无机材料学报 ›› 2020, Vol. 35 ›› Issue (2): 243-249.DOI: 10.15541/jim20190118 CSTR: 32189.14.10.15541/jim20190118
吴思1,2,梅雷2,胡孔球2,柴之芳2,3,聂长明1( ),石伟群2(
),石伟群2( )
)
收稿日期:2019-03-21
修回日期:2019-04-27
出版日期:2020-02-20
网络出版日期:2019-09-20
作者简介:吴 思(1993-), 女, 硕士研究生. E-mail: wusi@ihep.ac.cn
WU Si1,2,MEI Lei2,HU Kong-Qiu2,CHAI Zhi-Fang2,3,NIE Chang-Ming1( ),SHI Wei-Qun2(
),SHI Wei-Qun2( )
)
Received:2019-03-21
Revised:2019-04-27
Published:2020-02-20
Online:2019-09-20
Supported by:摘要:
本工作报道了一种含新型八核铀酰(U8)团簇单元([(UO2)8O4(μ3-OH)2(μ2-OH)2] 4+)的草酸铀酰配合物, 该化合物中, U型有机配体链可以增强铀酰之间的交联度, 具有稳定多核铀酰团簇的作用。通过与另外两种含单核和双核的铀酰配位化合物比较, 发现八核铀酰团簇单元的形成是一个pH调控的过程。理化性质分析显示, 荧光、红外、拉曼的信号峰都出现了不同程度的重叠和宽化, 表明八个铀酰离子具有较高的相似度, 这与此多核铀酰团簇的近平面分子构型密切相关。
中图分类号:
吴思,梅雷,胡孔球,柴之芳,聂长明,石伟群. pH调控合成U型配体介导的八核铀酰草酸网络[J]. 无机材料学报, 2020, 35(2): 243-249.
WU Si,MEI Lei,HU Kong-Qiu,CHAI Zhi-Fang,NIE Chang-Ming,SHI Wei-Qun. pH-dependent Synthesis of Octa-nuclear Uranyl-oxalate Network Mediated by U-shaped Linkers[J]. Journal of Inorganic Materials, 2020, 35(2): 243-249.
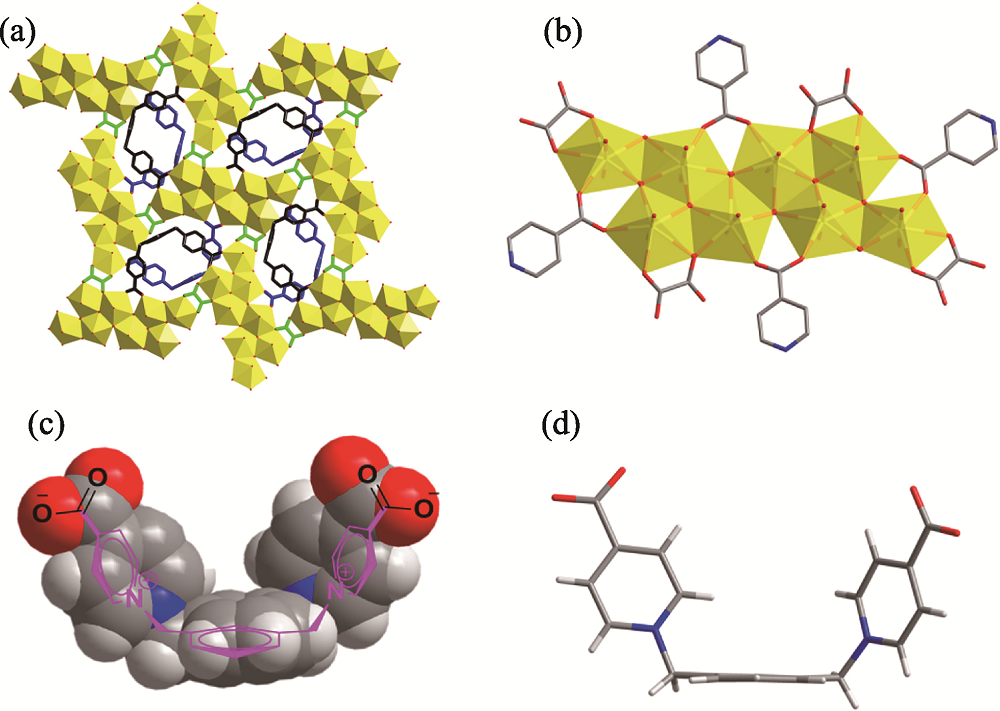
Fig. 1 Octa-nuclear uranyl-oxalate network reinforced by U-shaped zwitterionic dicarboxylate linkers (a) two-dimensional coordination network; (b) octa-nuclear uranyl (U8) motif, [(UO2)8O4(μ3-OH)2(μ2-OH)2]4+; (c) U-shaped linker in a space- filling mode overlapped with its molecular structure; (d) U-shaped linker in a stick mode Color codes: uranyl polyhedra in yellow; U-shaped linkers in dark or blue
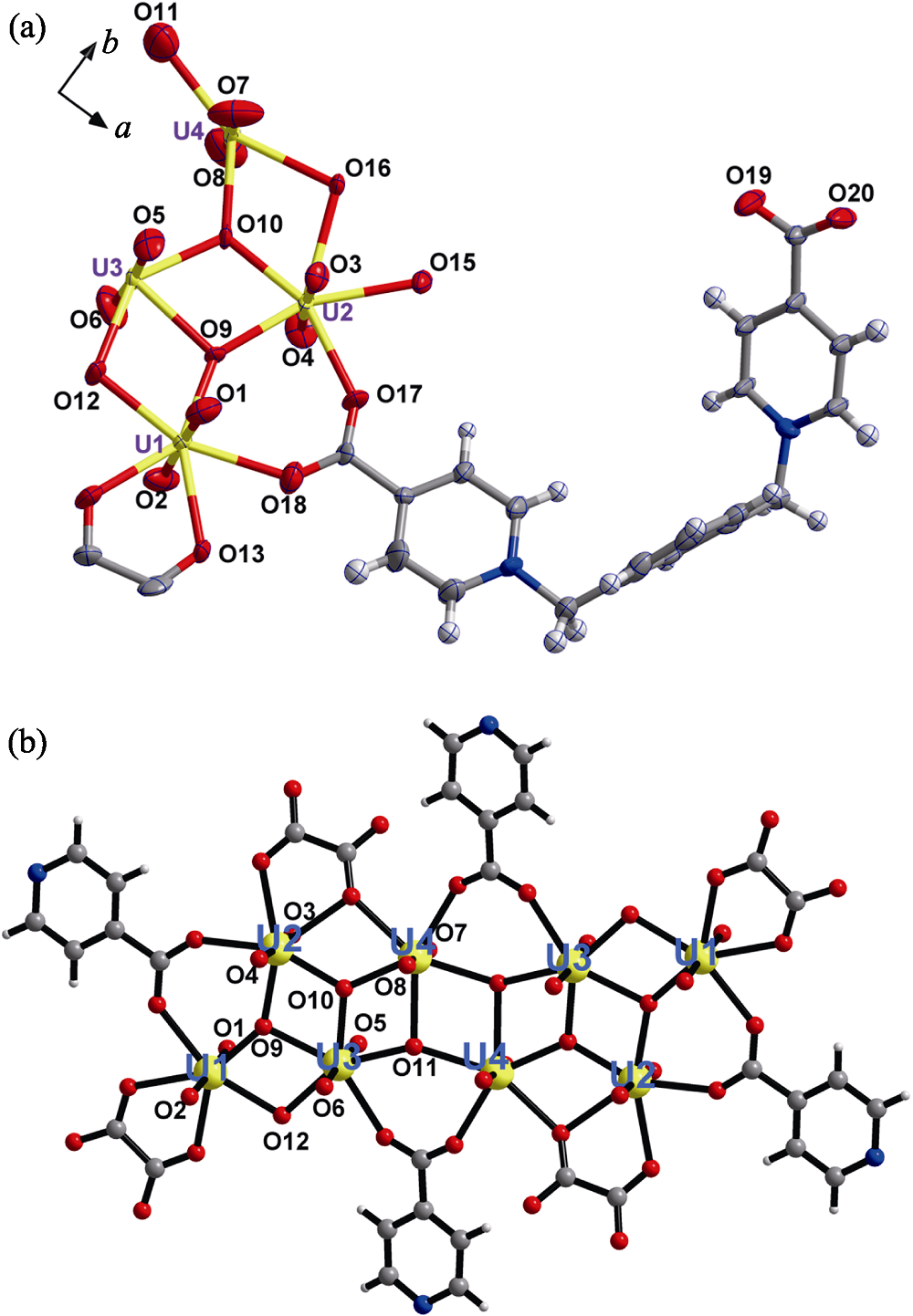
Fig. 2 Crystal structure of compound 1 (a) ORTEP view of compound 1 with the 30% probability level for thermal ellipsoids; (b) octa-nuclear uranyl (U8) motif in compound 1 showing detailed coordination spheres of all uranyl centers Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray
