无机材料学报 ›› 2017, Vol. 32 ›› Issue (2): 127-134.DOI: 10.15541/jim20160182 CSTR: 32189.14.10.15541/jim20160182
郑 譞1,2, 龚春丽1, 刘 海1, 汪广进1,2, 程 凡1, 郑根稳1, 文 胜1, 熊传溪2
收稿日期:2012-05-30
修回日期:2012-09-12
出版日期:2017-02-20
网络出版日期:2017-01-13
作者简介:郑 譞(1988–), 男, 博士研究生, 实验师. E-mail:63474559@qq.com
基金资助:ZHENG Xuan1,2, GONG Chun-Li1, LIU Hai1, WANG Guang-Jin1,2, CHENG Fan1, ZHENG Gen-Wen1, WEN Sheng1, XIONG Chuan-Xi2
Received:2012-05-30
Revised:2012-09-12
Published:2017-02-20
Online:2017-01-13
About author:ZHENG Xuan. E-mail:63474559@qq.com
Supported by:摘要:
以聚多巴胺包覆碳纳米管为载体, 借助聚多巴胺超强的粘附性, 利用简单的溶液浸渍法制备了磷钼酸负载碳纳米管(PMA@CNTs)复合物。通过傅里叶变换红外光谱(FTIR)、X射线衍射(XRD)、X射线光电子能谱(XPS)、扫描电镜(SEM)、透射电镜(TEM)和电化学测试等对复合物的组成、结构、形态和超级电容性能进行了表征。结果表明: 聚多巴胺可将磷钼酸均匀且牢固地负载在碳纳米管上。在0.5 mol/L的H2SO4电解液中, 复合物的最大比容量为511.7 F/g, 最大能量密度可达66.8 Wh/kg, 相应的功率密度为1000 W/kg。经过1000次循环, 比容量无任何衰减。以上研究结果说明PMA@CNTs复合物在电化学储能领域拥有极好的发展前景。
中图分类号:
郑 譞,龚春丽, 刘 海, 汪广进, 程 凡, 郑根稳, 文 胜, 熊传溪. 磷钼酸负载碳纳米管复合物的制备及其超级电容性能[J]. 无机材料学报, 2017, 32(2): 127-134.
ZHENG Xuan, GONG Chun-Li, LIU Hai, WANG Guang-Jin, CHENG Fan, ZHENG Gen-Wen, WEN Sheng, XIONG Chuan-Xi. Preparation of Phosphomolybdic Acid Coated Carbon Nanotubes and Its Supercapacitive Properties[J]. Journal of Inorganic Materials, 2017, 32(2): 127-134.

图1 多巴胺氧化自聚合机理[25-27]及PMA@CNTs复合物的制备过程示意图
Fig. 1 Illustration of oxidative self-polymerization mechanism of dopamine[25-27] and preparation procedure of PMA@CNTs hybrids
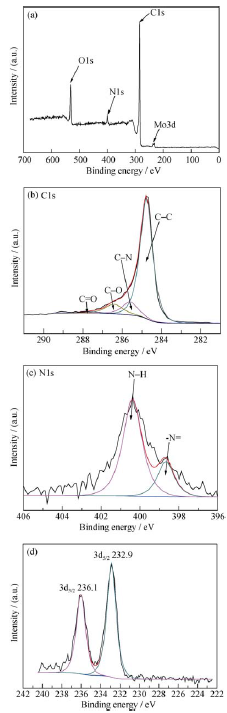
图4 PMA50@CNTs的(a)XPS宽谱扫描图, (b) C1s分峰图, (c)N1s分峰图, (d)Mo3d分峰图
Fig. 4 XPS spectra of PMA50@CNTs: (a) wide scan; peak deconvolution of (b) C1s, (c) N1s, and (d) Mo3d
| Element | at/% | wt/% |
|---|---|---|
| C | 82.99 | 79.93 |
| N | 3.73 | 5.96 |
| O | 7.05 | 11.37 |
| P | 1.86 | 0.86 |
| MO | 2.19 | 1.87 |
| Total | 100.00 | |
表1 PMA50@CNTs的EDX元素组成
Table1 EDX compositions of PMA50@CNTs
| Element | at/% | wt/% |
|---|---|---|
| C | 82.99 | 79.93 |
| N | 3.73 | 5.96 |
| O | 7.05 | 11.37 |
| P | 1.86 | 0.86 |
| MO | 2.19 | 1.87 |
| Total | 100.00 | |
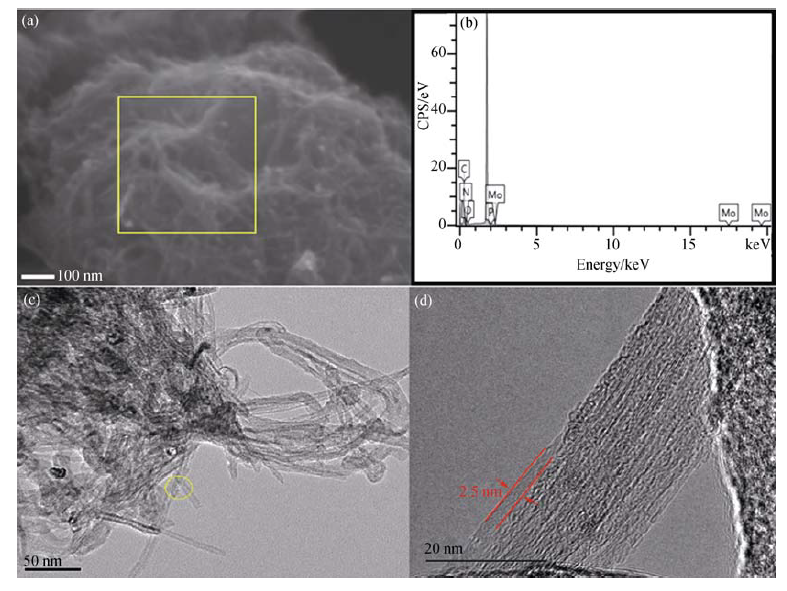
图5 (a)PMA50@CNTs的SEM照片, (b)PMA50@CNTs的EDX图谱, (c)、(d)PMA50@CNTs的TEM照片
Fig. 5 SEM images of PMA50@CNTs (a), EDXspectra of PMA50@CNTs (b), and TEM images of PMA50@CNTs (c), (d)
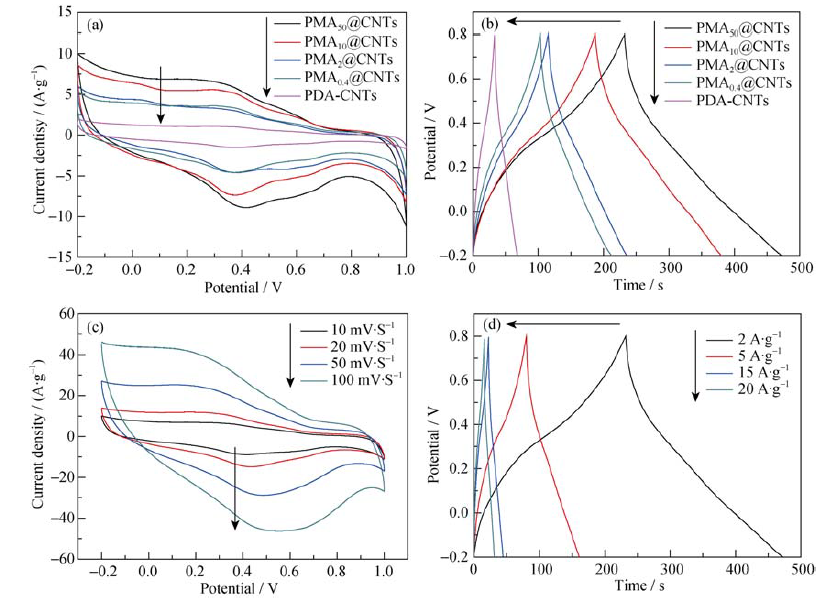
