无机材料学报 ›› 2023, Vol. 38 ›› Issue (5): 511-520.DOI: 10.15541/jim20220684 CSTR: 32189.14.10.15541/jim20220684
所属专题: 【能源环境】燃料电池(202506)
张祥松( ), 刘业通, 王永瑛, 武子瑞, 刘振中, 李毅(
), 刘业通, 王永瑛, 武子瑞, 刘振中, 李毅( ), 杨娟(
), 杨娟( )
)
收稿日期:2022-11-16
修回日期:2023-01-04
出版日期:2023-01-11
网络出版日期:2023-01-11
通讯作者:
李 毅, 讲师. E-mail: liyi5482@ujs.edu.cn;作者简介:张祥松(1996-), 男, 硕士研究生. E-mail: jsdaujszxs1996@163.com
基金资助:
ZHANG Xiangsong( ), LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi(
), LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi( ), YANG Juan(
), YANG Juan( )
)
Received:2022-11-16
Revised:2023-01-04
Published:2023-01-11
Online:2023-01-11
Contact:
LI Yi, lecturer. E-mail: liyi5482@ujs.edu.cn;About author:ZHANG Xiangsong (1996-), male, Master candidate. E-mail: jsdaujszxs1996@163.com
Supported by:摘要:
氨具有低成本、易液化和高体积能量密度等特点, 是一种有吸引力的无碳燃料, 用其制成的直接氨燃料电池也备受科研人员青睐, 却受限于阳极氨氧化缓慢的动力学过程。本工作采用无表面活性剂的简单方法通过纳米颗粒(NPs)自组装制备了三维多孔网络结构的PtIr合金气凝胶高效氨氧化催化剂。该结构提供了丰富开放的互联质子传输通道和额外的催化活性位点, 有助于氨电催化氧化中NH3分子的去质子化过程。当Pt和Ir物质的量比为80/20时, PtIr合金气凝胶展现出最优的氨氧化(AOR)催化活性。实验通过研究NH3浓度和工作温度对催化剂氨氧化性能的影响发现, Pt80Ir20合金气凝胶催化剂的AOR性能随着氨水浓度或温度的上升而增强, 如当氨水浓度为0.5 mol/L时, 合金催化剂在0.5 V电位下的质量比活性为44.03 A·g-1, 约是0.05 mol/L 氨水中的4倍。当温度上升至80 ℃ 时, 合金催化剂在0.5 V电位下的质量比活性为148.73 A·g-1, 约为25 ℃下的12倍。在此温度变化区间, 其AOR起始电位下降了40 mV。利用Arrhenius方程计算发现, Pt80Ir20合金气凝胶催化剂的AOR反应活化能比商业Pt/C催化剂降低了~9.43 kJ·mol-1。此外, 催化材料的稳定性测试结果表明, Pt80Ir20合金气凝胶催化剂经2000次循环伏安测试后的峰值质量比活性损失为~50.6%, 优于商业Pt/C催化剂(~74.9%)。
中图分类号:
张祥松, 刘业通, 王永瑛, 武子瑞, 刘振中, 李毅, 杨娟. 自组装制备PtIr合金气凝胶及其高效电催化氨氧化性能[J]. 无机材料学报, 2023, 38(5): 511-520.