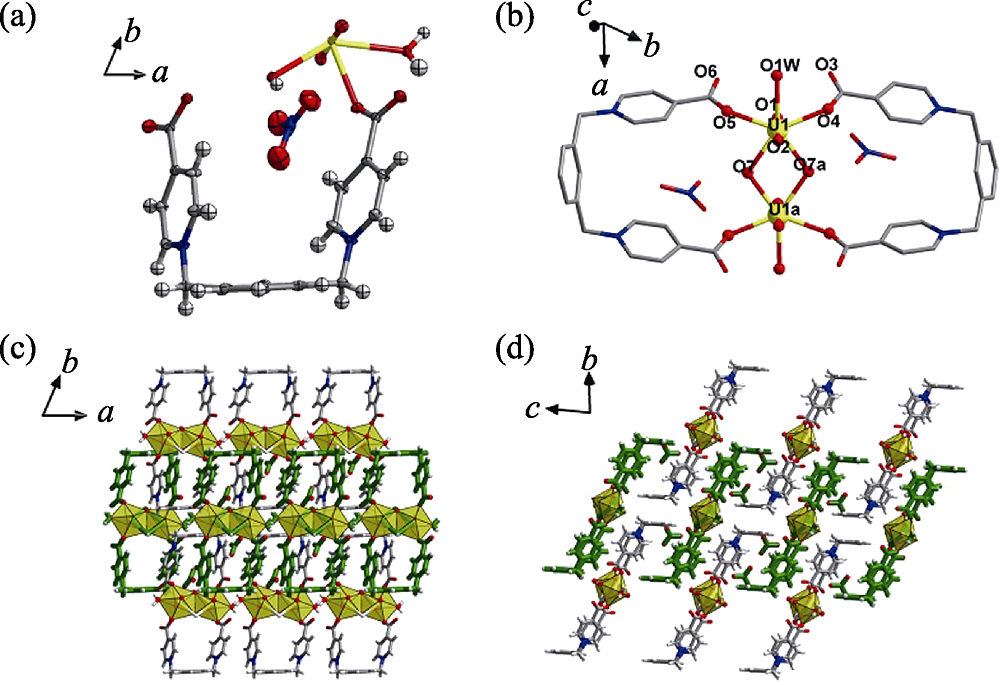
Fig. 3 Crystal structure of compound 2 (a) ORTEP view of compound 2 with the 30% probability level for thermal ellipsoids; (b) coordination environment of each uranyl center for dimeric uranyl motif; (c-d) crystal lattice stacking for compound 2 viewed for c axis (c) and a axis (d) Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray; the U-shaped linkers in green
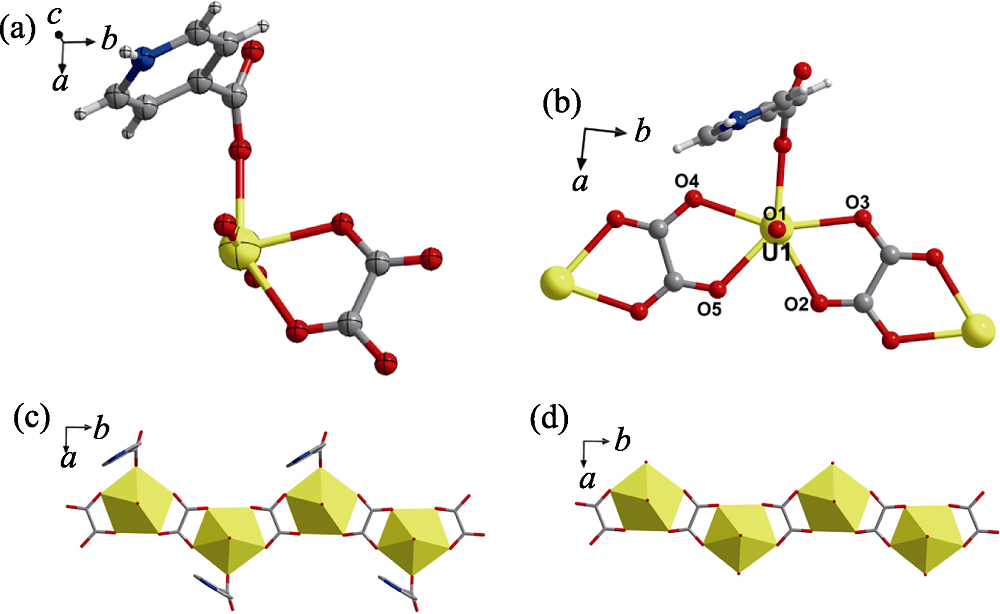
Fig. 4 Crystal structure of compound 3 (a) ORTEP view of compound 3 with the 30% probability level for thermal ellipsoids; (b) coordination environments of uranyl center; (c-d) the extended structure based on one-dimensional oxalate-bridging monomeric uranyl chain with (c) or without (d) terminal isonicotinate ligands Color codes: uranium atoms or polyhedras in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms in pale gray
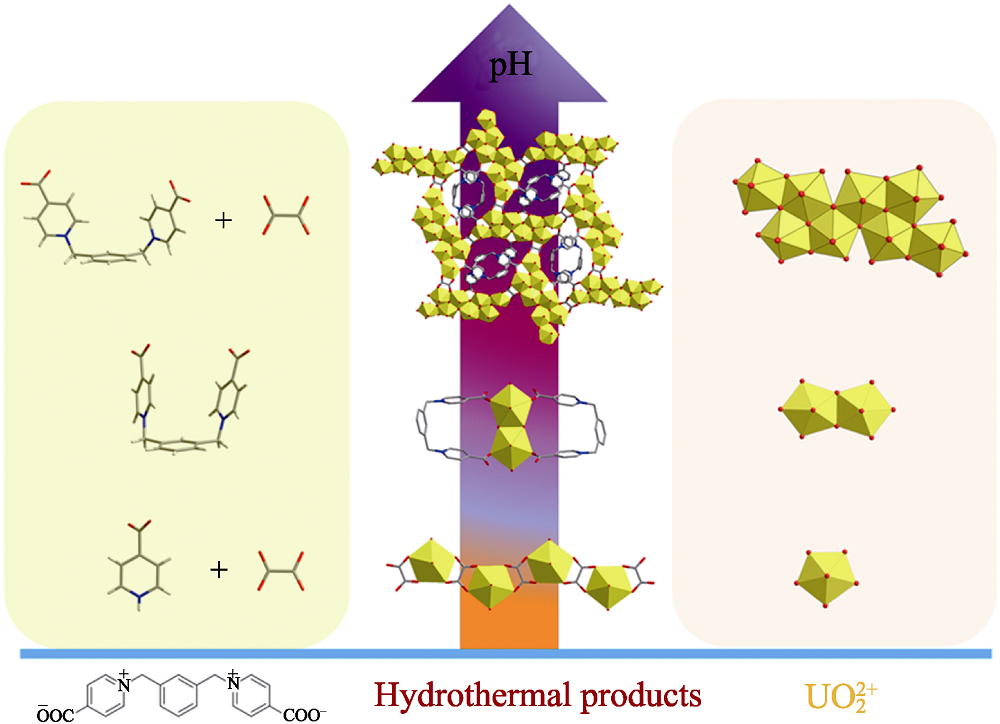
Fig. 5 pH-dependent regulation of hydrothermal reactions of m-Xyl-BPy4CA linkers and uranyl Color codes: uranium polyhedras in yellow; oxygen atoms in red; carbon atoms in gray; nitrogen atoms in blue
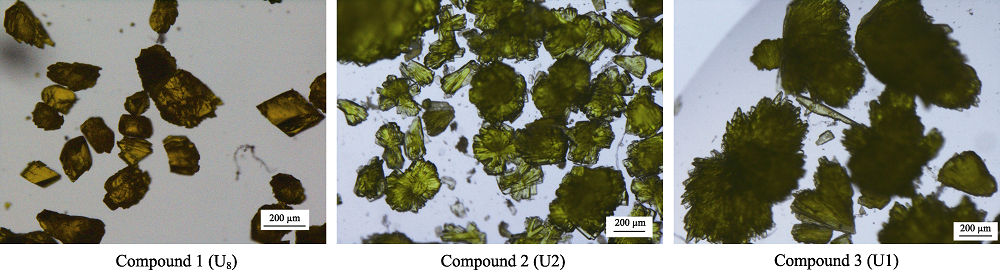
Fig. S1 Different optical morphologies of 1 with octa-nuclear uranyl (U8) motifs, 2 with binuclear uranyl (U2) motifs and 3 with monomeric uranyl (U1) motifs
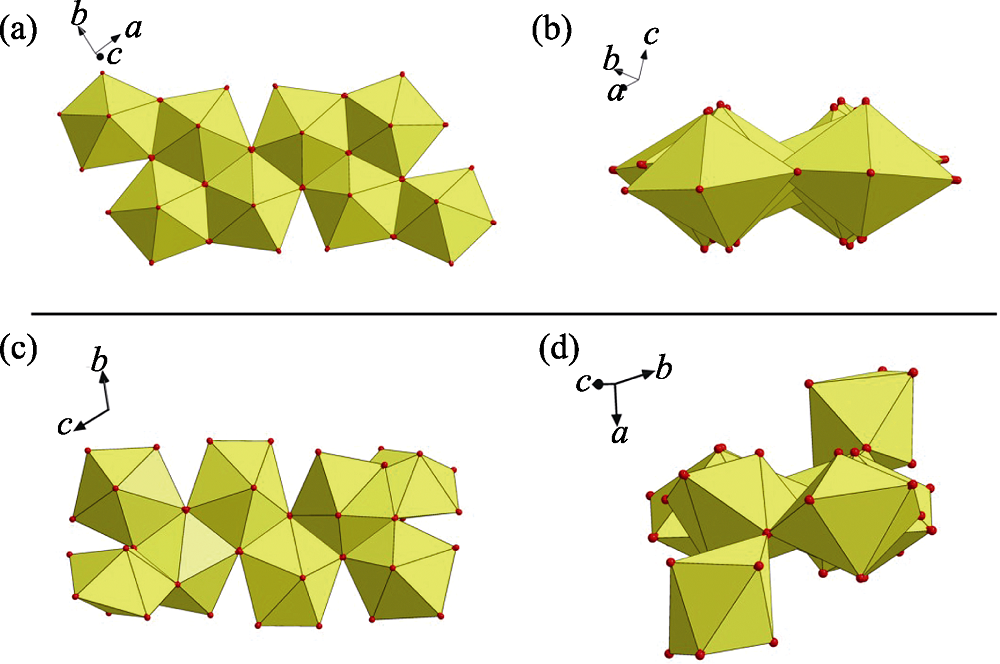
Fig. S5 (a) A nearly planar geometry of U8 motif found in this work; (b) a non-planar U8 motif with cation-cation interactions (CCIs) reported by Loiseau, et al[1]
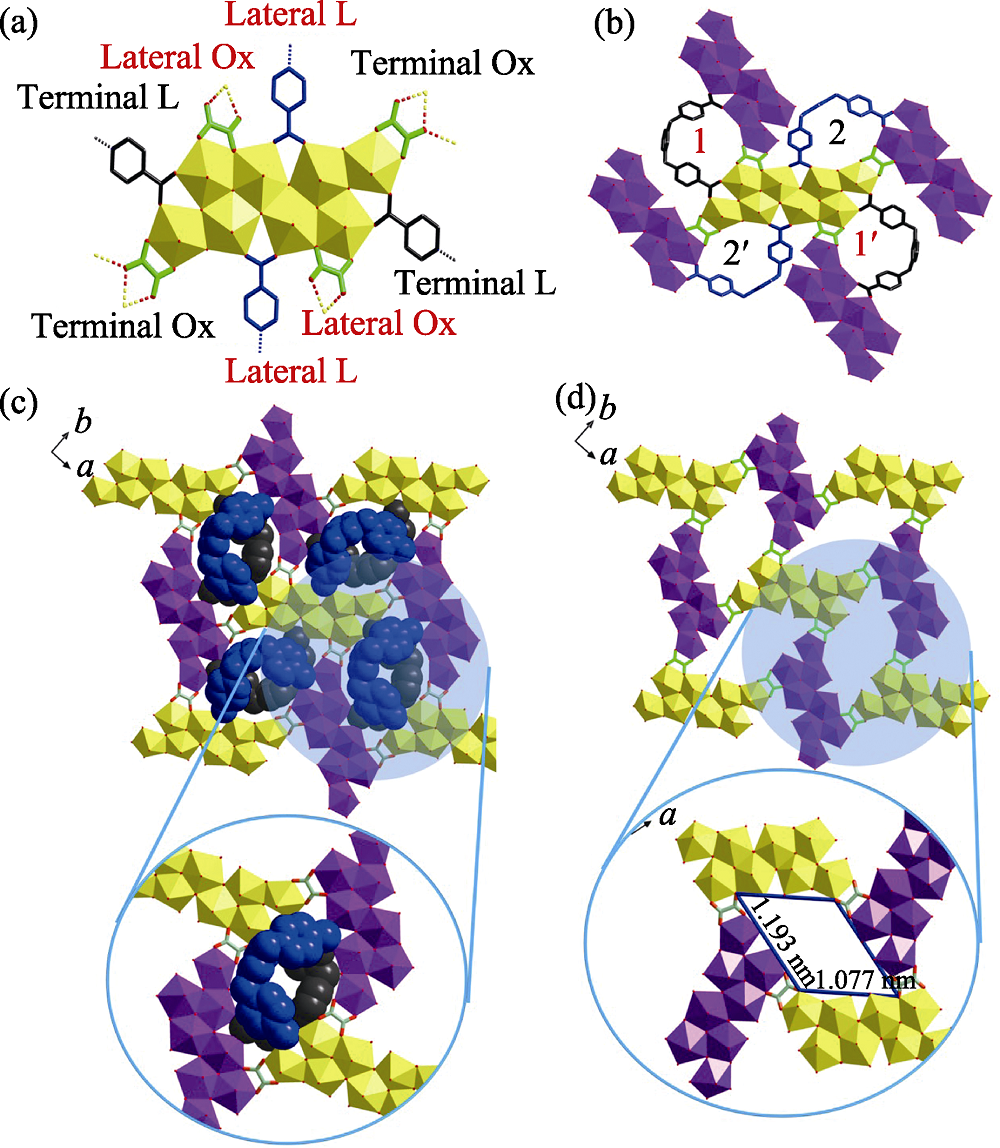