图7 PDA-CNTs、PMA0.4@CNTs、PMA2@CNTs、PMA10@CNTs和PMA50@CNTs在10 mV/s扫速下的循环伏安曲线(a)和在2 A/g电流密度下的恒流充放电曲线(b)以及PMA50@CNTs在不同扫速下的循环伏安曲线(c), 不同电流密度下的恒流充放电曲线(d)
Fig. 7 (a) Cyclic voltammograms at scan rate of 10 mV/s) and (b) galvanostatic charge-dischargecyclic curves at current density of 2 A/g for PDA-CNTs, PMA0.4@CNTs, PMA2@CNTs, PMA10@CNTs andPMA50@CNTs coin cell, and (c) cyclic voltammograms at different scan rates and (d) charge/discharge curves at different current densities for PMA50@CNTs Supercapacitors in 0.5 mol/L H2SO4 electrolytes
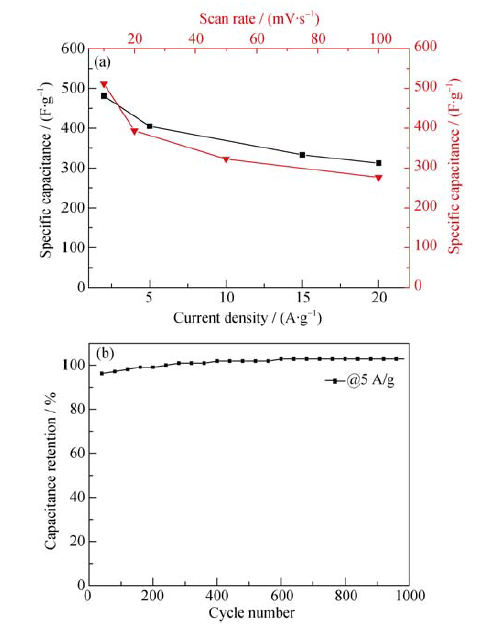
图8 (a)PMA50@CNTs在不同电流密度和扫速下的比容量曲线和(b)PMA50@CNTs在5 A/g电流密度下的循环稳定性测试结果
Fig. 8 (a) Specific capacitance of the PMA50@CNT selectrode as a function of scan rate and current density and (b) cycling stability of the PMA50@CNTs electrode at a current density of 5 A/g
| [1] | NAKAMURA O, KODAMA T, OGINO I,et al.High-conducting solid proton conductors: dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals.Chemistry Letters, 1979, 1(1): 17-18. |
| [2] | BURAKOV V S, BUTSEN A V, BRUSER V,et al. Synthesis of tungsten carbide nanopowder via submerged discharge method.Journal of Nanoparticle Research, 2008, 10(5): 881-886. |
| [3] | CLAIRE C C, SEBASTIENS, LAURENT R.Photocatalysis with polyoxometalates associated to porphyrins under visible light: an application of charge transfer in electrostatic complexes.Journal of Physical Chemistry A, 2010, 114(22): 6394-6400. |
| [4] | DECKER B, HARTMANNTHOMPSON C, CARVER P I,et al. Multilayer sulfonated polyhedral oligosilsesquioxane (s-poss)- sulfonated polyphenylsulfone (S-PPSU) composite proton exchange membranes.Chemistry of Materials, 2009, 22(3): 942-948. |
| [5] | POPE M T, MULLER A.Polyoxometalate chemistry: an old field with new dimensions in several disciplines.AngewandteChemie International Edition, 1991, 30(1): 34-48. |
| [6] | SU Y, LIU C, SHAN Y,et al.Promotion effect of molybdophorsphoric acid on oxygenreduction for cathode of DMFC.Chem. J. Chin. Univ., 2005, 26(6): 1114-1117. |
| [7] | GOTZ M, WENDT H.Binary and ternary anode catalyst formulations including the elements W, Sn and Mo for PEMFCs operated on methanol or reformate gas.ElectrochimicaActa, 1998, 43(24): 3637-3644. |
| [8] | HUANG X L, LIN Z, LIAN X Y,et al. Preparation and electrocatalytic properties of Pd/PMo12-GN composite towards formic acid oxidation.Journal of Inorganic Materials, 2014, 29(7): 722-728. |
