无机材料学报 ›› 2018, Vol. 33 ›› Issue (6): 653-658.DOI: 10.15541/jim20170350 CSTR: 32189.14.10.15541/jim20170350
所属专题: 电催化研究
王辉, 俞有幸
收稿日期:2017-07-20
修回日期:2017-10-25
出版日期:2018-06-20
网络出版日期:2018-05-24
作者简介:王 辉(1992-),男,硕士. E-mail: sy1501232@buaa.edu.cn
基金资助:WANG Hui, YU You-Xing
Received:2017-07-20
Revised:2017-10-25
Published:2018-06-20
Online:2018-05-24
About author:WANG Hui. E-mail: sy1501232@buaa.edu.cn
Supported by:摘要:
采用KOH溶液在通电条件下对Fe3N纳米颗粒表面改性的方法, 探究了碱化处理对Fe3N纳米颗粒电催化性能的影响。采用XRD、TEM、EDX、XPS、拉曼光谱和傅立叶变换红外光谱对碱化前后的Fe3N样品进行形貌和成分的表征, 采用时间电流曲线、LSV曲线、Tafel斜率、交流阻抗法和CV曲线对碱化前后的Fe3N样品进行电催化制氢(HER)性能的分析。结果表明, 用KOH处理的Fe3N样品, 平均晶粒尺寸由(80±10) nm缩小为(70±10) nm, 形状由破碎的链状结构变为椭圆形结构, 物相由ε-Fe3N相部分转变为α-Fe2O3相; 尺寸、形貌和成分的改变, 使得碱化后的样品有更多的电催化活性位点暴露。由电流密度为10 mA/cm2的过电位0.429 V降为0.204 V, Tafel斜率由103 mV/dec降为95 mV/dec。过电势降低, 交流阻抗变小, 电化学活性面积增大, 表明KOH碱化处理后的样品电催化制氢的能力得到大大提高。
中图分类号:
王辉, 俞有幸. KOH碱化处理对Fe3N纳米颗粒电催化制氢性能影响[J]. 无机材料学报, 2018, 33(6): 653-658.
WANG Hui, YU You-Xing. KOH Alkalized Fe3N Nanoparticles on Electrocatalytic Hydrogen Evolution Reaction[J]. Journal of Inorganic Materials, 2018, 33(6): 653-658.
| Element | N K/at% | O K/at% | Fe K/at% |
|---|---|---|---|
| Fe3N | 22.80 | 4.83 | 72.37 |
| Alkalized Fe3N | 13.50 | 12.30 | 74.20 |
表1 Fe3N纳米颗粒碱化前后不同元素相对含量对比
Tabel 1 Different elements relative content of Fe3N nanoparticles and alkalized Fe3N nanoparticles
| Element | N K/at% | O K/at% | Fe K/at% |
|---|---|---|---|
| Fe3N | 22.80 | 4.83 | 72.37 |
| Alkalized Fe3N | 13.50 | 12.30 | 74.20 |
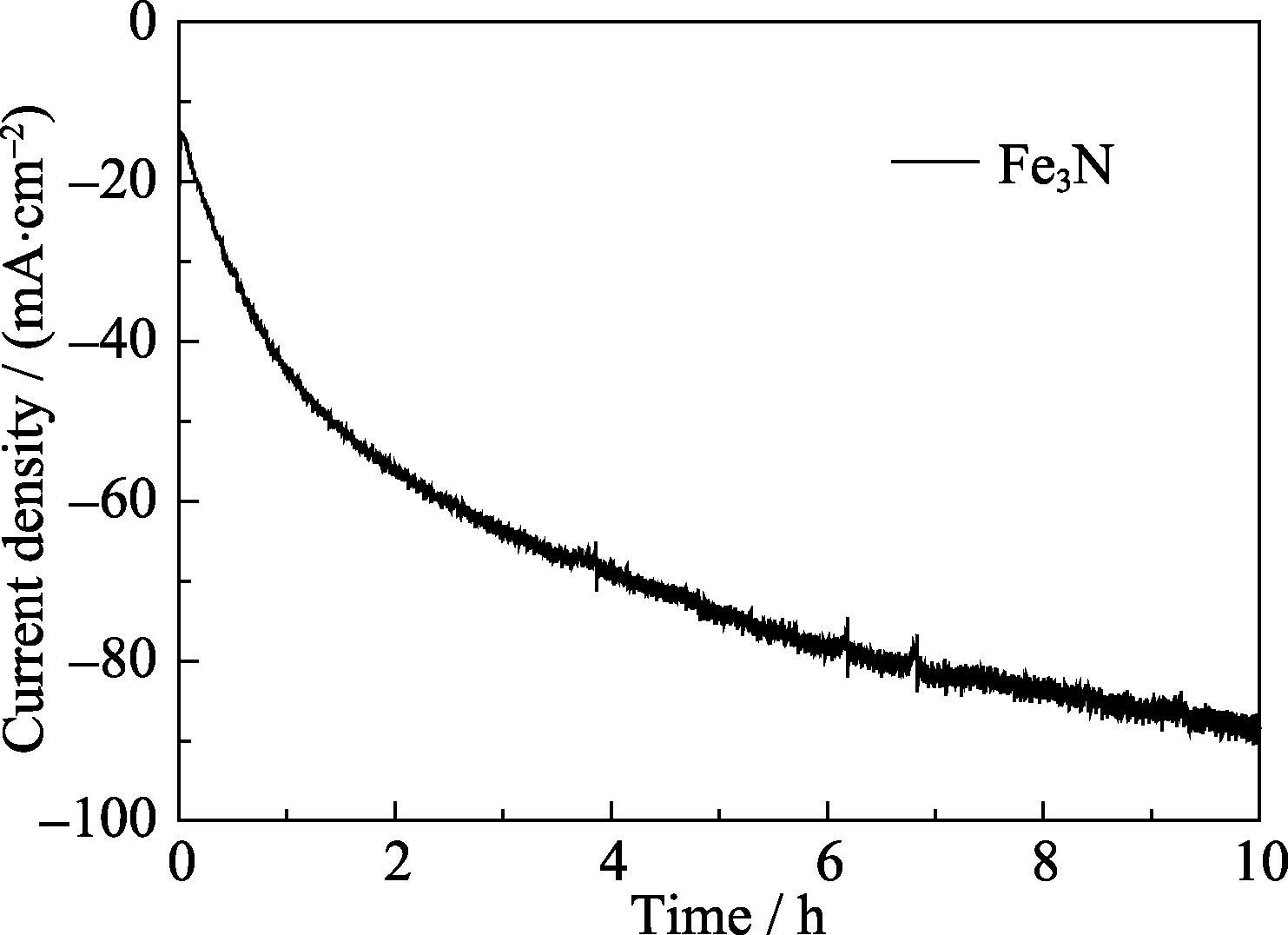
图6 Fe3N纳米颗粒在1.0 mol/L KOH溶液中-0.3 V的电压条件下的时间电流曲线