ZHANG Xiangsong, LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi, YANG Juan. Self-assembled Platinum-iridium Alloy Aerogels and Their Efficient Electrocatalytic Ammonia Oxidation Performance[J]. Journal of Inorganic Materials, 2023, 38(5): 511-520.
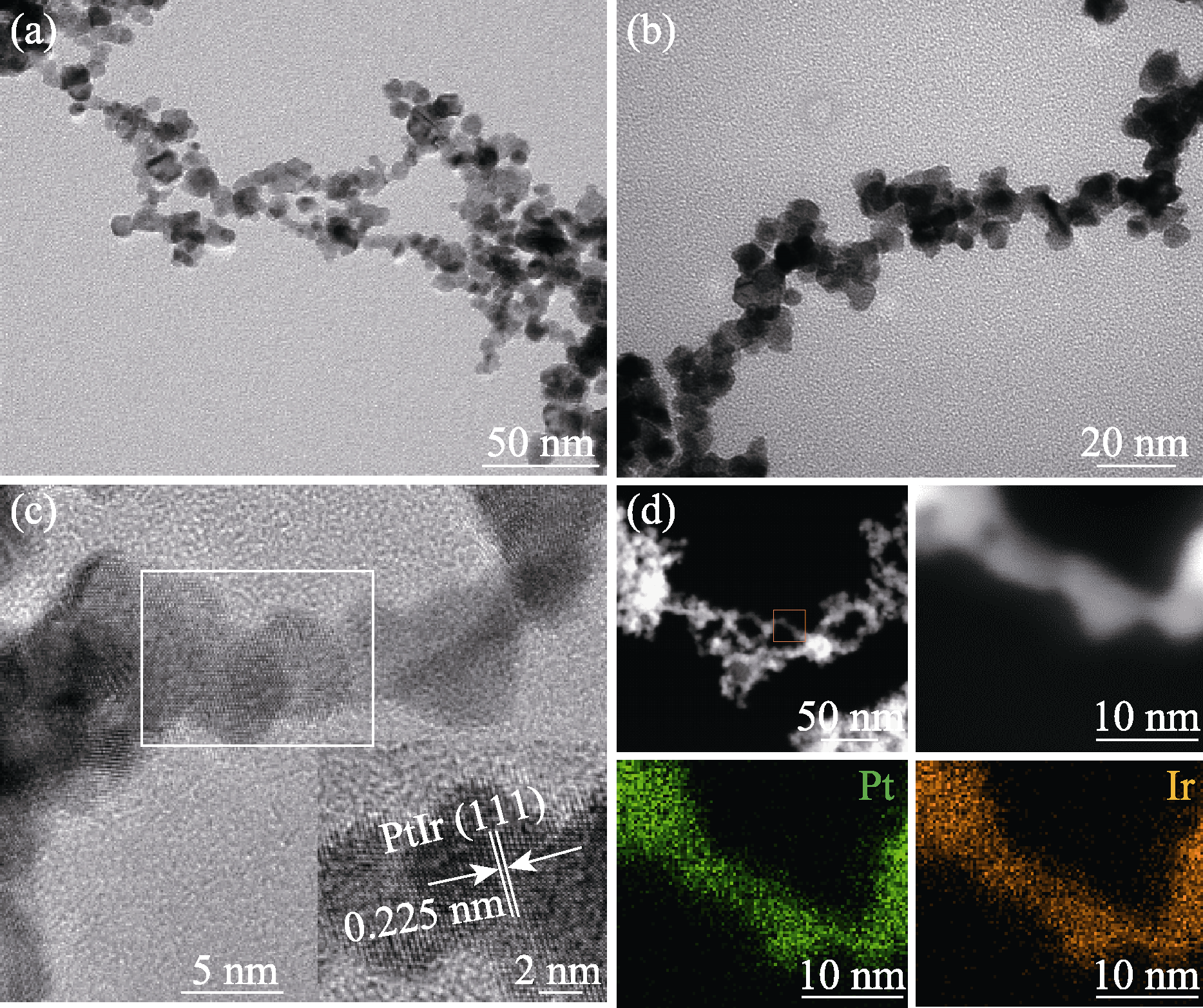
图2 Pt80Ir20合金气凝胶的结构和形貌表征
Fig. 2 Structure and composition of Pt80Ir20 alloy aerogel (a, b) TEM images; (c) HR-TEM image; (d) HAADF-STEM image and corresponding mapping images
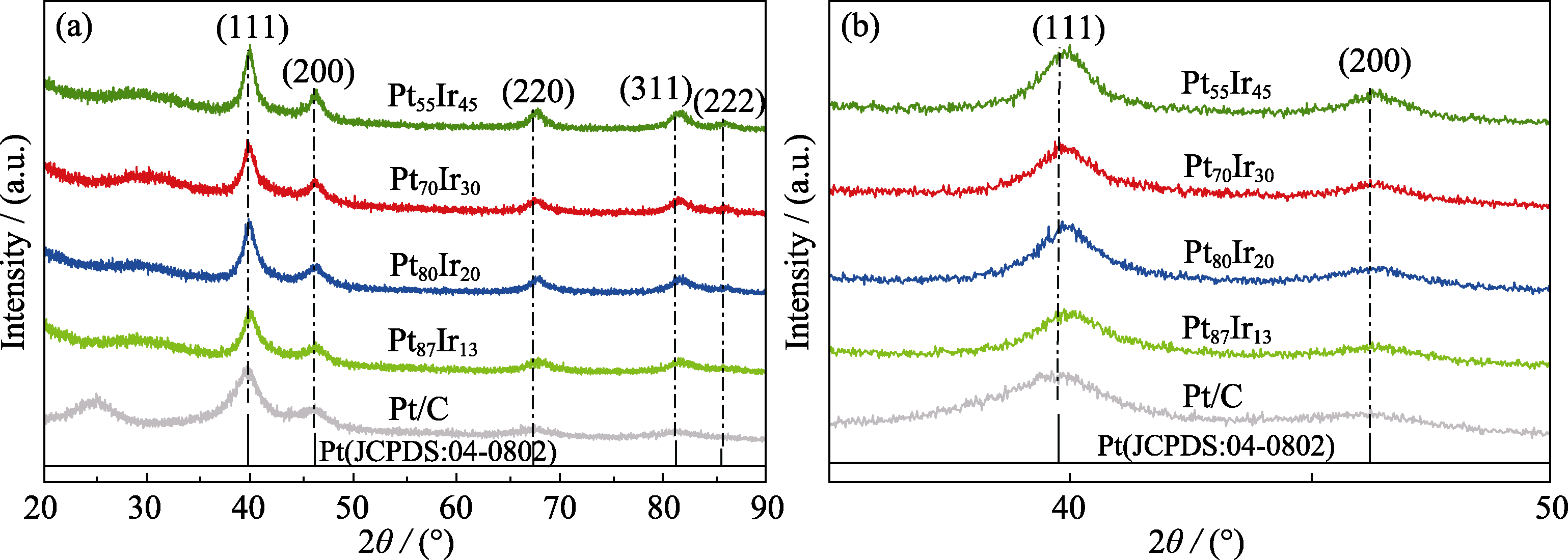
图3 不同催化剂的XRD谱图(a)及其局部(2θ=35°~50°)XRD放大图谱(b)
Fig. 3 (a) XRD patterns of different catalysts, commercial Pt/C, and (b) corresponding enlarged XRD patterns in the range of 2θ=35°-50°
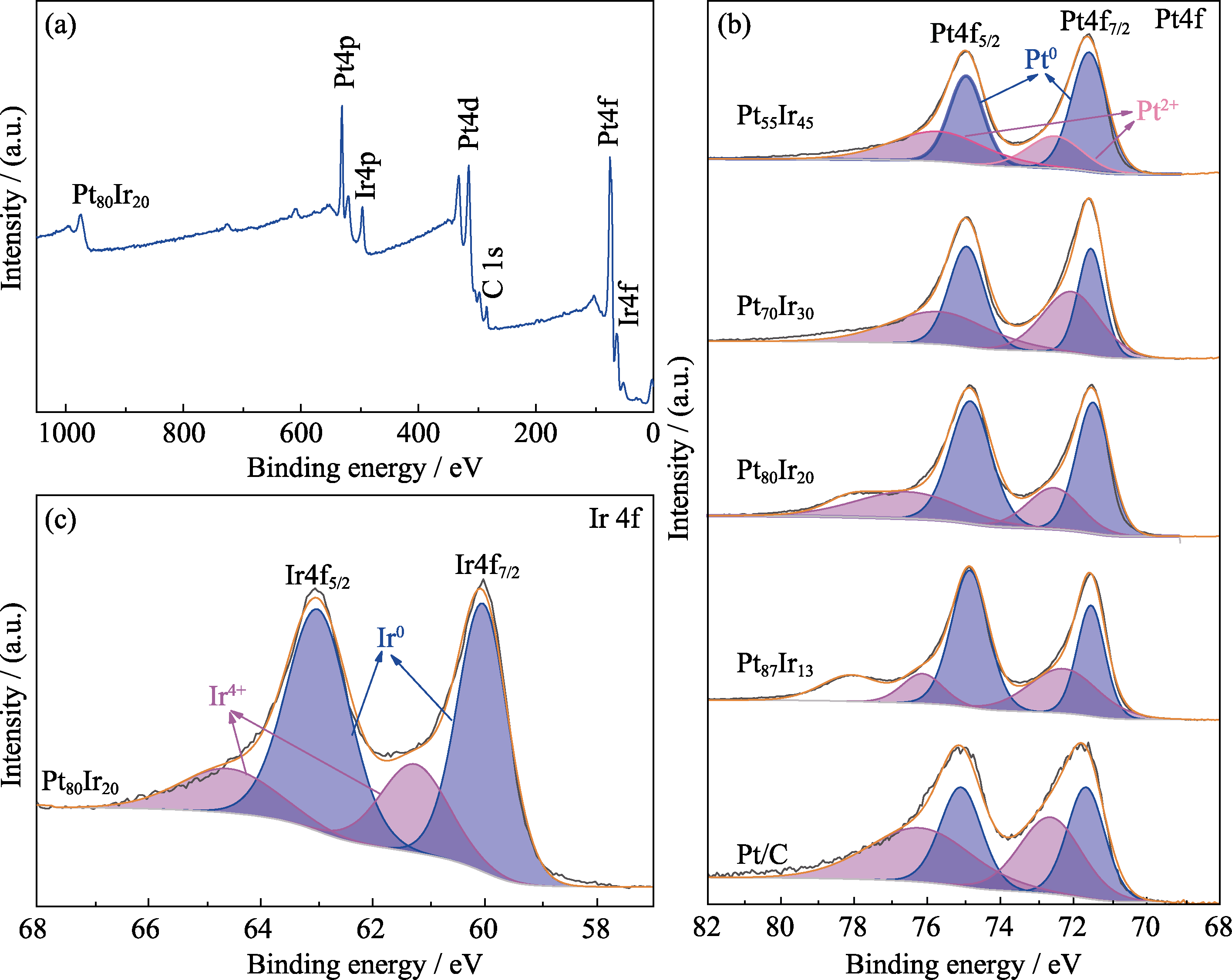
图4 (a)Pt80Ir20合金气凝胶的XPS全谱图, (b)不同样品的Pt4f XPS谱图, (c)Pt80Ir20合金气凝胶的Ir4f XPS谱图
Fig. 4 (a) XPS survey spectrum of Pt80Ir20 aerogel, (b) Pt4f XPS spectra of various Pt-based catalysts, and (c) Ir4f XPS spectrum of Pt80Ir20 aerogel
| Sample | Pt/% | Ir/% | Pt/Ir |
|---|---|---|---|
| Pt55Ir45 | 54.33 | 45.67 | 1.19 |
| Pt70Ir30 | 67.24 | 32.76 | 2.05 |
| Pt80Ir20 | 79.34 | 20.66 | 3.84 |
| Pt87Ir13 | 88.36 | 11.64 | 7.59 |
表S1 不同样品的XPS测试元素含量(%, 原子分数)
Table S1 Elemental quantification (%, in atom) determined by XPS for different Pt100-xIrx aerogel catalysts
| Sample | Pt/% | Ir/% | Pt/Ir |
|---|---|---|---|
| Pt55Ir45 | 54.33 | 45.67 | 1.19 |
| Pt70Ir30 | 67.24 | 32.76 | 2.05 |
| Pt80Ir20 | 79.34 | 20.66 | 3.84 |
| Pt87Ir13 | 88.36 | 11.64 | 7.59 |
| Sample | Pt4f5/2/eV | ∆1/eV | Pt4f7/2/eV | ∆2/eV |
|---|---|---|---|---|
| Commercial Pt/C | 75.10 | - | 71.70 | - |
| Pt87Ir13 aerogel | 74.85 | -0.25 | 71.53 | -0.17 |
| Pt80Ir20 aerogel | 74.88 | -0.22 | 71.50 | -0.20 |
| Pt70Ir30 aerogel | 74.92 | -0.18 | 71.53 | -0.17 |
