无机材料学报 ›› 2023, Vol. 38 ›› Issue (6): 647-655.DOI: 10.15541/jim20220625 CSTR: 32189.14.10.15541/jim20220625
所属专题: 【能源环境】氢能材料(202506)
收稿日期:2022-10-24
修回日期:2022-12-25
出版日期:2022-12-28
网络出版日期:2022-12-28
通讯作者:
曹宝月, 副教授. E-mail: 231052@slxy.edu.cn作者简介:孙强强(1985-), 男, 博士, 副教授. E-mail: sqq3c118@slxy.edu.cn
基金资助:
SUN Qiangqiang( ), CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue(
), CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue( )
)
Received:2022-10-24
Revised:2022-12-25
Published:2022-12-28
Online:2022-12-28
Contact:
CAO Baoyue, associate professor. E-mail: 231052@slxy.edu.cnAbout author:SUN Qiangqiang (1985-), male, PhD, associate professor. E-mail: sqq3c118@slxy.edu.cn
Supported by:摘要:
镍基电极材料是碱性电解水中最具工业应用前景的过渡金属催化剂, 而其缓慢的析氢反应动力学及低活失活问题仍亟待解决。本研究以泡沫镍(NF)为基底, 采用一步循环伏安法制备了主晶相为独立分相的多晶态金属镍铜合金、夹杂有少量非晶态V2O5相、具有三维多孔团簇结构的金属镍铜负载钒氧化物电催化剂(VOx-NiCu/NF)。纳米颗粒、团簇交织形成的微米孔及泡沫镍的一级微孔共同构成了VOx-NiCu/NF的三级多孔微纳结构, 使其电催化活性面积增加了28倍, 并在析氢反应中表现出优异的催化性能。在碱性介质中, 获得-10 mA·cm-2的析氢电流密度, VOx-NiCu/NF需要的过电势(η10)仅为35 mV, 表现出类铂的催化活性, 具有优异的长效稳定性及强劲的耐用性。电极表面形成的多孔团簇结构, 显著增加了催化活性位点并为物质传递提供大量通道。镍铜合金及非晶态V2O5相, 在一定程度协同改善了材料的固有析氢活性。理想的组成及独特的结构特性提高了VOx-NiCu/NF的催化性能, 其中结构优势对其最优效能起主导作用。动力学分析发现, VOx-NiCu/NF在析氢过程遵循Volmer-Heyrovsky机理, 即表面活性氢原子的电化学脱附为电荷转移过程的决速步骤, 为后续深入研究催化机制奠定了基础。
中图分类号:
孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655.
SUN Qiangqiang, CHEN Zixuan, YANG Ziyue, WANG Yimeng, CAO Baoyue. Amorphous Vanadium Oxide Loaded by Metallic Nickel-copper towards High-efficiency Electrocatalyzing Hydrogen Production[J]. Journal of Inorganic Materials, 2023, 38(6): 647-655.