Fig. S6 (a-b) Eight-connected U8 motif with four oxalate (Ox) and four m-Xyl-BPy4CA (L) moieties extends from four directions through oxalate ligands (a), which thus connecting four adjacent ones with each oxalate ligand going together with a U-shaped bidentate m-Xyl-BPy4CA linker (b); (c) U8-based uranyl-oxalate 2D network (enlarged diagram: a minimum rhombic loop); (d) U8-based uranyl-oxalate 2D network with all the cross-linking m-Xyl-BPy4CA linkers omitted for clarity (enlarged diagram: a minimum rhombic loop in size of 1.193 nm× 1.077 nm)
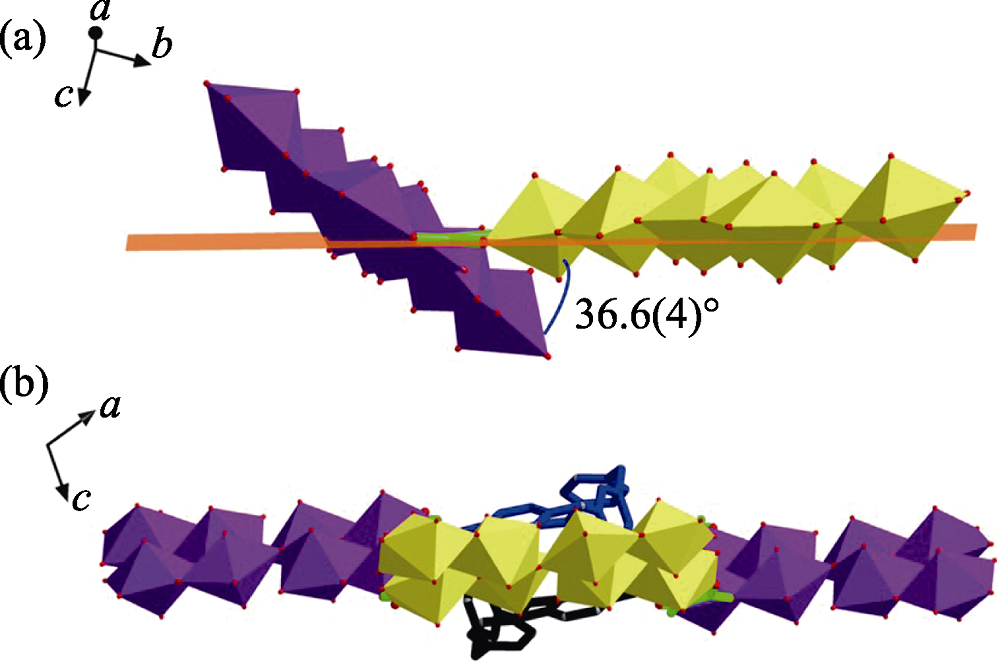
Fig. S7 Each U8 motif displays a different overall orientation from that of its adjacent U8 with an angle of inclination of 36.6(4)° (a), resulting in a distortion of the rhombic loop (b)
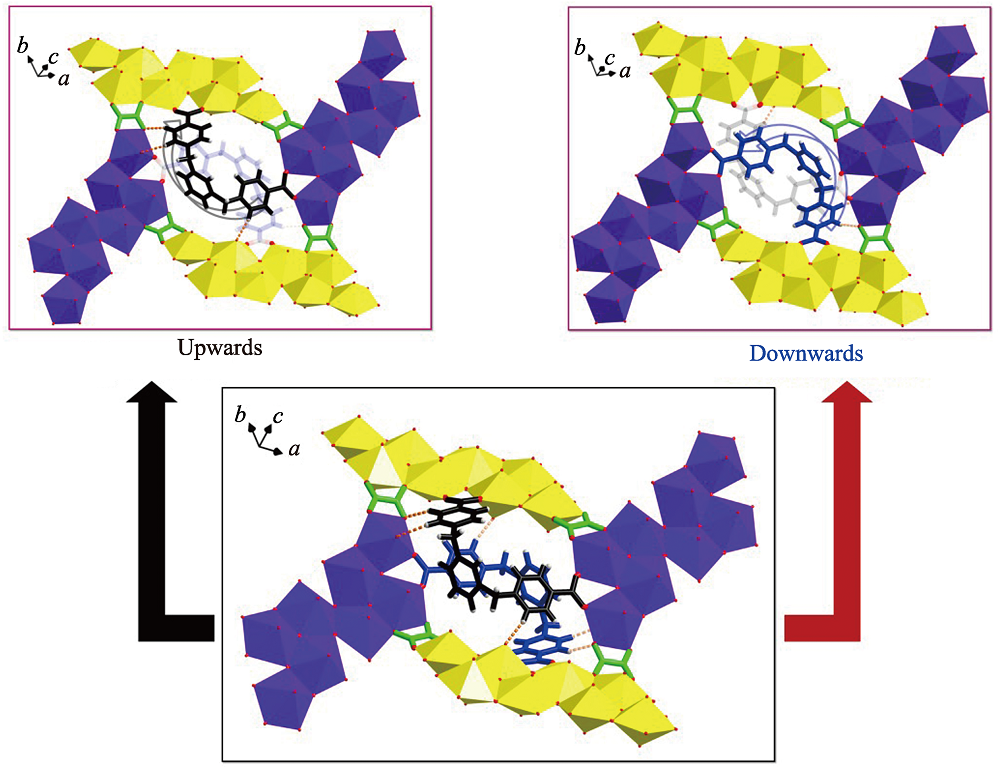
Fig. S9 Two ‘U’-shaped bidentate m-Xyl-BPy4CA ligands located in the cavity of rhombic loop crosslink all the four U8 motifs through coordination bonds and hydrogen bonds (bottom) where one m-Xyl-BPy4CA ligand points upwards (top left) and the other points downwards from the opposite direction (top right)
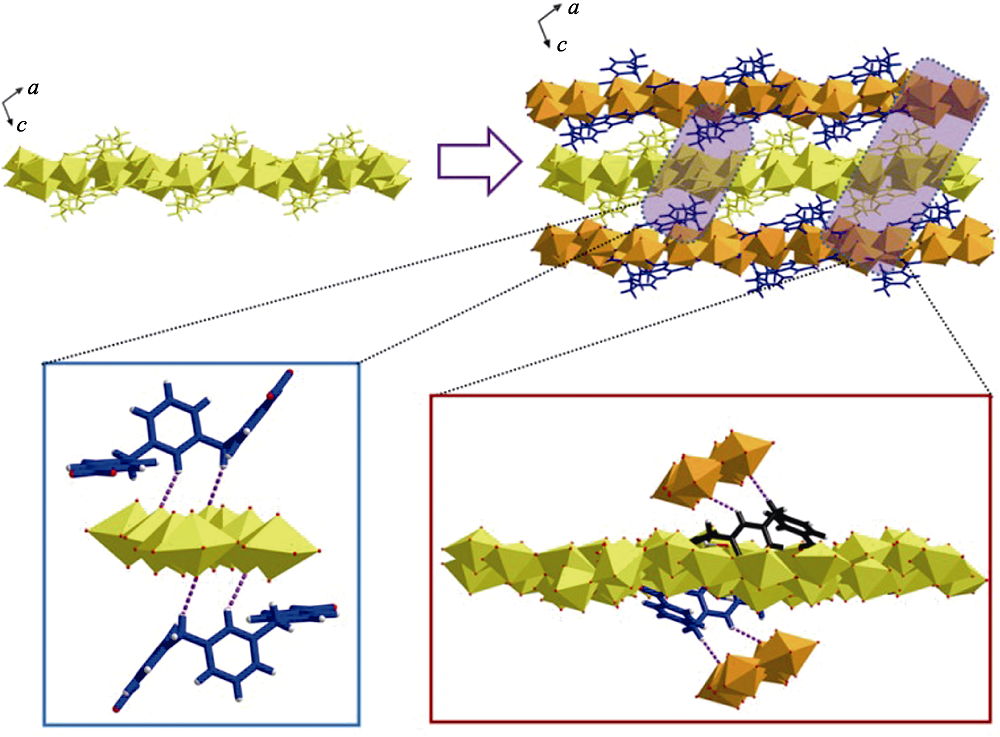
Fig. S10 Hydrogen bonds between adjacent layers of 2D sheets through U8 motifs that interact with neighboured m-Xyl-BPy4CA from another sheet or m-Xyl-BPy4CA interacting with neighboured uranyl group from another sheet
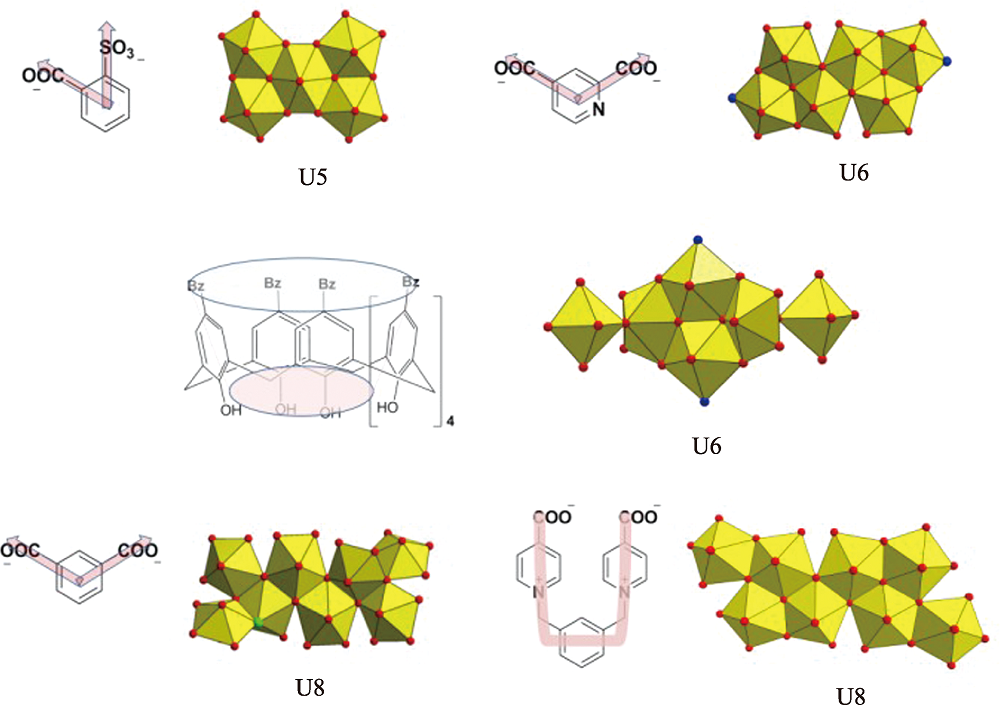
Fig. S11 Some examples of high-nuclear uranyl motif based on nonlinear multi-topic organic ligands, as suggested by the cases of pentanuclear (U5), hexanuclear (U6) and octanuclear (U8) uranyl motifs derived from sulfobenzoate precursors[2], ortho-position or meta-position aromatic/heteroaromatic dicarboxylate[3,4], calixarene ligand[3] and U-shaped linkers used in this work