| Pt55Ir45 aerogel | 74.93 | -0.17 | 71.57 | -0.13 |
表S2 不同样品与商业Pt/C样品Pt4f结合能的对比
Table S2 Comparison of binding energy between Pt4f with different Pt-based catalysts
| Sample | Pt4f5/2/eV | ∆1/eV | Pt4f7/2/eV | ∆2/eV |
|---|---|---|---|---|
| Commercial Pt/C | 75.10 | - | 71.70 | - |
| Pt87Ir13 aerogel | 74.85 | -0.25 | 71.53 | -0.17 |
| Pt80Ir20 aerogel | 74.88 | -0.22 | 71.50 | -0.20 |
| Pt70Ir30 aerogel | 74.92 | -0.18 | 71.53 | -0.17 |
| Pt55Ir45 aerogel | 74.93 | -0.17 | 71.57 | -0.13 |
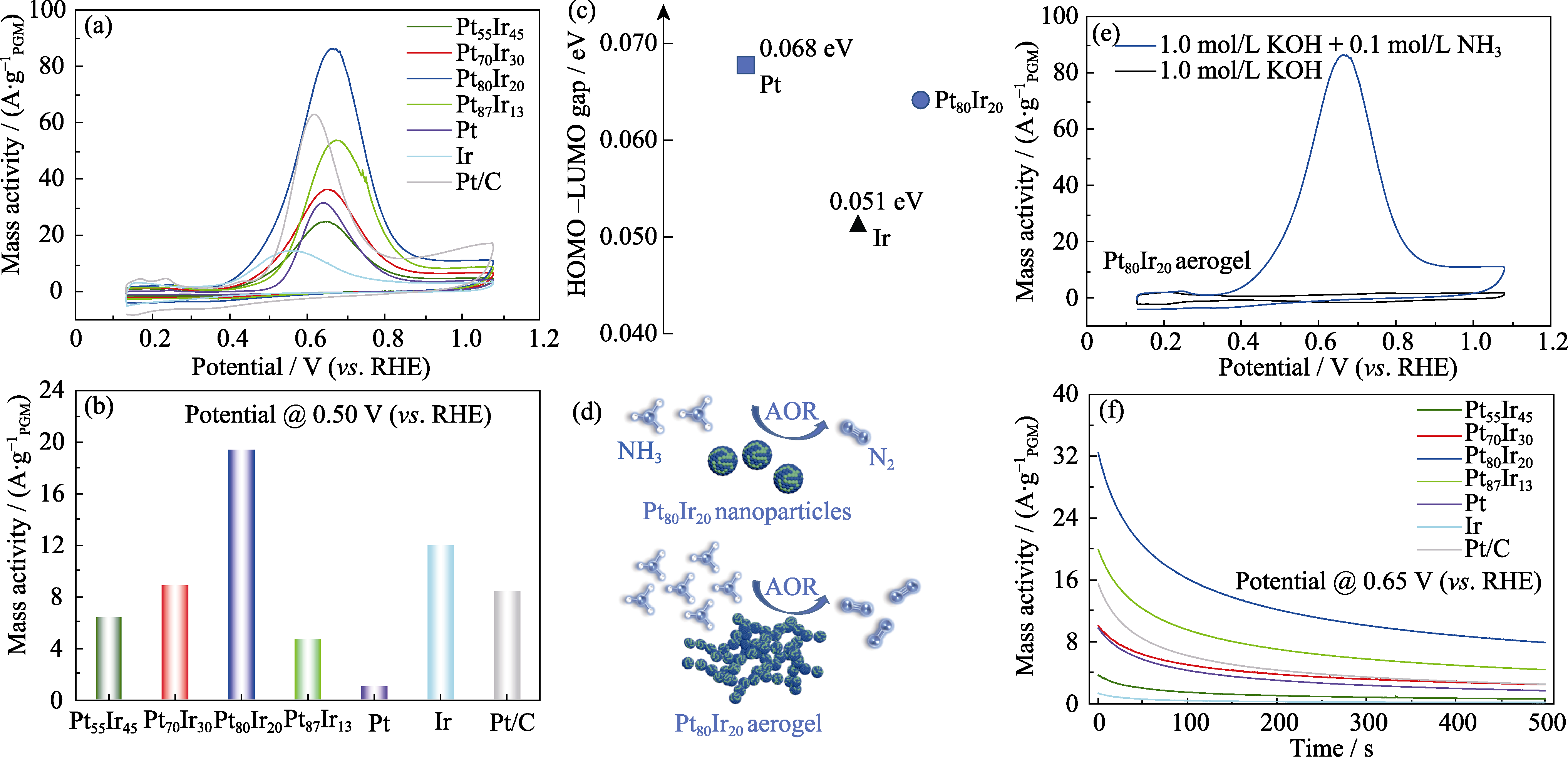
图5 (a)不同催化剂室温下的AOR催化CV曲线, (b)不同催化剂在0.5 V电极电势下的AOR活性对比, (c)Pt, Ir和Pt80Ir20纳米颗粒的最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的能量差, (d)Pt80Ir20合金纳米颗粒与Pt80Ir20合金气凝胶的电催化氨氧化示意图, (e)优选Pt80Ir20合金气凝胶催化剂在有/无NH3条件下的CV曲线, (f)不同催化剂的恒电位曲线
Fig. 5 (a) CV curves of Pt100-xIrx aerogels and commercial Pt/C catalysts under room temperature, (b) AOR activity comparison for Pt100-xIrx aerogels and commercial Pt/C catalysts at 0.5 V(vs. RHE), (c) energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Pt, Ir and Pt80Ir20 nanoparticles, (d) schematic diagram of electrocatalytic ammonia oxidation of Pt80Ir20 alloy nanoparticles and Pt80Ir20 alloy aerogel, (e) CV curves of the Pt80Ir20 catalyst in the presence and absence of NH3; (f) CA curves of Pt100-xIrx aerogels and commercial Pt/C catalysts Colorful figures are available on website
| Sample | Onset potential/V | Mass activity at 0.5 V (vs. RHE)/(mA·mgPGM-1) | Peak mass activity/ (mA·mgPGM-1) | Ref. |
|---|---|---|---|---|
| Pt87Ir13 aerogel | 0.411 | 4.7 | 53.7 | This work |
| Pt80Ir20 aerogel | 0.368 | 19.4 | 86.3 | This work |