Fig. 6 Current-time (I-t) curve obtained for HER with Fe3N nanoparticles at overpotential of -0.3 V (vs. RHE) in 1.0 mol/L KOH
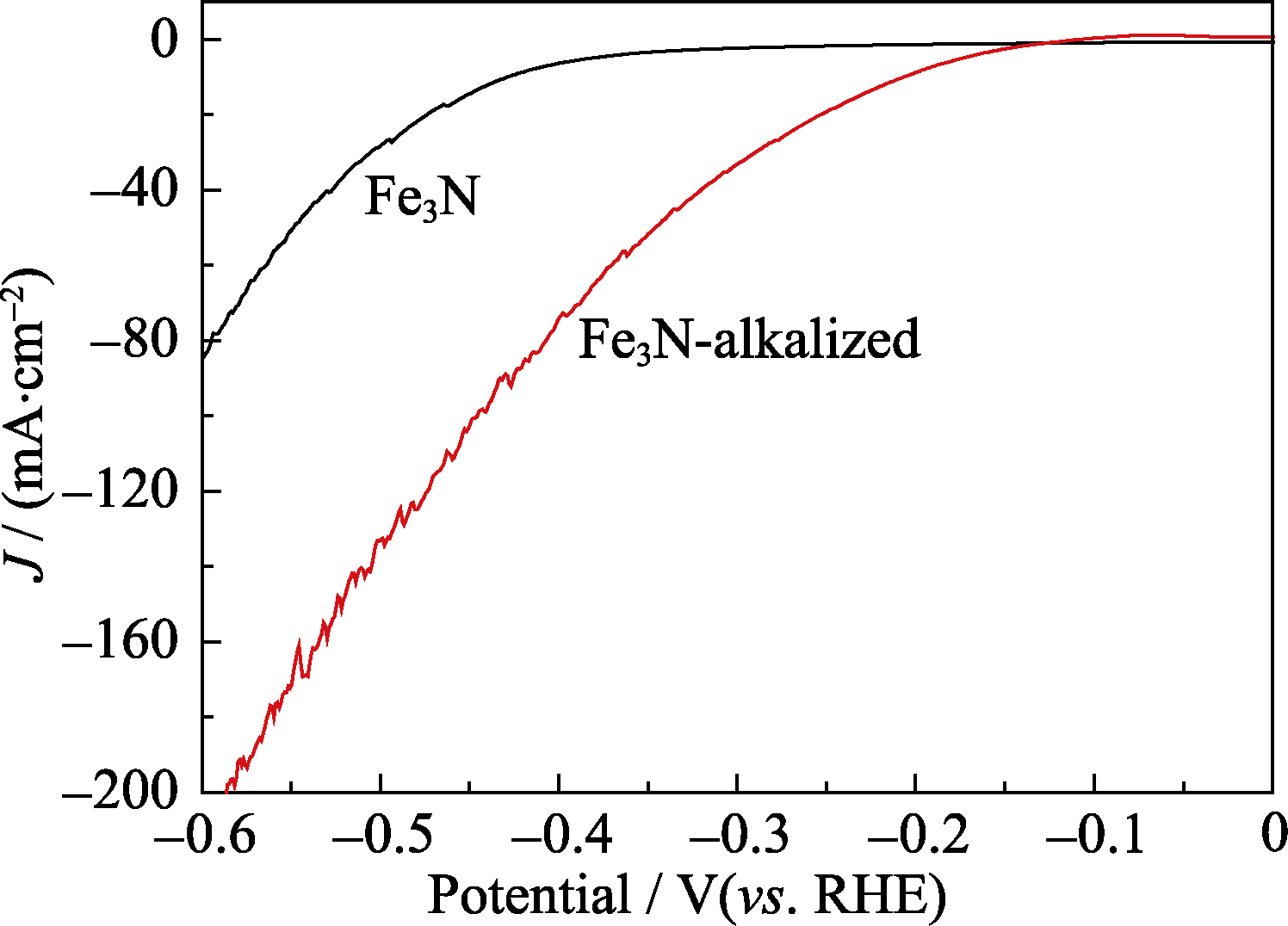
图7 Fe3N纳米颗粒碱化前后的线性扫描伏安法曲线, 扫描速度为5 mV/s
Fig. 7 Linear sweep voltammetry (LSV) curves for HER of Fe3N and alkalized Fe3N nanoparticles measured at 5 mV/s scan rate
| [1] | SUN S, ZHANG G, GAUQUELIN N,et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Scientific Reports, 2013, 3(5): 65-65. |
| [2] | CHENG N, STAMBULA S, WANG D,et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nature Communications, 2016, 7: 13638. |
| [3] | HOLLADAY J D, HU J, KING D L,et al. An overview of hydrogen production technologies. Catalysis Today, 2009, 139(4): 244-260. |
| [4] | STAFFELL I, GREEN R.The cost of domestic fuel cell micro- CHP systems.International Journal of Hydrogen Energy, 2013, 38(2): 1088-1102. |
| [5] | URSUA A, GANDIA L M, SANCHIS P.Hydrogen production from water electrolysis: current status and future trends.Proceedings of the IEEE, 2012, 100(2): 410-426. |
| [6] | SUBBARAMAN R, TRIPKOVIC D, CHANG K C,et al. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr (oxy) oxide catalysts. Nature Materials, 2012, 11(6): 550-557. |
| [7] | CHOI C H, KIM M, KWON H C,et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nature Communications, 2016, 7: 10922. |
| [8] | HE F, LI K, YIN C,et al. Single Pd atoms supported by graphitic carbon nitride, a potential oxygen reduction reaction catalyst from theoretical perspective. Carbon, 2017, 114: 619-627. |