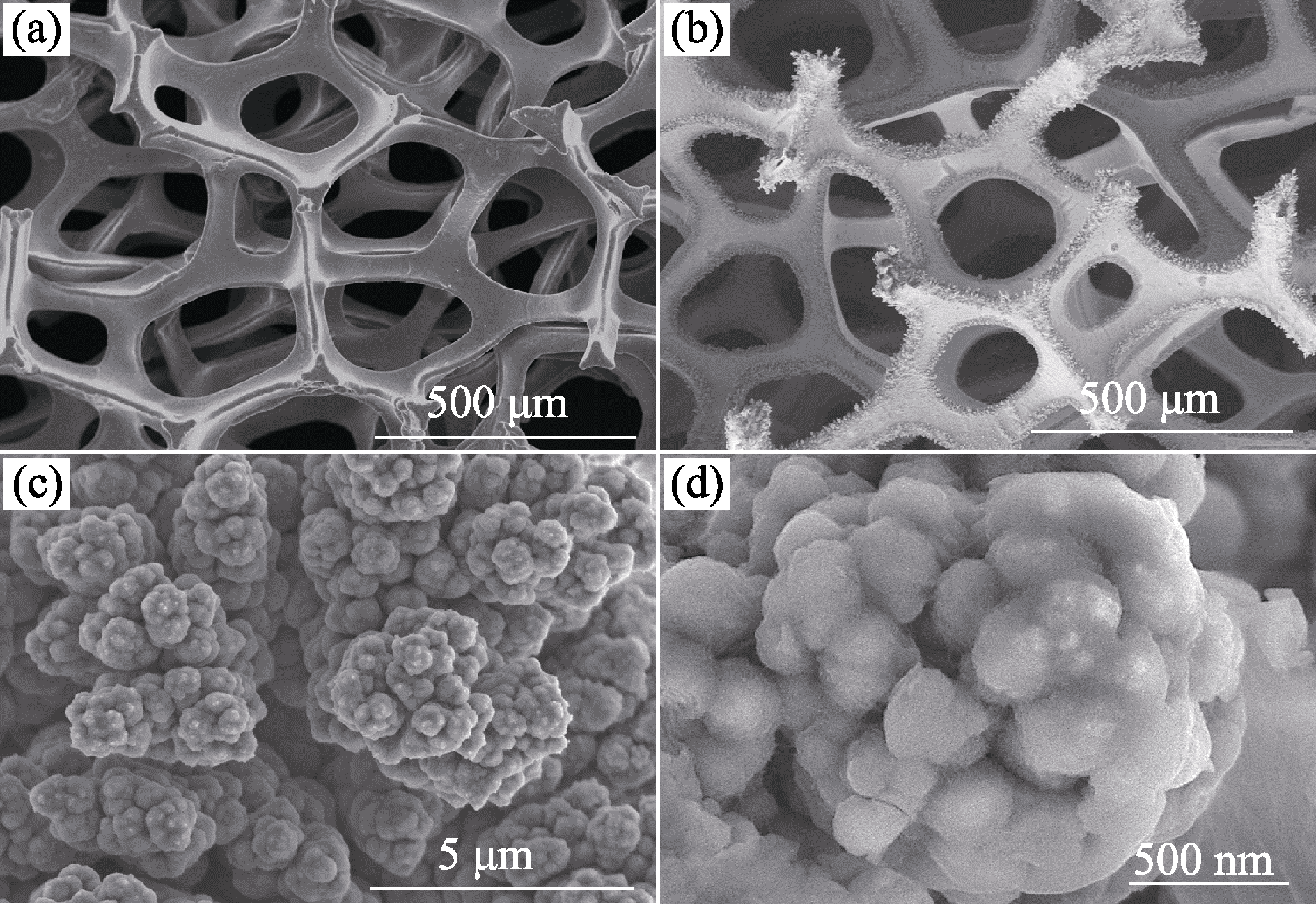
图1 (a)空白泡沫镍及(b~d)VOx-NiCu/NF在不同放大倍数下的SEM照片
Fig. 1 Different magnification SEM images of bare NF (a) and optimal VOx-NiCu/NF magnified by (b-d) 60000 times
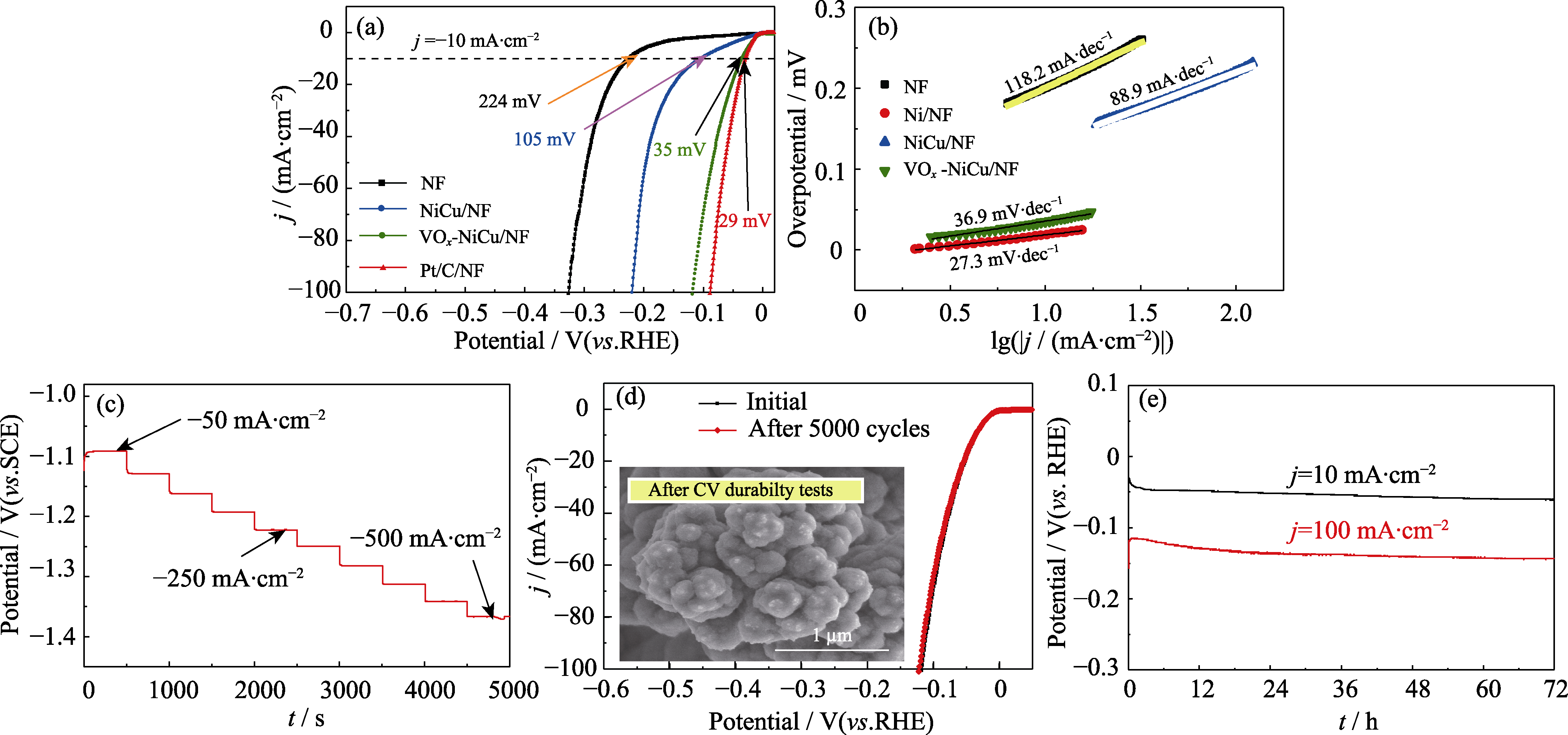
图5 VOx-NiCu/NF的(a)析氢极化曲线,(b)Tafel曲线及以(c)多电流阶跃法,(d)循环伏安法,(e)计时电位法测试的72 h催化稳定性结果
Fig. 5 (a) LSV curves and (b) Tafel plots of electrodes towards HER, (c, d) durability and stability tests of VOx-NiCu/NF towards the HER by (c) multicurrent-step, (d) CV curves before and after 5000 cycles with insert showing SEM image after 5000 cycles, and (e) chronopotentiometry of VOx-NiCu/NF for 72 h Colorful figures are available on website
| Material | Tafel slope/ (mV·dec-1) | η10/mV | Ref. |
|---|---|---|---|
| VOx-NiCu/NF | 36.9 | 35 | This work |
| Co@N-CNT | 94.0 | 44 | [ |
| FeCoNi-HNTAs | 37.5 | 58 | [ |
| NiFeV/NF | 62.0 | 125 | [ |
| Ni-Ce-Pr-Ho/NF | 121.6 | 78 | [ |
| N/C/MoP | 51.3 | 169 | [ |
| CoxPy/NixPy-NPC | 84.0 | 126 | [ |
| Ni/Mo2C/NC | 63.0 | 180 | [ |
| Ni-B/graphene | 148 | 187 | [ |
| CoS2/MoS2/NC | 80.0 | 215 | [ |
表1 VOx-NiCu/NF与近期文献报道的析氢催化剂(1 mol·L-1 KOH溶液)性能
Table 1 HER activities of VOx-NiCu/NF with recently reported electrocatalysts in 1 mol·L-1 KOH alkaline solution
| Material | Tafel slope/ (mV·dec-1) | η10/mV | Ref. |
|---|---|---|---|
| VOx-NiCu/NF | 36.9 | 35 | This work |
| Co@N-CNT | 94.0 | 44 | [ |
| FeCoNi-HNTAs | 37.5 | 58 | [ |
| NiFeV/NF | 62.0 | 125 | [ |
| Ni-Ce-Pr-Ho/NF | 121.6 | 78 | [ |