| Pt70Ir30 aerogel | 0.361 | 8.9 | 31.5 | This work |
| Pt55Ir45 aerogel | 0.358 | 6.4 | 24.8 | This work |
| Pt | 0.511 | 1.1 | 31.5 | This work |
| Ir | 0.354 | 12.0 | 14.6 | This work |
| Commercial Pt/C | 0.495 | 8.4 | 62.9 | This work |
| Commercial PtIr/C | 0.428 | 10.4 | 25.1 | [S1] |
| Ir-decorate Pt NCs/C | ~ 0.43 | - | 100 | [S2] |
| Polycrystalline PtIr | ~ 0.41 | - | - | [S3] |
| PtRh/C(Pt:Rh = 9:1) | 0.44 | 9.0 | 93.8 | [S4] |
| Pt-decorated Ni particles | ~0.50 | - | 75.3 | [S5] |
表S3 不同样品的AOR性能对比
Table S3 Comparison of AOR activity between different catalysts
| Sample | Onset potential/V | Mass activity at 0.5 V (vs. RHE)/(mA·mgPGM-1) | Peak mass activity/ (mA·mgPGM-1) | Ref. |
|---|---|---|---|---|
| Pt87Ir13 aerogel | 0.411 | 4.7 | 53.7 | This work |
| Pt80Ir20 aerogel | 0.368 | 19.4 | 86.3 | This work |
| Pt70Ir30 aerogel | 0.361 | 8.9 | 31.5 | This work |
| Pt55Ir45 aerogel | 0.358 | 6.4 | 24.8 | This work |
| Pt | 0.511 | 1.1 | 31.5 | This work |
| Ir | 0.354 | 12.0 | 14.6 | This work |
| Commercial Pt/C | 0.495 | 8.4 | 62.9 | This work |
| Commercial PtIr/C | 0.428 | 10.4 | 25.1 | [S1] |
| Ir-decorate Pt NCs/C | ~ 0.43 | - | 100 | [S2] |
| Polycrystalline PtIr | ~ 0.41 | - | - | [S3] |
| PtRh/C(Pt:Rh = 9:1) | 0.44 | 9.0 | 93.8 | [S4] |
| Pt-decorated Ni particles | ~0.50 | - | 75.3 | [S5] |
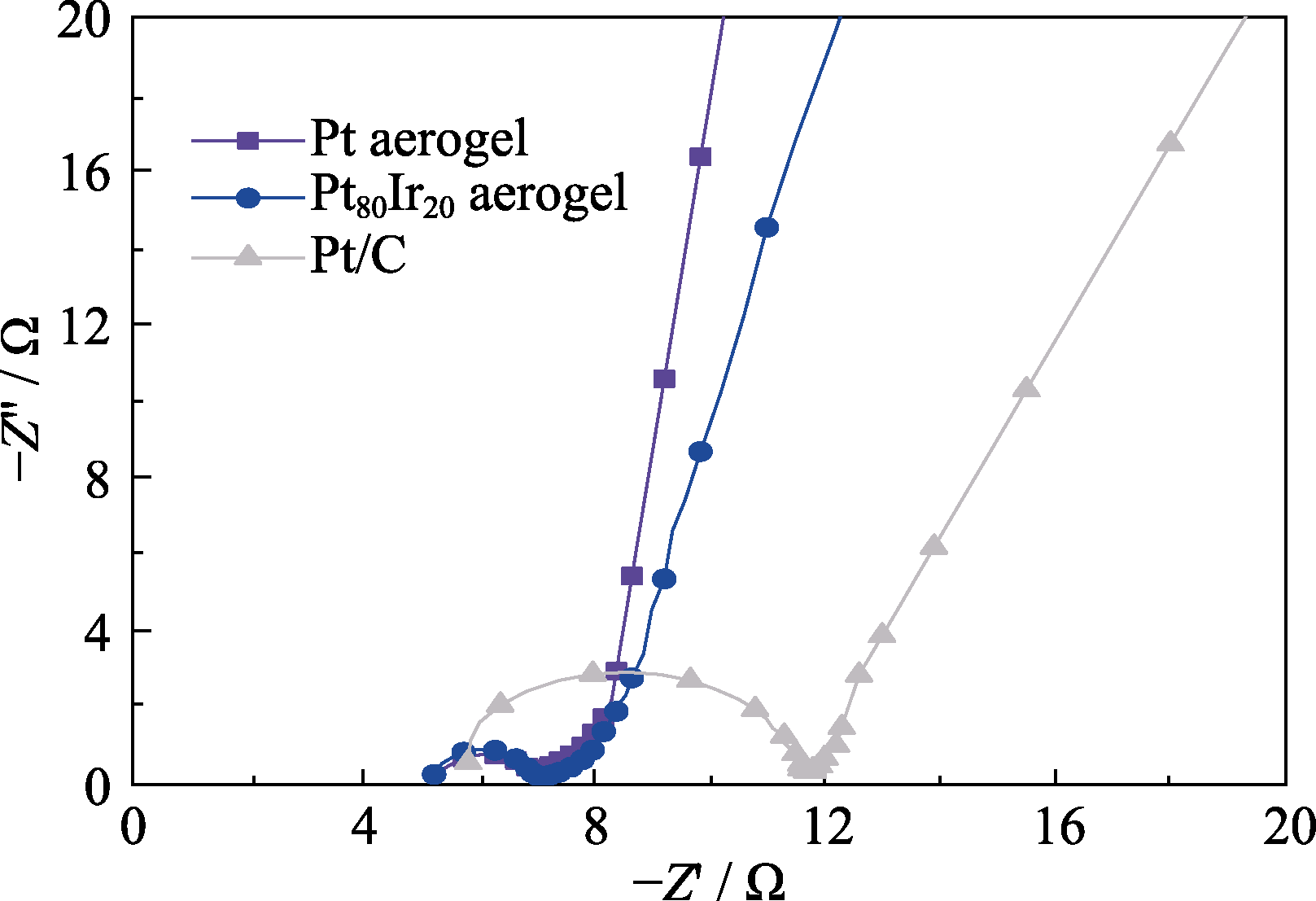
图S6 Pt(紫罗兰色),Pt80Ir20(蓝色)气凝胶以及商业Pt/C催化剂(灰色)的EIS阻抗曲线
Fig. S6 Nyquist plots of EIS spectra measured for Pt (violet), Pt80Ir20 aerogel (blue) and commercial Pt/C (gray) in 1.0 mol/L KOH electrolyte at the open circuit potential
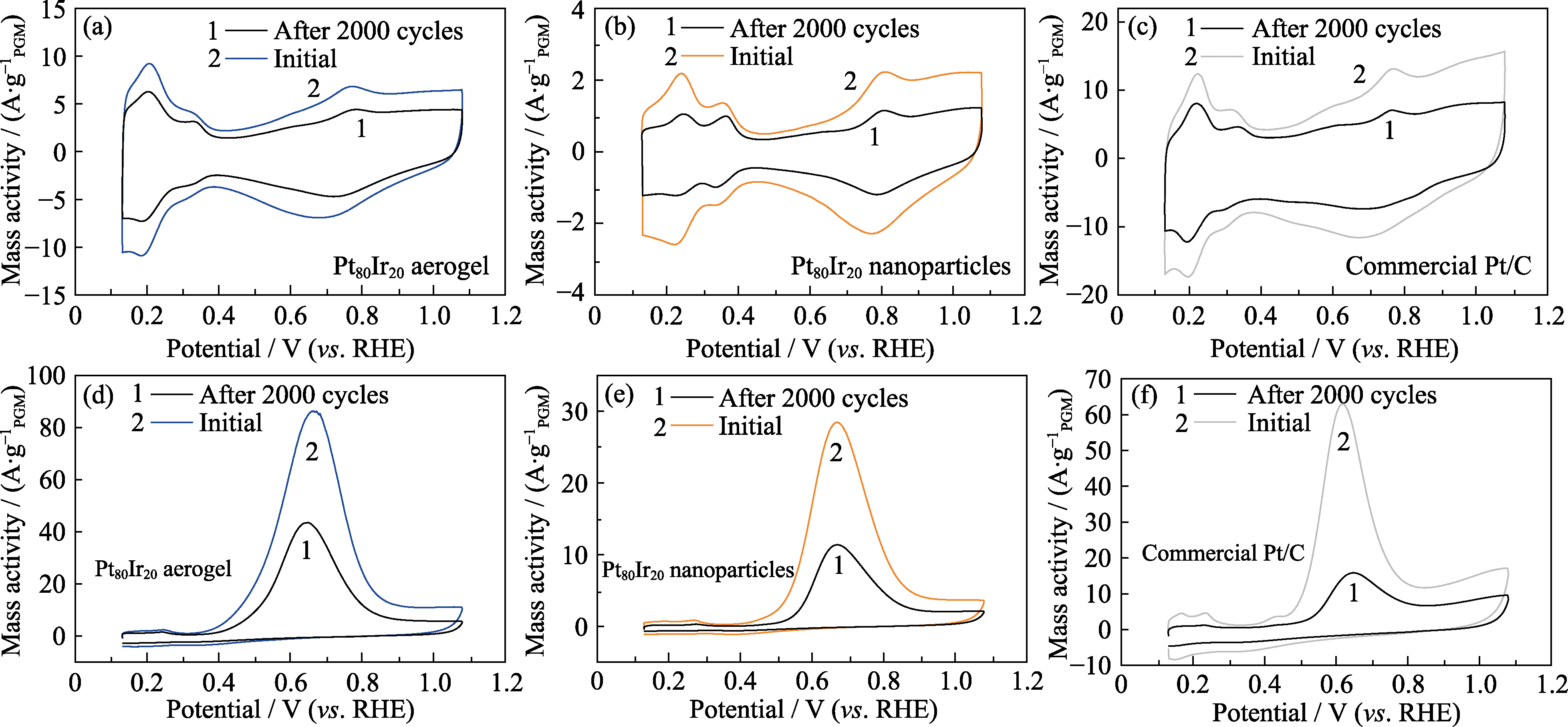