| [9] | YANG S, TAK Y J, KIM J,et al. Support effects in single-atom platinum catalysts for electrochemical oxygen reduction. ACS Catalysis, 2017, 7(2): 1301-1307. |
| [10] | LIU R, ZHANG L Q, YU C, ,et al. Atomic-level-designed catalytically active palladium atoms on ultrathin gold nanowires. Advanced Materials. 2017, 29(7): 604571-1-8. |
| [11] | DANILOVIC N, SUBBARAMAN R, CHANG K C,et al. Frontispiece: using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angewandte Chemie International Edition, 2014, 53(51): 14016-14021. |
| [12] | MCPHERSON I J, VINCENT K A.Electrocatalysis by hydrogenases: lessons for building bio-inspired devices.Journal of the Brazilian Chemical Society, 2014, 25(3): 427-441. |
| [13] | ECKENHOFF W T, MCNAMARA W R, DU P,et al. Cobalt complexes as artificial hydrogenases for the reductive side of water splitting. Biochimica Et Biophysica Acta (BBA)-Bioenergetics, 2013, 1827(8): 958-973. |
| [14] | GONG M, LI Y, WANG H,et al. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. Journal of the American Chemical Society, 2013, 135(23): 8452-8455. |
| [15] | SONG F, HU X.Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis.Nature Communications, 2014, 5: 4477. |
| [16] | ZOU X, ZHANG Y.Noble metal-free hydrogen evolution catalysts for water splitting.Chemical Society Reviews, 2015, 44(15): 5148-5180. |
| [17] | MIN J P, JIN H L, HEMBRAM K,et al. Oxygen reduction electrocatalysts based on coupled iron nitride nanoparticles with nitrogen-doped carbon. Catalysts, 2016, 6(6): 86. |