| N/C/MoP | 51.3 | 169 | [ |
| CoxPy/NixPy-NPC | 84.0 | 126 | [ |
| Ni/Mo2C/NC | 63.0 | 180 | [ |
| Ni-B/graphene | 148 | 187 | [ |
| CoS2/MoS2/NC | 80.0 | 215 | [ |

图6 VOx-NiCu/NF的(a)循环伏安曲线,(b)双电容曲线,(c)交流阻抗谱图(插图为等效电路)及(d)归一化的LSV曲线
Fig. 6 (a) CV curves, (b) double-layer capacitance curves, (c) Nyquist plots with insert showing equivalent circuit and LSV curves by ECSA normalization of VOx-NiCu/NF towards HER Colorful figures are available on website

图S7 (a)经过72 h长效稳定测试VOx-NiCu/NF的析氢活性及微观结构, (b)96 h的长时稳定性测试
Fig. S7 (a) Chemical activity and microstructure of VOx-NiCu/NF after a 72-h unintermittent running for HER, and (b) a 96-h unintermittent running for HER.
| [1] |
WANG J, FENG X C, HEDMAN D, et al. Enhancing the hydrogen evolution reaction on MoS2 flakes by cold plasma treatment. Electrochemistry Communications, 2022, 137: 107250.
DOI URL |
| [2] |
HA T D C, DO H H, LEE H, et al. A GO/CoMo3S13 chalcogel heterostructure with rich catalytic Mo-S-Co bridge sites for the hydrogen evolution reaction. Nanoscale, 2022, 14(26):9331.
DOI URL |
| [3] |
CHANDRASEKARAN S, YAO L, DENG L B, et al. Recent advances in metal sulfides: from controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chemical Society Reviews, 2019, 48(15):4178.
DOI PMID |
| [4] |
ZHANG J Q, ZHAO Y F, XIN G, et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nature Catalysis, 2018, 1(12):985.
DOI |
| [5] |
WANG D L, Li H P, DU N, et al. Single platinum atoms immobilized on monolayer tungsten trioxide nanosheets as an efficient electrocatalyst for hydrogen evolution reaction. Advanced Functional Materials, 2021, 31(23):2009770.