图6 2000次CV循环后的电化学活性面积以及室温AOR性能对比
Fig. 6 Electrochemical active areas of catalysts and AOR performance before and after 2000 CV cycles (a, d) Pt80Ir20 aerogel; (b, e) Pt80Ir20 nanoparticles; (c, f) Commercial Pt/C
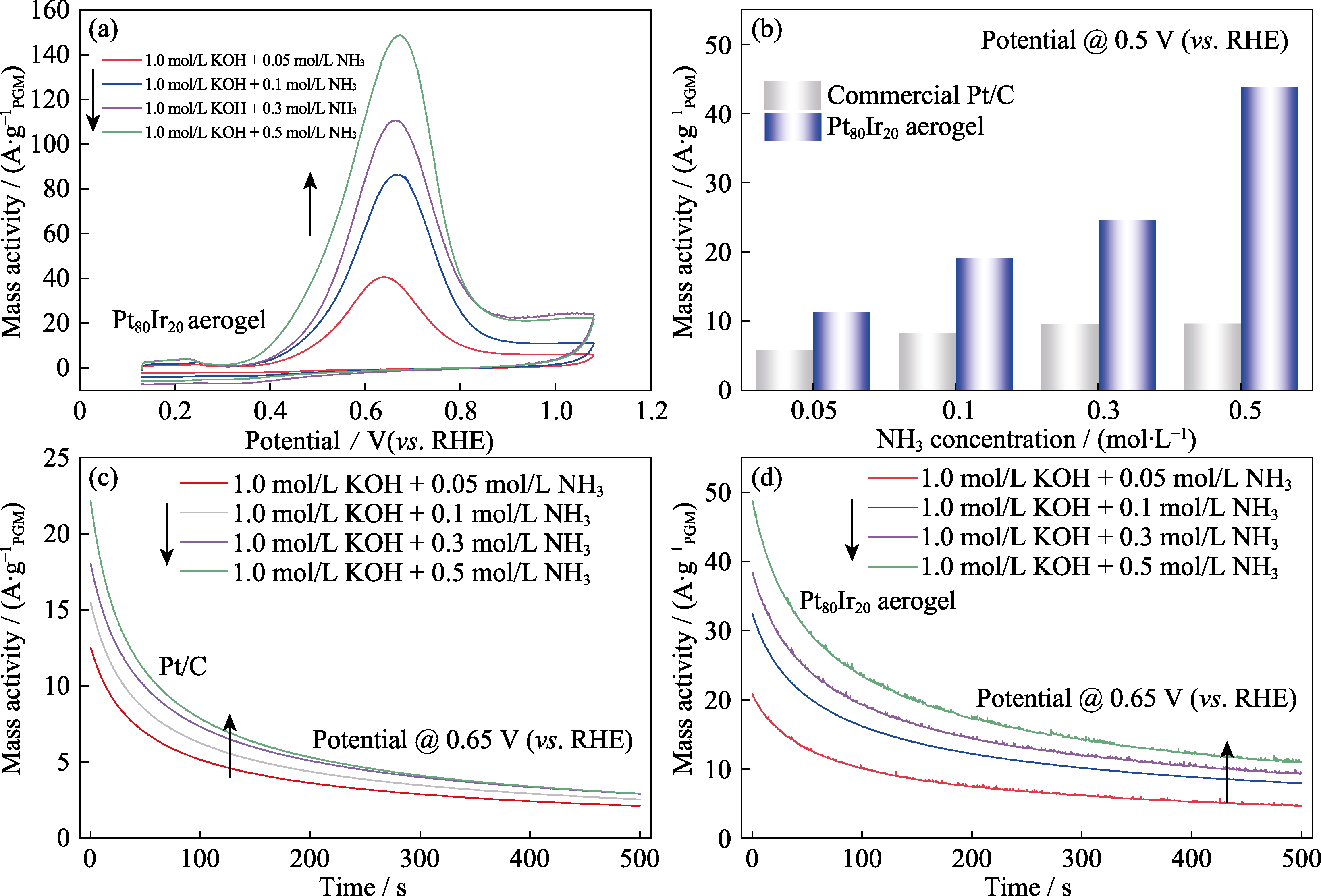
图7 (a)不同NH3浓度下的Pt80Ir20合金气凝胶催化剂的AOR性能, (b)不同NH3浓度(mol/L)下, Pt80Ir20合金气凝胶与商业Pt/C在0.5 V(vs. RHE)电位下AOR活性对比, 不同NH3浓度下的Pt80Ir20合金气凝胶(c)与商业Pt/C(d)在0.65 V(vs. RHE)电位下的CA曲线
Fig. 7 (a) CV curves of the Pt80Ir20 aerogel tested in different NH3 concentrations, (b) AOR activity comparison for Pt80Ir20 aerogel and commercial Pt/C in different NH3 concentrations at 0.5 V(vs. RHE), and (c, d) CA curves of the Pt80Ir20 aerogel (c) and commercial Pt/C (d) at 0.65 V(vs. RHE)
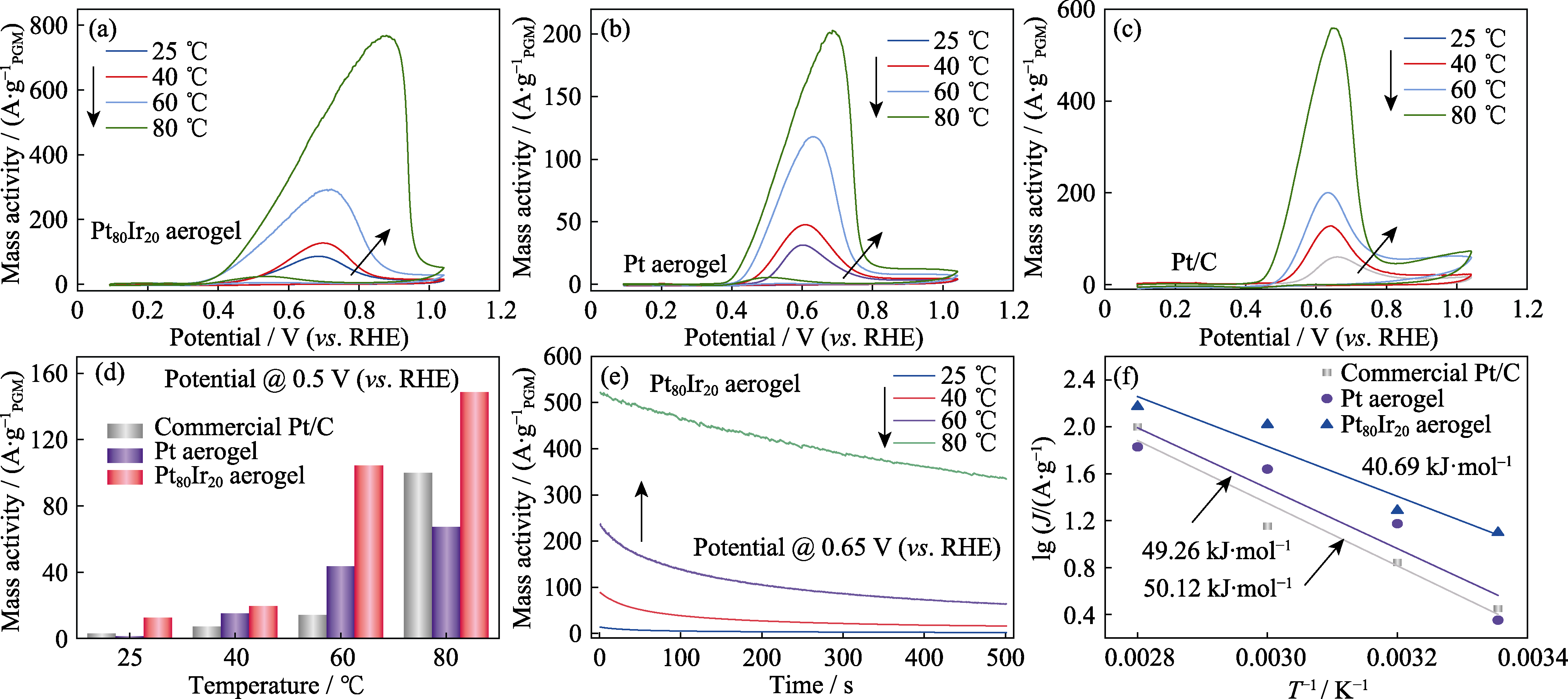
图8 (a~c)不同温度下的催化剂的AOR性能, (d)商业Pt/C、Pt气凝胶与Pt80Ir20合金气凝胶在0.5 V(vs. RHE)电极电势下的AOR活性对比, (e)不同工作温度下的Pt80Ir20合金气凝胶的恒电位曲线, (f)商业Pt/C催化剂、Pt气凝胶和Pt80Ir20合金气凝胶在0.5 V(vs. RHE)电极电势下的阿仑尼乌斯图
Fig. 8 (a-c) CV curves of catalysts at different temperatures, (d) AOR activity comparison for commercial Pt/C, Pt aerogel and Pt80Ir20 aerogel catalysts at different temperatures at 0.5 V(vs. RHE), (e) CA curves of the Pt80Ir20 aerogel at different temperatures at 0.65 V (vs. RHE), (f) Arrhenius plots for NH3 oxidation on commercial Pt/C, Pt aerogel and Pt80Ir20 aerogel catalysts at 0.5 V(vs. RHE)
| [1] | LI Y, WANG H H, PRIEST C, et al. Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions. Adv. Mater., 2020, 33(6): 2000381. |
| [2] |
JIN H, LEE S, SOHN Y. Capping agent-free synthesis of surface engineered Pt nanocube for direct ammonia fuel cell. Int. J. Energy Res., 2021, 45(12): 18281.
DOI URL |