| [18] | BHATTACHARYYA S.ChemInform abstract: iron nitride family at reduced dimensions: a review of their synthesis protocols and structural and magnetic properties.Cheminform, 2015, 46(12): 1601-1622. |
| [19] | MIN J P, JIN H L, HEMBRAM K P S S,et al. Oxygen reduction electrocatalysts based on coupled iron nitride nanoparticles with nitrogen-doped carbon. Catalysts, 2016, 6(6): 86. |
| [20] | QIAN Y, DU P, WU P,et al. Chemical nature of catalytic active sites for the oxygen reduction reaction on nitrogen-doped carbon- supported non-noble metal catalysts. Journal of Physical Chemistry C, 2016, 120(18): 9884-9896. |
| [21] | BEZERRA C W B, ZHANG L, LIU H,et al. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. Journal of Power Sources, 2007, 173(2): 891-908. |
| [22] | MUTHUSWAMY N, BUAN M E M, WALMSLEY J C,et al. Evaluation of ORR active sites in nitrogen-doped carbon nanofibers by KOH post treatment. Catalysis Today, 2018, 301(S1): 11-16. |
| [23] | YU F, ZHOU H, ZHU Z,et al. Three-dimensional nanoporous iron nitride film as an efficient electrocatalyst for water oxidation. ACS Catalysis, 2017, 7(3): 2052-2057. |
| [24] | DONG G, FANG M, WANG H,et al. Insight into the electrochemical activation of carbon-based cathodes for hydrogen evolution reaction. Journal of Materials Chemistry A, 2015, 3(24): 13080-13086. |
| [25] | WANG J, XU F, JIN H, et al. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Advanced Materials. 2017, 29(14): 1605838-1-35. |
| [26] | TANG C, WANG W, SUN A,et al. Sulfur-decorated molybdenum carbide catalysts for enhanced hydrogen evolution. ACS Catalysis, 2015, 5(11):6956-6963. |