DOI URL |
| [6] |
GONG M, WANG D Y, CHEN C C, et al. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Research, 2016, 9(1):28.
DOI URL |
| [7] |
PLETCHER D, LI X H. Prospects for alkaline zero gap water electrolysers for hydrogen production. International Journal of Hydrogen Energy, 2011, 36(23):15089.
DOI URL |
| [8] |
SYMES D, TAYLOR-COX C, HOLYFIELD L, et al. Feasibility of an oxygen-getter with nickel electrodes in alkaline electrolysers. Materials for Renewable and Sustainable Energy, 2014, 3(2):27.
DOI URL |
| [9] | LI Y P, WANG W T, CHENG M Y, et al. Environmentally benign general synthesis of nonconsecutive carbon-coated RuP2 porous microsheets as efficient bifunctional electrocatalysts under neutral conditions for energy-saving H2 production in hybrid water electrolysis. Catalysis Science & Technology, 2022, 12(13):4339. |
| [10] |
SUN Y T, DING S, XU S S, et al. Metallic two-dimensional metal-organic framework arrays for ultrafast water splitting. Journal of Power Sources, 2021, 494: 229733.
DOI URL |
| [11] | KONKENA B, MASA J, XIA W, et al. MoSSe@reduced graphene oxide nanocomposite heterostructures as efficient and stable electrocatalysts for the hydrogen evolution reaction. Nano Energy, 2016, l29: 46. |
| [12] |
FABER M S, DZIEDZIC R, LUKOWSKI M A, et al. High- performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. Journal of the American Chemical Society, 2014, 136(28):10053.