| [3] |
LI Y, PILLAI H S, WANG T, et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci., 2021, 14(3): 1449.
DOI URL |
| [4] |
LI Y, LI X, PILLAI H S, et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation. ACS Catal., 2020, 10(7): 3945.
DOI URL |
| [5] |
ZHAO Y, SETZLER B P, WANG J H, et al. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule, 2019, 3(10): 2472.
DOI URL |
| [6] |
PILLAI H S, XIN H. New insights into electrochemical ammonia oxidation on Pt(100) from first principles. Ind. Eng. Chem. Res., 2019, 58(25): 10819.
DOI URL |
| [7] |
KANG Y M, WANG W, LI J M, et al. High performance PtxEu alloys as effective electrocatalysts for ammonia electro-oxidation. Int. J. Hydrogen. Energy, 2017, 42(30): 18959.
DOI URL |
| [8] |
XUE Q, ZHAO Y, ZHU J Y, et al. PtRu nanocubes as bifunctional electrocatalysts for ammonia electrolysis. J. Mater. Chem. A, 2021, 9(13): 8444.
DOI URL |
| [9] | CHEN R Y, ZHENG S S, YAO Y D, et al. Performance of direct ammonia fuel cell with PtIr/C, PtRu/C, and Pt/C as anode electrocatalysts under mild conditions. Int. J. Hydrogen. Energy, 2021 (46): 27749. |
| [10] |
GOTTESFELD S. The direct ammonia fuel cell and a common pattern of electrocatalytic processes. J. Electrochem. Soc., 2018, 165(15): J3405.
DOI URL |
| [11] | SONG L, LIANG Z X, MA Z, et al. Temperature-dependent kinetics and reaction mechanism of ammonia oxidation on Pt, Ir, and PtIr alloy catalysts. J. Electrochem. Soc., 2018, 165: 3095. |
| [12] |
SACRE N, DUCA M, GARBARION S, et al. Tuning Pt-Ir interactions for NH3 electrocatalysis. ACS Catal., 2018, 8(3): 2508.
DOI URL |