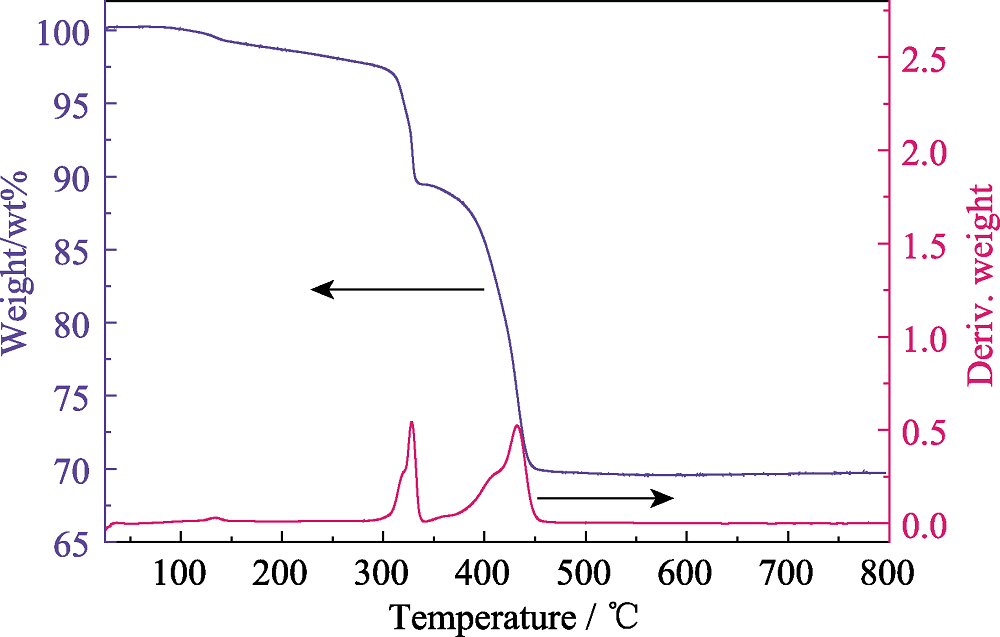
Fig. S13 Thermogravimetric analysis (TGA) of compounds 1, where 1 starts to decompose at ~295 ℃, and finally transforms to U3O8 with residual weight of 69.31% (theoretical value: 70.25%)
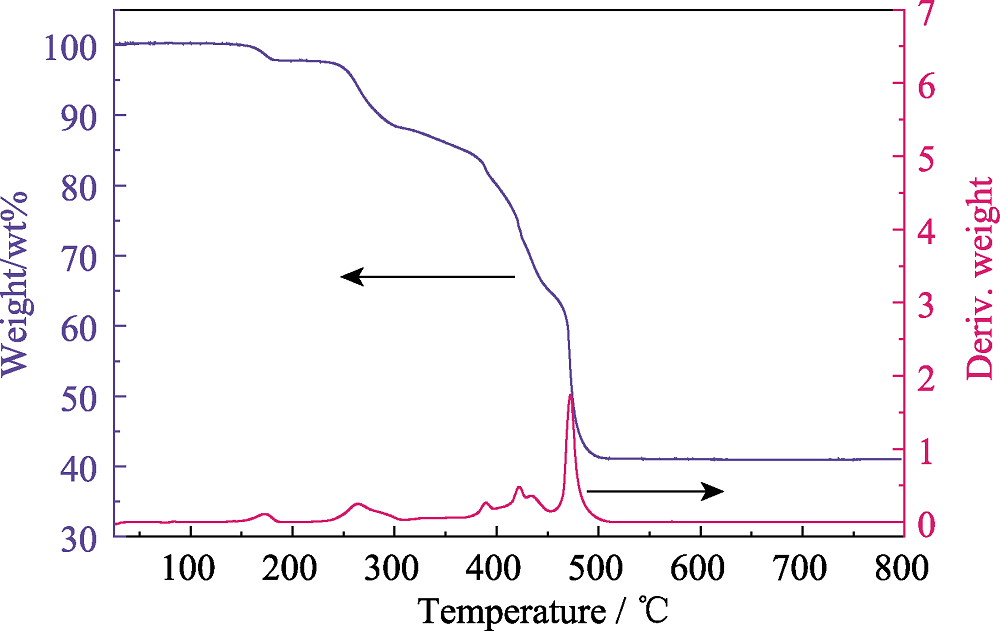
Fig. S14 Thermogravimetric analysis (TGA) of compounds 2, where 2 starts to decompose at ~233 ℃, and finally transforms to U3O8 with residual weight of 40.95% (theoretical value: 40.20%)
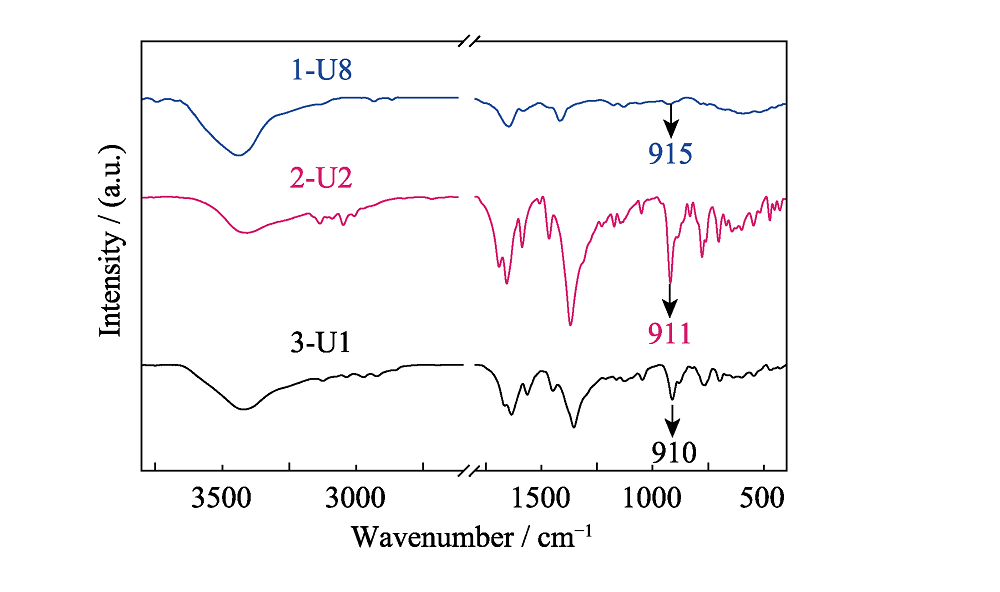
Fig. S15 Fourier transform infrared (IR) spectra of compounds 1 (U8 motif, blue line), 2 (U2 motif, red line) and 3 (U1 motif, black line) with characteristic symmetric ν1vibrations at 915, 911 and 910 nm, respectively
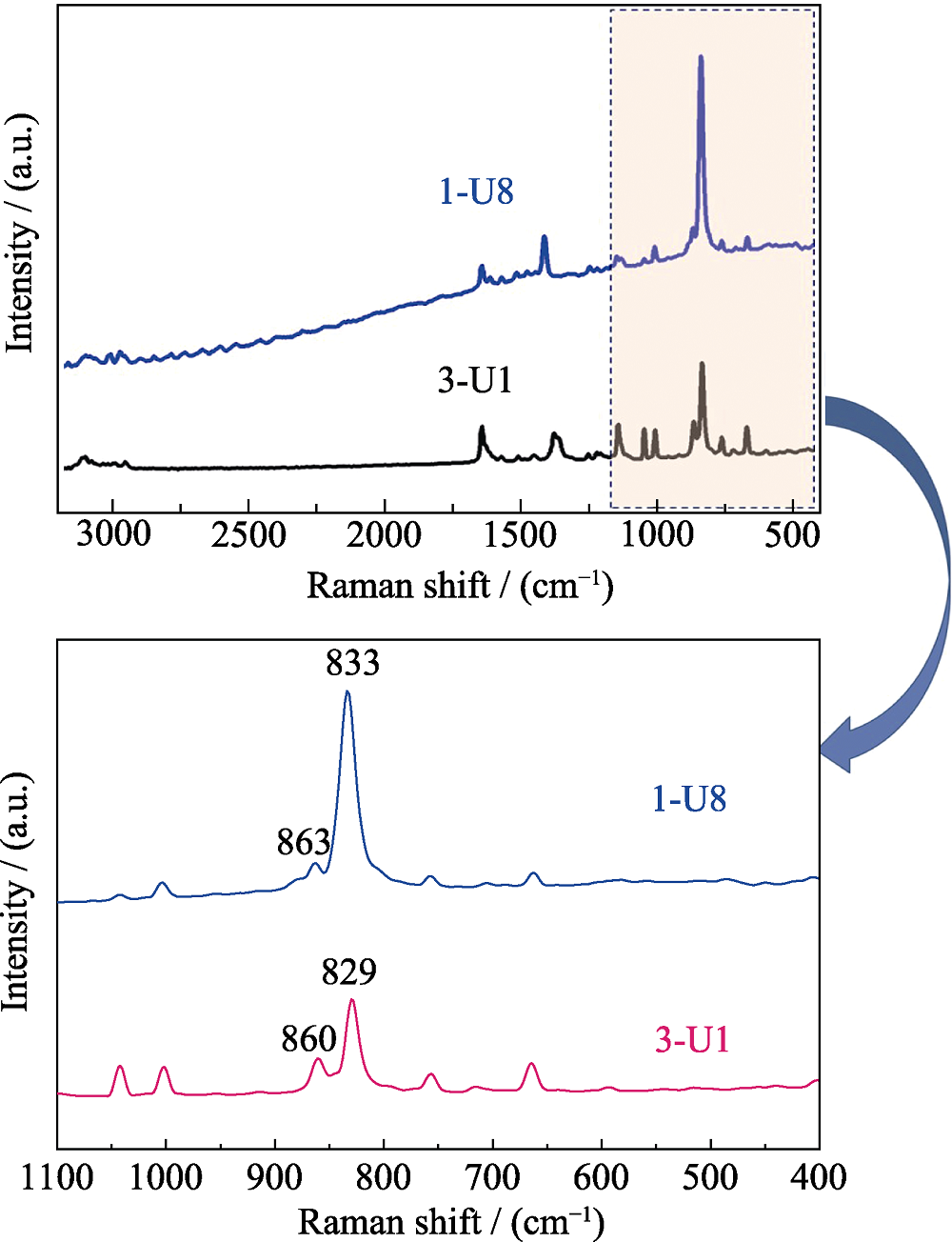
Fig. S16 The Raman spectra of compounds s 1 (U8 motif) and 3 (U1 motif) with characteristic asymmetric ν3 vibrations (1: 833 and 863 cm-1; 3: 829 and 860 cm-1)
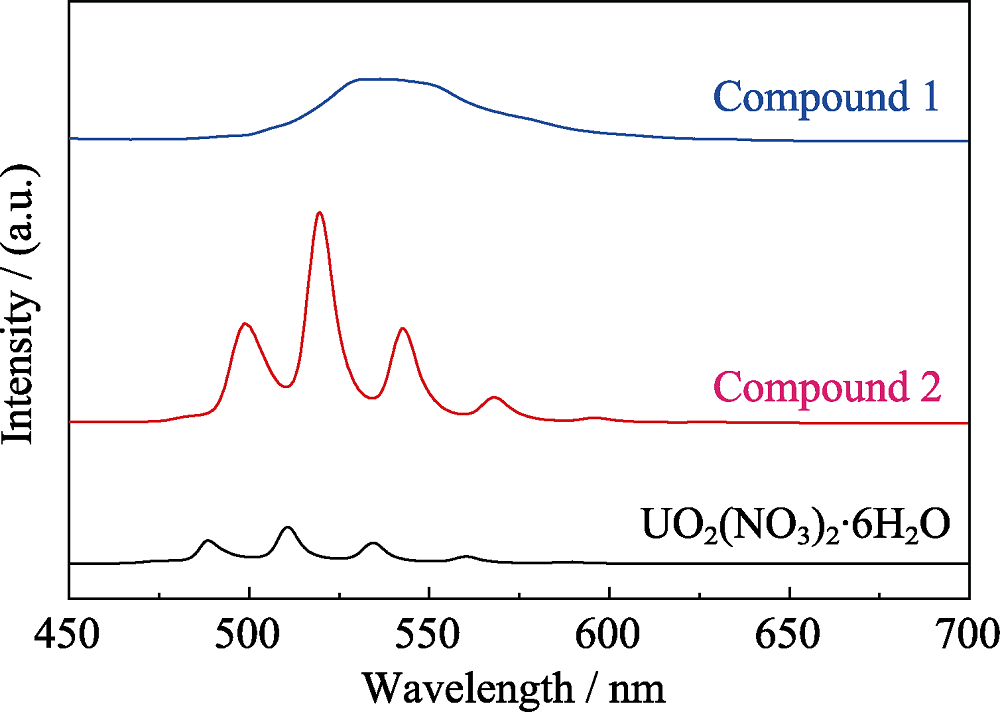
Fig. S17 Solid-state fluorescence spectra of compound 1 and 2 as compared to that of uranyl nitrate (UO2(NO3)2): 1, a broad peak ranging from 530 to 550 nm; 2, five main emission bands located at 499, 520, 543, 568 and 596 nm; UO2(NO3)2, 488, 511, 534, 561 and 589 nm
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
Table S1 Selected bond distances related to uranyl centers in compounds 1, 2 and 3
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
Table S2 Distances and angles for hydrogen bonds observed in compounds 1 and 2
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
Table S3 Crystal data and structure refinement for compounds 1, 2 and 3
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