| [9] | AND E C, GOMEZGARCIA C J.Polyoxometalate-based molecular materials.Chemical Reviews, 1998, 98(1): 273-296. |
| [10] | WON B K, VOITL T, RODRIGUEZ R G J,et al.Powering fuel cells with CO via aqueous polyoxometalates and gold catalysts.Science, 2004, 305(5688): 1280-1283. |
| [11] | LIU S, KURTH D G, VOLKMER D.Polyoxometalates as pH-sensitive probes in self-assembled multilayers.Chemical Communications, 2002, 9(9): 976-977. |
| [12] | LIU S L, DIRK V, KURTH D G.Smart polyoxometalate-based nitrogen monoxide sensors.Analytical Chemistry, 2004, 76(15): 4579-4582. |
| [13] | DOUVAS A M, ELENI M, NIKOS G,et al.Polyoxometalate-based layered structures for charge transport control in molecular devices.ACS Nano, 2008, 2(2): 733-742. |
| [14] | ELEFTHERIOS K, DOUVAS A M, DIMITRIS V,et al.Molecular storage elements for proton memory devices.Advanced Materials, 2008, 20(23): 4568-4574. |
| [15] | CORONADO E, GIMENEZ S C, GOMEZ G C J.Recent advances in polyoxometalate-containing molecular conductors.Coordination Chemistry Reviews, 2005, 249(17): 1776-1796. |
| [16] | WANG X L, ZHANG H, WANG E B,et al.Phosphomolybdate-polypyrrole composite bulk-modified carbon paste electrode for a hydrogen peroxide amperometric sensor.Mater. Lett. , 2004, 58(10): 1661-1664. |
| [17] | LIU Z, WU Q Y, SONG X L,et al. Solid high-proton conductors based on heteropoly acids.Progress in Chemistry, 2009, 21(5): 982-989. |
| [18] | QI W, LIU W, LIU S,et al. Heteropoly acid/carbon nanotube hybrid materials as efficient solid-acid catalysts.Chemcatchem, 2014, 6(9): 2613-2620. |
| [19] | WEN S, GUAN W, KAN Y,et al. Theoretical insights into [PMo12O40]3- grafted on single-walled carbon nanotubes.Physical Chemistry Chemical Physics, 2013, 15(23): 9177-9185. |
| [20] | AVCI A K, TRIMM D L, KARAKAYA M.Microreactor catalytic combustion for chemicals processing.Catalysis Today, 2010, 155(1/2): 66-74. |
| [21] | QIN L C, ZHAO X, HIRAHARA K,et al. The smallest carbon nanotube.Nature, 2000, 408(6808): 95-120. |
| [22] | MOORE V C, STRANO M S, HAROZ E H,et al. Individually suspended single-walled carbon nanotubes in various surfactants.Nano Letters, 2003, 3(10): 1379-1382. |