| [13] | ESTEJAB A, BOTTE G. Ammonia oxidation kinetics on bimetallic clusters of platinum and iridium: a theoretical approach. Mol. Catal., 2018, 445: 279. |
| [14] |
DOUK A S, SARAVANI H. Porous 3D inorganic superstructure of Pd-Ir aerogel as advanced support-less anode electrocatalyst toward ethanol oxidation. ACS Omega, 2020, 5: 22031.
DOI PMID |
| [15] |
ZHU C, DU D, EYCHMULLER A. Engineering ordered and nonordered porous noble metal nanostructures: synthesis, assembly, and their applications in electrochemistry. Chem. Rev., 2015, 115: 8896.
DOI PMID |
| [16] |
ZHU C Z, GUO S J, DONG S J. PdM (M= Pt, Au) bimetallic alloy nanowires with enhanced electrocatalytic activity for electro- oxidation of small molecules. Adv. Mater., 2012, 24: 2326.
DOI URL |
| [17] | CAI B, DIANAT A, HUBNER R, et al. Multimetallic hierarchical aerogels: shape engineering of the building blocks for efficient electrocatalysis. Adv. Mater., 2017, 29: 1605254. |
| [18] |
HE FEI, LI YA, LUO JIN, et al. Development of SiO2/C and SiC/C composites featuring aerogel structures. J. Inorg. Mater., 2017, 32(5): 449.
DOI URL |
| [19] |
LIU W, HERRMAANN A K, BIGALL N C, et al. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res., 2015, 48: 154.
DOI URL |
| [20] |
RAJIB S, AHMED A F, INDIKA U A. Oxidative self-assembly of Au/Ag/Pt alloy nanoparticles into high-surface area, mesoporous, and conductive aerogels for methanol electro-oxidation. Chem. Mater., 2022, 34(13): 5874.
DOI URL |
| [21] |
LIU W, HAUBOLD D, RUTKOWSKI B, et al. Self-supporting hierarchical porous PtAg alloy nanotubular aerogels as highly active and durable electrocatalysts. Chem. Mater., 2016, 28: 6477.
