alumino-silicate glass at high temperatures. Journal of the European Ceramic Society, 2019,39:4245-4254. |
| [46] | TURCER L R, KRAUSE A R, GARCES H F, et al. Environmental-barrier coating ceramics for resistance against attack by molten calcia-magnesia-aluminosilicate (CMAS) glass: Part I, YAlO3 and γ-Y2Si2O7. Journal of the European Ceramic Society, 2018,38:3905-3913. |
| [47] | TURCER L R, KRAUSE A R, GARCES H F, et al. Environmental-barrier coating ceramics for resistance against attack by molten calcia-magnesia-aluminosilicate (CMAS) glass: Part II, β-Yb2Si2O7 and β-Sc2Si2O7. Journal of the European Ceramic Society, 2018,38:3914-3924. |
| [48] | SUN L C, LUO Y X, TIAN Z L, et al. High temperature corrosion of (Er0.25Tm0.25Yb0.25Lu0.25)2Si2O7 environmental barrier coating material subjected to water vapor and molten calcium-magnesium- aluminosilicate (CMAS). Corrosion Science, 2020,175:108881. |
| [49] | FELSCHE J. The Crystal Chemistry of the Rare-earth Silicates. Rare Earths. Structure and Bonding, Vol 13. Berlin, Heidelberg: Springer, 1973. |
| [50] | SUN L C, LUO Y X, REN X M, et al. A multicomponent γ-type (Gd1/6Tb1/6Dy1/6Tm1/6Yb1/6Lu1/6)2Si2O7 disilicate with outstanding thermal stability. Materials Research Letters, 2020,8(11):424-430. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 余乐洋阳, 赵芳霞, 张舒心, 徐以祥, 牛亚然, 张振忠, 郑学斌. 感应等离子球化技术制备喷涂用高熵硼化物粉体[J]. 无机材料学报, 2025, 40(7): 808-816. |
| [3] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [4] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [5] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [6] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [7] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [8] | 梁锐辉, 钟鑫, 洪督, 黄利平, 牛亚然, 郑学斌. Yb2O3改性硅黏结层的环境障涂层体系耐高温水氧腐蚀行为研究[J]. 无机材料学报, 2025, 40(4): 425-432. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [13] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [14] | 樊文楷, 杨潇, 李宏华, 李永, 李江涛. 无压烧结制备(Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2Zr2O7高熵陶瓷及其高温抗CMAS腐蚀性能[J]. 无机材料学报, 2025, 40(2): 159-167. |
| [15] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||