| [23] | AND Y S, THOMAS E L.High-concentration dispersion of single-wall carbon nanotubes.Macromolecules, 2004, 37(13): 4815-4820. |
| [24] | OU Y Y, HUANG M H.High-density assembly of gold nanoparticles on multiwalled carbon nanotubes using 1-pyrenemethylamine as interlinker.Journal of Physical Chemistry B, 2006, 110(5): 2031-2036. |
| [25] | TOMA F M, SARTOREL A, IURLO M,et al.Efficient water oxidation at carbon nanotube-polyoxometalateelectrocatalytic interfaces.Nature Chemistry, 2010, 2(10): 826-831. |
| [26] | LEE H, RHO J, MESSERSMITH P B.Facile conjugation of biomolecules onto surfacesvia mussel adhesive protein inspired coatings.Advanced Materials, 2009, 21(4): 431-434. |
| [27] | DALSIN J L, HU B H, AND B P L,et al.Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces.Journal of the American Chemical Society, 2003, 125(125): 4253-4258. |
| [28] | LEE H, DELLATORE S M, MILLER W M.Mussel-inspired surface chemistry for multifunctional coatings.Science, 2007, 318(5849): 426-430 |
| [29] | XI Z Y, XU Y Y, ZHU L P,et al.A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine).Journal of Membrane Science, 2009, 327(1): 244-253. |
| [30] | FEI B, QIAN B, YANG Z,et al. Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine.Carbon, 2008, 46(13): 1795-1797. |
| [31] | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity.Pure & Applied Chemistry, 1982, 54(11): 2201-2218. |
| [32] | LIU C, YU Z, NEFF D,et al.Graphene-based supercapacitor with an ultrahigh energy density.Nano Letters, 2010, 10(12): 4863-4868. |
| [33] | ZHAO X, ZHU J, LING L,et al. Enhanced electroactivity of pdnanocrystals supported on H3PMo12O40/carbon for formic acidelectrooxidation.J. Power Sources, 2012, 210(4): 392-396. |
| [34] | BURZIO L A, WAITE J H.Cross-linking in adhesive quinoproteins: studies with model decapeptides.Biochemistry, 2000, 39(36): 11147-11153. |
| [35] | 齐明立. 含Keggin结构磷钼酸盐杂化材料的合成、晶体结构与性质研究. 哈尔滨: 哈尔滨师范大学硕士学位论文, 2013. |
| [36] | SJOSTROM H, STAFSTROM S, BOMAN M,et al. Superhard and elastic carbon nitride thin films having fullerenelike microstructure.Physical Review Letters, 1995, 75(7): 1336-1339. |
| [37] | TAN K L, WOON L L, WONG H K,et al.Surface modification of plasma-pretreated poly(tetrafluoroethylene) films by graft copolymerization.Macromolecules, 1993, 26(11): 2832-2836. |