DOI URL |
| [22] |
DOUK A S, SARAVANI H, NOROOZIFAR M. Three-dimensional assembly of building blocks for the fabrication of Pd aerogel as a high performance electrocatalyst toward ethanol oxidation. Electrochim. Acta, 2018, 275: 182.
DOI URL |
| [23] |
LYU ZI YE, TANG YI PING, CAO HUA ZHEN, et al. Effect of V doping on electrocatalytic performance of Ni-Co-S on bacterial cellulose-derived carbon aerogel. J. Inorg. Mater., 2020, 35(10): 1142.
DOI |
| [24] | CAI B, SAYEVICH V, GAPONIK N, et al. Emerging hierarchical aerogels: self-assembly of metal and semiconductor nanocrystals. Adv. Mater., 2018, 30(33): 1707518. |
| [25] |
BURPO F J, NAGELLI E A, MORRIS L A, et al. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res., 2017, 32(22): 4153.
DOI URL |
| [26] | MIAO B Q, LIU Y C, DING Y, et al. Rhodium nanodendrites catalyzed alkaline methanol oxidation reaction in direct methanol fuel cells. SM&T, 2022, ( 31):e00379. |
| [27] |
MIAO J, ZHAO X J, HU H Y, et al. Porous palladium phosphide nanotubes for formic acid electrooxidation. Carbon Energy, 2022, 4: 283.
DOI URL |
| [28] |
QIAO Z, WANG S, LI X, et al. 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: a balance between graphitization and hierarchical porosity. Energy Environ. Sci., 2019, 12: 2830.
DOI URL |
| [29] |
LIU Q T, LI Y C, ZHENG L R, et al. Sequential synthesis and active-site coordination principle of precious metal single-atom catalysts for oxygen reduction reaction and PEM fuel cells. Adv. Energy Mater., 2020, 10(20): 2000689.
DOI URL |
| [30] |
ALMANA N, PHIVILAY S P, LAVEILLE P, et al. Design of a core-shell Pt-SiO2 catalyst in a reverse microemulsion system: distinctive kinetics on CO oxidation at low temperature. J. Catal., 2016, 340: 368.
DOI URL |
| [31] |
SIDDHARTH K, HONG Y M, QIN X P, et al. Surface engineering in improving activity of Pt nanocubes for ammonia electrooxidation reaction. Appl. Catal. B, 2020, 269: 118821.
DOI URL |
| [32] |
ESTEJAB A, G. BOTTE G. DFT calculations of ammonia oxidation reactions on bimetallic clusters of platinum and iridium. Comput. Theor. Chem., 2016, 1091: 31.
DOI URL |
| [33] |
CHAN Y T, SIDDHARTH K, SHAO M H. Investigation of cubic Pt alloys for ammonia oxidation reaction. Nano Res., 2020, 13(7): 1920.
DOI |
| [34] |
LIU Z Z, LI Y, ZHANG X S, et al. Surface structure engineering of PtPd nanoparticles for boosting ammonia oxidation electrocatalysis. ACS Appl. Mater. Interfaces, 2022, 14: 28816.
DOI URL |
| [1] | 周厚霖, 宋志庆, 田国, 高兴森. 生长条件对BiFeO3纳米岛内自组装铁电拓扑畴形成的影响[J]. 无机材料学报, 2025, 40(6): 667-674. |
| [2] | 袁利萍, 吴袁泊, 俞佳静, 张世琰, 孙铱, 胡云楚, 范友华. 磷钼酸插层水滑石复合CNFs气凝胶的制备及其隔热保温性能[J]. 无机材料学报, 2025, 40(4): 415-424. |
| [3] | 罗艺, 夏书海, 牛波, 张亚运, 龙东辉. 柔性有机硅气凝胶的制备及其高温无机化转变研究[J]. 无机材料学报, 2022, 37(12): 1281-1288. |
| [4] | 彭飞, 姜勇刚, 冯坚, 蔡华飞, 冯军宗, 李良军. 耐高温氧化铝气凝胶隔热复合材料研究进展[J]. 无机材料学报, 2021, 36(7): 673-684. |
| [5] | 李华鑫, 陈俊勇, 肖洲, 乐弦, 余显波, 向军辉. 纳米材料形貌和性能调控的仿生自组装研究进展[J]. 无机材料学报, 2021, 36(7): 695-710. |
| [6] | 张晓山, 王兵, 吴楠, 韩成, 吴纯治, 王应德. 高温隔热用微纳陶瓷纤维研究进展[J]. 无机材料学报, 2021, 36(3): 245-256. |
| [7] | 张泽,王晓栋,沈军. 气凝胶骨架结构的有机-无机交联度对其力学、热学性能的影响[J]. 无机材料学报, 2020, 35(4): 454-460. |
| [8] | 罗燚,冯军宗,冯坚,姜勇刚,李良军. 新型碳材料质子交换膜燃料电池Pt催化剂载体的研究进展[J]. 无机材料学报, 2020, 35(4): 407-415. |
| [9] | 柳凤琦, 冯坚, 姜勇刚, 李良军. 氮化硼气凝胶的制备及其应用进展[J]. 无机材料学报, 2020, 35(11): 1193-1202. |
| [10] | 丁卓峰, 杨永强, 李在均. 组氨酸功能化碳点/石墨烯气凝胶的制备及超级电容器性能[J]. 无机材料学报, 2020, 35(10): 1130-1136. |
| [11] | 吕子夜, 唐谊平, 曹华珍, 郑国渠, 侯广亚. V掺杂对Ni-Co-S/细菌纤维素基碳气凝胶电催化性能的影响[J]. 无机材料学报, 2020, 35(10): 1142-1148. |
| [12] | 彭新村, 王智栋, 曾梦丝, 刘云, 邹继军, 朱志甫, 邓文娟. SiO2纳米球的粒径均一性研究及其在硅光学共振纳米柱阵列中的应用[J]. 无机材料学报, 2019, 34(7): 734-740. |
| [13] | 朱召贤, 王飞, 姚鸿俊, 董金鑫, 龙东辉. 遮光剂掺杂Al2O3-SiO2气凝胶/莫来石纤维毡复合材料的高温隔热性能研究[J]. 无机材料学报, 2018, 33(9): 969-975. |
| [14] | 王景平, 成方媛, 杜显锋, 徐友龙. 表面自组装法制备高比容Al2O3/TiO2复合膜[J]. 无机材料学报, 2018, 33(6): 617-622. |
| [15] | 王勇, 于云, 冯爱虎, 江峰, 胡学兵, 宋力昕. Nafion改性多级孔径石墨烯气凝胶制备与性能研究[J]. 无机材料学报, 2018, 33(4): 469-474. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||