DOI URL |
| [13] |
CHEN S, WANG C L, LIU S, et al. Boosting hydrazine oxidation reaction on CoP/Co Mott-Schottky electrocatalyst through engineering active sites. Journal of Physical Chemistry Letters, 2021, 12(20):4849.
DOI PMID |
| [14] |
GONG M, ZHOU W, KENNEY M J, et al. Blending Cr2O3 into NiO-Ni electrocatalyst for sustained water splitting. Angewandte Chemie International Edition, 2015, 54(41):11989.
DOI URL |
| [15] |
LI Y B, TAN X, CHEN S, et al. Processable surface modification of nickel-heteroatom (N, S) bridge sites for promoted alkaline hydrogen evolution. Angewandte Chemie International Edition, 2019, 58(2):461.
DOI URL |
| [16] |
GONG M, ZHOU W, TSAI M C, et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nature Communications, 2014, 5: 4695.
DOI PMID |
| [17] |
SUN Q Q, DONG Y J, WANG Z L, et al. Synergistic nanotubular copper-doped nickel catalysts for hydrogen evolution reactions. Small, 2018, 14(14):1704137.
DOI URL |
| [18] |
FLEISCHMANN S, JACKEL N, ZEIGER M, et al. Enhanced electrochemical energy storage by nanoscopic decoration of endohedral and exohedral carbon with vanadium oxide via atomic layer deposition. Chemistry of Materials, 2016, 28(8):2802.
DOI URL |
| [19] |
CONCEPCION P, KNOZINGER H, LOPEZ NIETO J M, et al. Characterization of supported vanadium oxide catalysts. nature of the vanadium species in reduced catalysts. Journal of Physical Chemistry B, 2002, 106(10):2574.
DOI URL |
| [20] |
CHEN J L, CHANG C C, HO Y K, et al. Behind the color switching in gas ochromic VO2. Physical Chemistry Chemical Physics, 2015, 17(5):3482.
DOI URL |
| [21] |
LIU J N, CUI J S, SUN J H, et al. Hierarchical nickel-vanadium nanohybrid with strong electron transfer for accelerated hydrogen evolution reaction. Applied Surface Science, 2020, 528: 146982.
DOI URL |
| [22] |
PENG X Y, HUANG C, ZHANG B, et al. Vanadium carbide nanodots anchored on N doped carbon nanosheets fabricated by spatially confined synthesis as a high-efficient electrocatalyst for hydrogen evolution reaction. Journal of Power Sources, 2021, 490: 229551.
DOI URL |
| [23] |
WEN L L, YU J, XING C C, et al. Flexible vanadium-doped Ni2P nanosheet arrays grown on carbon cloth for an efficient hydrogen evolution reaction. Nanoscale, 2019, 11(10):4198.
DOI URL |
| [24] |
HE D Y, CAO L Y, HUANG J F, et al. In-situ optimizing the valence configuration of vanadium sites in NiV-LDH nanosheet arrays for enhanced hydrogen evolution reaction. Journal of Energy Chemistry, 2020, 47: 263.
DOI URL |
| [25] |
DEY K K, JHA S, KUMAR A, et al. Layered vanadium oxide nanofibers as impressive electrocatalyst for hydrogen evolution reaction in acidic medium. Electrochimica Acta, 2019, 312: 89.
DOI URL |
| [26] |
JIANG L L, XU S S, XIA B K, et al. Defect engineering of graphene hybrid catalysts for oxygen reduction reactions. Journal of Inorganic Materials, 2022, 37(2):215.
DOI |
| [27] |
PENG Y H, GENG Z G, ZHAO S T, et al. Pt single atoms embedded in the surface of Ni nanocrystals as highly active catalysts for selective hydrogenation of nitro compounds. Nano Letters, 2018, 18(6):3785.
DOI PMID |
| [28] |
LIANG J, FAN Z Y, CHEN S, et al. Hierarchical NiCo2O4 nanosheets@halloysite nanotubes with ultrahigh capacitance and long cycle stability as electrochemical pseudocapacitor materials. Chemistry of Materials, 2014, 26(15):4354.