| [1] | ALTMAIER M, GAONA X, FANGHANEL T , et al. Recent advances in aqueous actinide chemistry and thermodynamics. Chemical Reviews, 2013,113(2):901-943. |
| [2] | JONES M B, GAUNT A J . Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chemical Reviews, 2013,113(2):1137-1198. |
| [3] | WANG K X, CHEN J S . Extended structures and physicochemical properties of uranyl-organic compounds. Accounts of Chemical Research, 2011,44(7):531-540. |
| [4] | ANDREWS M B, CAHILL C L . Uranyl bearing hybrid materials: synthesis, speciation, and solid-state structures. Chemical Reviews, 2013,113(2):1121-1136. |
| [5] | YANG W T, PARKER T G, SUN Z M , et al. Structural chemistry of uranium phosphonates. Coordination Chemistry Reviews, 2015,303(1):86-109. |
| [6] | LOISEAU T, MIHALCEA I, HENRY N , et al. The crystal chemistry of uranium carboxylates. Coordination Chemistry Reviews, 2014,266(35):69-109. |
| [7] | RAI D, FELMY A R, RYAN J L , et al. Uranium (IV) hydrolysis constants and solubility product of UO2·xH2O(am). Inorganic Chemistry, 1990,29(2):260-264. |
| [8] | AHRLAND S . On the complex chemistry of the uranyl ion Ι. The hydrolysis of the 6-valent uranium in aqueous solutions. Acta Chemica Scandinavica, 1949,3(4):374-400. |
| [9] | ZANONATO P, DI BERNARDO P, BISMONDO A , et al. Hydrolysis of uranium (VI) at variable temperatures (10-85 ℃). Journal of the American Chemical Society, 2004,126(17):5515-5522. |
| [10] | SALMON L, THUERY P, EPHRITIKHINE M , et al. Crystal structure of the first octanuclear uranium (IV) complex with compartmental schiff base ligands. Polyhedron, 2004,23(4):623-627. |
| [11] | MIHALCEA I, HENRY N, CLAVIER N , et al. Occurence of an octanuclear motif of uranyl isophthalate with cation-cation interactions through edge-sharing connection mode. Inorganic Chemistry, 2011,50(13):6243-6249. |