| [38] | III J R F, KUO M C, TURNER J A,et al.The use of the heteropoly acids, H3PMo12O40 and H3PW12O40, for the enhanced electrochemical oxidation of methanol for direct methanol fuel cells.ElectrochimicaActa, 2008, 53(14): 4927-4933. |
| [39] | SUPPES G M, DEORE B A, FREUND M S.Porous conducting polymer/heteropolyoxometalate hybrid material for electrochemicalsupercapacitor applications. Langmuir, 2008, 24(3): 1064-1069. |
| [40] | WU Z S, SUN Y, TAN Y Z,et al. Three-dimensional graphene- based macro- and mesoporous frameworks for high-performance electrochemical capacitive energy storage.Journal of the American Chemical Society, 2012, 134(48): 19532-19535. |
| [41] | LIN T, CHEN I W, LIU F,et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage.Science, 2015, 350(6267): 1508-1513. |
| [1] | 陈义, 邱海鹏, 陈明伟, 徐昊, 崔恒. SiC/SiC复合材料基体硼改性方法及其力学性能研究[J]. 无机材料学报, 2025, 40(5): 504-510. |
| [2] | 袁利萍, 吴袁泊, 俞佳静, 张世琰, 孙铱, 胡云楚, 范友华. 磷钼酸插层水滑石复合CNFs气凝胶的制备及其隔热保温性能[J]. 无机材料学报, 2025, 40(4): 415-424. |
| [3] | 穆爽, 马沁, 张禹, 沈旭, 杨金山, 董绍明. Yb2Si2O7改性SiC/SiC复合材料的氧化行为研究[J]. 无机材料学报, 2025, 40(3): 323-328. |
| [4] | 杨舒琪, 杨存国, 牛慧祝, 石唯一, 舒珂维. GeP3/科琴黑复合材料作为钠离子电池高性能负极材料[J]. 无机材料学报, 2025, 40(3): 329-336. |
| [5] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [6] | 栾新刚, 何典蔚, 涂建勇, 成来飞. 2D平纹和3D针刺C/SiC复合材料的低速冲击破坏行为和失效机理[J]. 无机材料学报, 2025, 40(2): 205-214. |
| [7] | 王文婷, 徐敬军, 马科, 李美栓, 李兴超, 李同起. 原位反应/热压合成Ti2AlC-20TiB2复合材料在1000~1300 ℃空气中的高温氧化行为[J]. 无机材料学报, 2025, 40(1): 31-38. |
| [8] | 全文心, 余艺平, 方冰, 李伟, 王松. 管状C/SiC复合材料高温空气氧化行为与宏细观建模研究[J]. 无机材料学报, 2024, 39(8): 920-928. |
| [9] | 何思哲, 王俊舟, 张勇, 费嘉维, 吴爱民, 陈意峰, 李强, 周晟, 黄昊. 高频低损耗的Fe/亚微米FeNi软磁复合材料[J]. 无机材料学报, 2024, 39(8): 871-878. |
| [10] | 孙海洋, 季伟, 王为民, 傅正义. TiB-Ti周期序构复合材料设计、制备及性能研究[J]. 无机材料学报, 2024, 39(6): 662-670. |
| [11] | 吴晓晨, 郑瑞晓, 李露, 马浩林, 赵培航, 马朝利. SiCf/SiC陶瓷基复合材料高温环境损伤原位监测研究进展[J]. 无机材料学报, 2024, 39(6): 609-622. |
| [12] | 粟毅, 史扬帆, 贾成兰, 迟蓬涛, 高扬, 马青松, 陈思安. 浆料浸渍辅助PIP工艺制备C/HfC-SiC复合材料的微观结构及性能研究[J]. 无机材料学报, 2024, 39(6): 726-732. |
| [13] | 赵日达, 汤素芳. 多孔碳陶瓷化改进反应熔渗法制备陶瓷基复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 623-633. |
| [14] | 方光武, 谢浩元, 张华军, 高希光, 宋迎东. CMC-EBC损伤耦合机理及一体化设计研究进展[J]. 无机材料学报, 2024, 39(6): 647-661. |
| [15] | 张幸红, 王义铭, 程源, 董顺, 胡平. 超高温陶瓷复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 571-590. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||