DOI URL |
| [29] |
HAO J H, YANG W S, HUANG Z P, et al. Superhydrophilic and superaerophobic copper phosphide microsheets for efficient electrocatalytic hydrogen and oxygen evolution. Advanced Materials Interfaces, 2016, 3(16):1600236.
DOI URL |
| [30] |
SURYANTO B H R, WANG Y, HOCKING R K, et al. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nature Communications, 2019, 10: 5599.
DOI PMID |
| [31] | LUO P, ZHANG H J, LIU L, et al. Targeted synthesis of unique nickel sulfide (NiS, NiS2) microarchitectures and the applications for the enhanced water splitting system. ACS Applied Materials & Interfaces, 2017, 9(3):2500. |
| [32] |
XU H, FENG J X, TONG Y X, et al. Cu2O-Cu hybrid foams as high-performance electrocatalysts for oxygen evolution reaction in alkaline media. ACS Catalysis, 2017, 7(2):986.
DOI URL |
| [33] |
FAN K, JI Y F, ZOU H Y, et al. Hollow iron-vanadium composite spheres: a highly efficient iron-based water oxidation electrocatalyst without the need for nickel or cobalt. Angewandte Chemie International Edition, 2017, 56(12):3289.
DOI URL |
| [34] |
MANILEVICH F D, KOZIN L F, MASHKOVA N V, et al. Regularities of hydrogen evolution on steel cathodes covered with galvanic nickel coatings containing vanadium-pentoxide inclusions. Protection of Metals and Physical Chemistry of Surfaces, 2014, 50: 178.
DOI URL |
| [35] |
KUMAR M, JEONG D I, SARWAR N, et al. Cobalt supported nitrogen-doped carbon nanotube as efficient catalyst for hydrogen evolution reaction and reduction of 4-nitrophenol. Applied Surface Science, 2022, 572: 151450.
DOI URL |
| [36] |
LI H Y, CHEN S M, ZHANG Y, et al. Systematic design of superaerophobic nanotube array electrode comprised of transition- metal sulfides for overall water splitting. Nature Communications, 2018, 9: 2452.
DOI |
| [37] |
DINH K N, ZHENG P L, DAI Z F, et al. Ultrathin porous NiFeV ternary layer hydroxide nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting. Small, 2018, 14(8):1703257.
DOI URL |
| [38] |
LIU W, TAN W Y, HE H W, et al. One-step electrodeposition of Ni-Ce-Pr-Ho/NF as an efficient electrocatalyst for hydrogen evolution reaction in alkaline medium. Energy, 2022, 250: 123831.
DOI URL |
| [39] |
WANG C, LI W, WANG X D, et al. Open N-doped carbon coated porous molybdenum phosphide nanorods for synergistic catalytic hydrogen evolution reaction. Nano Research, 2022, 15(3): 1824.
DOI |
| [40] |
ZHU L, HUANG Y H, WANG B L, et al. N-doped porous carbon-supported CoxPy/NixPy catalyst with enhanced catalytic activity for hydrogen evolution reaction in alkaline solution and neutral seawater. Journal of Solid State Electrochemistry, 2022, 26(1):233.
DOI |
| [41] |
YUAN Q, CHEN W H, HU R F, et al. Metal-polydopamine derived N-doped carbon nanorod wrapping Ni and Mo2C nanoparticles for efficient hydrogen evolution reaction. Materials Letters, 2022, 307: 130989.
DOI URL |
| [42] | WANG S, ZHAO R, XUE W D. Rapid synthesis of nickel boride/graphene by microwave thermal shock and its application in hydrogen evolution reaction. Journal of Physics: Conference Series, 2022, 2160: 012007. |
| [43] |
JI K, MATRAS-POSTOLEK K, SHI R X, et al. MoS2/CoS2 heterostructures embedded in N-doped carbon nanosheets towards enhanced hydrogen evolution reaction. Journal of Alloys and Compounds, 2022, 891: 161962.
DOI URL |
| [44] |
LU X Y, ZHAO C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nature Communications, 2015, 6: 6616.
DOI PMID |
| [45] |
BOLAR S, SHIT S, KUMAR J S, et al. Optimization of active surface area of flower like MoS2using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium. Applied Catalysis B: Environmental, 2019, 254: 432.