| [12] | PASQUALE S, SATTIN S, ESCUDERO-ADAN E C , et al. Giant regular polyhedra from calixarene carboxylates and uranyl. Nature Communications, 2012,3(1):785. |
| [13] | THUERY P . A highly adjustable coordination system: nanotubular and molecular cage species in uranyl ion complexes with kemp's triacid. Crystal Growth & Design, 2014,14(3):901-904. |
| [14] | WANG L H, SHANG R, ZHENG Z , et al. Two systems of [DabcoH2]2+/[PipH2]2+-uranyl-oxalate showing reversible crystal-to- crystal transformations controlled by the diammonium/uranyl/oxalate ratios in aqueous solutions ([DabcoH2]2+=1,4-diazabicyclo- [2.2.2]-octaneH2 and [PipH2]2+ = PiperazineH2). Crystal Growth & Design, 2013,13(6):2597-2606. |
| [15] | CHAPELET-ARAB B, NOWOGROCKI G, ABRAHAM E , et al. Crystal structure of new uranyl oxalates (NH4)2[UO2(C2O4)·2H2O] and (NH4)2-x(N2H5)x[UO2(C2O4)3]·3H2O (x=0 and x=1). Comparison with other uranyl oxalates. Radiochimica Acta, 2005,93(5):279-285. |
| [16] | GIESTING P A, PORTER N J, BURNS P C , et al. A series of sheet-structured alkali metal uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(8):589-599. |
| [17] | GIESTING P A, PORTER N J, BURNS P C , et al. Uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(4):252-259. |
| [18] | DUVIEUBOURG L, NOWOGROCKI G, ABRAHAM F , et al. Hydrothermal synthesis and crystal structures of new uranyl oxalate hydroxides: α- and β-[(UO2)2 (C2O4)(OH)2(H2O)2] and [(UO2)2((C2O4)(OH)2(H2O)2]·H2O. Journal of Solid State Chemistry, 2005,178(11):3437-3444. |
| [19] | THUERY P . Reaction of uranyl nitrate with carboxylic diacids under hydrothermal conditions. Crystal structure of complexes with L(+)-tartaric and oxalic acids. Polyhedron, 2007,26(1):101-106. |