| [27] | NASIBULIN A G, RACKAUSKAS S, JIANG H,et al. Simple and rapid synthesis of α-Fe2O3 nanowires under ambient conditions. Nano Research, 2009, 2(5): 373-379. |
| [1] | 杨鑫, 韩春秋, 曹玥晗, 贺桢, 周莹. 金属氧化物电催化硝酸盐还原合成氨研究进展[J]. 无机材料学报, 2024, 39(9): 979-991. |
| [2] | 李红兰, 张俊苗, 宋二红, 杨兴林. Mo/S共掺杂的石墨烯用于合成氨: 密度泛函理论研究[J]. 无机材料学报, 2024, 39(5): 561-568. |
| [3] | 景欣欣, 陈必清, 翟佳鑫, 袁美玲. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476. |
| [4] | 杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382. |
| [5] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [6] | 张祥松, 刘业通, 王永瑛, 武子瑞, 刘振中, 李毅, 杨娟. 自组装制备PtIr合金气凝胶及其高效电催化氨氧化性能[J]. 无机材料学报, 2023, 38(5): 511-520. |
| [7] | 胡越, 安琳, 韩鑫, 侯成义, 王宏志, 李耀刚, 张青红. RhO2修饰BiVO4薄膜光阳极的制备及其光电催化分解水性能[J]. 无机材料学报, 2022, 37(8): 873-882. |
| [8] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [9] | 付永胜, 毕敏, 李春, 孙敬文, 汪信, 朱俊武. 非贵金属/碳氮复合材料电催化析氧反应的研究进展[J]. 无机材料学报, 2022, 37(2): 163-172. |
| [10] | 吴静, 余立兵, 刘帅帅, 黄秋艳, 姜姗姗, ANTON Matveev, 王连莉, 宋二红, 肖蓓蓓. NiN4/Cr修饰的石墨烯电化学固氮电极[J]. 无机材料学报, 2022, 37(10): 1141-1148. |
| [11] | 苏莉, 杨建平, 兰悦, 王连军, 江莞. 纳米铁颗粒及其复合材料的界面设计及环境修复应用[J]. 无机材料学报, 2021, 36(6): 561-569. |
| [12] | 朱云娜, 陈必清, 程天舒, 杜婵, 张士民, 赵静. 非晶Nd-Ni-B/NF稀土复合电极材料的制备及其析氢性能[J]. 无机材料学报, 2021, 36(6): 637-644. |
| [13] | 周煜筑, 张友魁, 宋礼. 贵金属磷化物催化剂及其同步辐射X射线吸收谱[J]. 无机材料学报, 2021, 36(3): 225-244. |
| [14] | 朱勇, 顾军, 于涛, 何海佟, 姚睿. 铂钴合金纳米电催化剂的制备及性能研究[J]. 无机材料学报, 2021, 36(3): 299-305. |
| [15] | 张文进, 申倩倩, 薛晋波, 李琦, 刘旭光, 贾虎生. 具有高度有序氧空位的α-Fe2O3纳米带的制备及光电催化水氧化性能研究[J]. 无机材料学报, 2021, 36(12): 1290-1296. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||