DOI URL |
| [46] |
WANG L Y, LI Y B, SUN Q Q, et al. Ultralow Fe(III) ion doping triggered generation of Ni3S2 ultrathin nanosheet for enhanced oxygen evolution reaction. ChemCatChem, 2019, 11(7): 2011.
DOI URL |
| [47] |
LI Y B, TAN X, HOCKING R K, et al. Implanting Ni-O-VOx sites into Cu-doped Ni for low-overpotential alkaline hydrogen evolution. Nature Communications, 2020, 11: 2720.
DOI |
| [48] |
SUN T T, ZHANG C W, CHEN J F, et al. Three-dimensionally ordered macro-/mesoporous Ni as a highly efficient electrocatalyst for the hydrogen evolution reaction. Journal of Materials Chemistry A, 2015, 3(21):11367.
DOI URL |
| [1] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [2] | 王旭, 李翔, 寇华敏, 方伟, 吴庆辉, 苏良碧. 不同浓度Y3+离子掺杂对CaF2晶体性能的影响[J]. 无机材料学报, 2024, 39(9): 1029-1034. |
| [3] | 景欣欣, 陈必清, 翟佳鑫, 袁美玲. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476. |
| [4] | 张文宇, 郭瑞华, 岳全鑫, 黄雅荣, 张国芳, 关丽丽. 高熵磷化物双功能催化剂的制备及高效电解水性能[J]. 无机材料学报, 2024, 39(11): 1265-1274. |
| [5] | 朱云娜, 陈必清, 程天舒, 杜婵, 张士民, 赵静. 非晶Nd-Ni-B/NF稀土复合电极材料的制备及其析氢性能[J]. 无机材料学报, 2021, 36(6): 637-644. |
| [6] | 李兆, 孙强强, 陈索倩, 周春生, 曹静, 王永锋, 王亚楠. 水热合成镍铜复合磷化物及其电催化析氢与肼氧化性能[J]. 无机材料学报, 2020, 35(10): 1149-1156. |
| [7] | 苏琨, 张亚茹, 陆飞, 张君, 王熙. 铂修饰二氧化钛纳米片的制备及其光电催化析氢反应研究[J]. 无机材料学报, 2019, 34(11): 1200-1204. |
| [8] | 王辉, 俞有幸. KOH碱化处理对Fe3N纳米颗粒电催化制氢性能影响[J]. 无机材料学报, 2018, 33(6): 653-658. |
| [9] | 刘双宇, 徐 丽, 陈 新, 韩 钰, 刘海镇, 盛 鹏, 王 博, 赵广耀. 石墨烯负载团簇结构CoFe2O4及其电化学储锂性[J]. 无机材料学报, 2017, 32(9): 904-908. |
| [10] | 赵晓婵, 房 艳, 房春晖, 周永全, 戈海文, 朱发岩. 石墨烯包覆分子筛复合电极材料的制备及其性能研究[J]. 无机材料学报, 2017, 32(4): 386-392. |
| [11] | 王艳芝, 赵敏寿. Ti-V基固溶体/AB5型镧镁基合金复合储氢材料的结构与电化学性能[J]. 无机材料学报, 2012, 27(5): 463-468. |
| [12] | 崔朝军,吴广明,张明霞,孙娟萍,杨辉宇,沈 军. 锂钒氧化物纳米管的合成与表征[J]. 无机材料学报, 2009, 24(4): 787-792. |
| [13] | 周 艺,黄可龙1,朱志平,杨 波,夏畅斌,肖汉宁. 酸催化溶胶-凝胶法Eu2+、Gd3+共掺杂TiO2的制备及光催化活性[J]. 无机材料学报, 2008, 23(5): 1085-1088. |
| [14] | 陈昌国,刘渝萍,李兰. 锂离子电池中钒氧化物电极材料的研究现状[J]. 无机材料学报, 2004, 19(6): 1225-1230. |
| [15] | 刘建睿,王猛,尹大川,黄卫东. 锂离子电池正极材料LiV3O8的低温合成研究[J]. 无机材料学报, 2002, 17(3): 617-620. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||