| [20] | VOLOGZHANINA A V, SEREZHKINA L B, NEKLYUDOVA N A , et al. Synthesis and characterisation of a trinuclear uranyl complex: crystal structure of (CN3H6)5[(UO2)3O(OH)2(CH3COO)(C2O4)3]. Inorganica Chimica Acta, 2009,362(14):4921-4925. |
| [21] | CHUGH C A, SHARMA A, SHARMA A , et al. Kinetics and mechanism of thermal decomposition of uranyl oxalate. Asian Journal of Chemistry, 2011,23(4):1865-1866. |
| [22] | BARTLETT J R, COONEY R P , et al. On the determination of uranium oxygen bond lengths in dioxouranium (VI) compounds by raman-spectroscopy. Journal of Molecular Structure, 1989,193(1):295-300. |
| [23] | BRACHMANN A, GEIPEL G, BERNHARD G , et al. Study of uranyl (VI) malonate complexation by time resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochimica Acta, 2002,90(3):147-153. |
| [24] | MEI L, WANG C Z, ZHU L Z , et al. Exploring new assembly modes of uranyl terephthalate: templated syntheses and structural regulation of a series of rare 2d→3d polycatenated frameworks. Inorganic Chemistry, 2017,56(14):7694-7706. |
| [25] | NATRAJAN L S . Developments in the photophysics and photochemistry of actinide ions and their coordination compounds. Coordination Chemistry Reviews, 2012,256(15/16):1583-1603. |
| [26] | THUERY P, HARROWFIELD J . Solvent effects in solvo-hydrothermal synthesis of uranyl ion complexes with 1,3-adamantanediacetate. CrystEngComm, 2015,17(21):4006-4018. |
| [27] | THUERY P, HARROWFIELD J . Structural variations in the uranyl/4,4'-biphenyldicarboxylate system. rare examples of 2d→3d polycatenated uranyl-organic networks. Inorganic Chemistry, 2015,54(16):8093-8102. |
| [28] | THUERY P, RIVIERE E, HARROWFIELD J , et al. Uranyl and uranyl-3d block cation complexes with 1,3-adamantanedicarboxylate: crystal structures, luminescence, and magnetic properties. Inorganic Chemistry, 2015,54(6):2838-2850. |
| [1] | 王晓波, 朱于良, 薛稳超, 史汝川, 骆柏锋, 罗骋韬. PT含量变化对PMN-PT单晶的大功率性能影响[J]. 无机材料学报, 2025, 40(7): 840-846. |
| [2] | 汤新丽, 丁自友, 陈俊锐, 赵刚, 韩颖超. 基于稀土铕离子荧光标记的磷酸钙纳米材料体内分布与代谢研究[J]. 无机材料学报, 2025, 40(7): 754-764. |
| [3] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [4] | 王鲁杰, 张玉新, 李彤阳, 于源, 任鹏伟, 王建章, 汤华国, 姚秀敏, 黄毅华, 刘学建, 乔竹辉. 深海服役环境下碳化硅陶瓷材料的腐蚀及磨损行为[J]. 无机材料学报, 2025, 40(7): 799-807. |
| [5] | 余乐洋阳, 赵芳霞, 张舒心, 徐以祥, 牛亚然, 张振忠, 郑学斌. 感应等离子球化技术制备喷涂用高熵硼化物粉体[J]. 无机材料学报, 2025, 40(7): 808-816. |
| [6] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [7] | 孙晶, 李翔, 毛小建, 章健, 王士维. 月桂酸改性剂对氮化铝粉体抗水解性能的影响[J]. 无机材料学报, 2025, 40(7): 826-832. |
| [8] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [9] | 李文元, 徐佳楠, 邓瀚澳, 常爱民, 张博. 钒取代对LaTaO4陶瓷微观结构和微波介电性能的影响[J]. 无机材料学报, 2025, 40(6): 697-703. |
| [10] | 董晨雨, 郑维杰, 马一帆, 郑春艳, 温峥. 压电力显微镜表征Pb(Mg,Nb)O3-PbTiO3超薄膜弛豫特性[J]. 无机材料学报, 2025, 40(6): 675-682. |
| [11] | 何国强, 张恺恒, 王震涛, 包健, 席兆琛, 方振, 王昌昊, 王威, 王鑫, 姜佳沛, 李祥坤, 周迪. Ba(Nd1/2Nb1/2)O3: 一种被低估的K40微波介质陶瓷[J]. 无机材料学报, 2025, 40(6): 639-646. |
| [12] | 张家维, 陈宁, 程原, 王博, 朱建国, 金城. Bi4Ti3O12铋层状压电陶瓷的A/B位掺杂及其电学性能[J]. 无机材料学报, 2025, 40(6): 690-696. |
| [13] | 熊思宇, 莫尘, 朱肖伟, 朱国斌, 陈德钦, 刘来君, 施晓东, 李纯纯. 超低介电常数LiBxAl1-xSi2O6微波介质陶瓷的低温烧结[J]. 无机材料学报, 2025, 40(5): 536-544. |
| [14] | 崔宁, 张玉新, 王鲁杰, 李彤阳, 于源, 汤华国, 乔竹辉. (TiVNbMoW)Cx高熵陶瓷的单相形成过程与碳空位调控[J]. 无机材料学报, 2025, 40(5): 511-520. |
| [15] | 安然, 林锶, 郭世刚, 张冲, 祝顺, 韩颖超. 铁掺杂纳米羟基磷灰石的制备及紫外吸收性能研究[J]. 无机材料学报, 2025, 40(5): 457-465. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||