无机材料学报 ›› 2015, Vol. 30 ›› Issue (12): 1243-1253.DOI: 10.15541/jim20150223 CSTR: 32189.14.10.15541/jim20150223
何 飞1,2, 郁万军1,2, 方旻翰1,2, 赫晓东1,2, 李明伟3
收稿日期:2015-05-11
修回日期:2015-06-10
出版日期:2015-12-20
网络出版日期:2015-11-24
作者简介:何 飞(1978–), 男, 副教授. E-mail: hefei@hit.edu.cn
基金资助:
Received:2015-05-11
Revised:2015-06-10
Published:2015-12-20
Online:2015-11-24
About author:HE Fei. E-mail: hefei@hit.edu.cn
Supported by:摘要:
气凝胶材料具有高比表面积、高孔隙率、低密度、低热导率和高透过率等特性, 在隔热、隔声和光学等领域具有广泛的应用前景。但该类材料纤细骨架构成的多孔结构所呈现的高脆性是限制其应用的主要因素。本文依据硅氧烷先驱体含有不可水解基团数量的特征, 综述了采用全水解双先驱体、全/部分水解双先驱体和部分水解双先驱体等三类采用双硅氧烷先驱体制备气凝胶材料的研究现状, 分析了这三类气凝胶所呈现出的组织结构特征及其在力学、热学、光学和疏水性等方面的性能特点。通过对硅氧烷先驱体类型的选择和组合, 可以设计气凝胶材料的组织结构与性能, 为改善气凝胶材料的力学行为提出了新思路。
中图分类号:
何 飞, 郁万军, 方旻翰, 赫晓东, 李明伟. 基于双硅氧烷先驱体制备的氧化硅基气凝胶研究进展[J]. 无机材料学报, 2015, 30(12): 1243-1253.
HE Fei, YU Wan-Jun, FANG Min-Han, HE Xiao-Dong, LI Ming-Wei. An Overview on Silica Aerogels Synthesized by Siloxane Co-precursors[J]. Journal of Inorganic Materials, 2015, 30(12): 1243-1253.
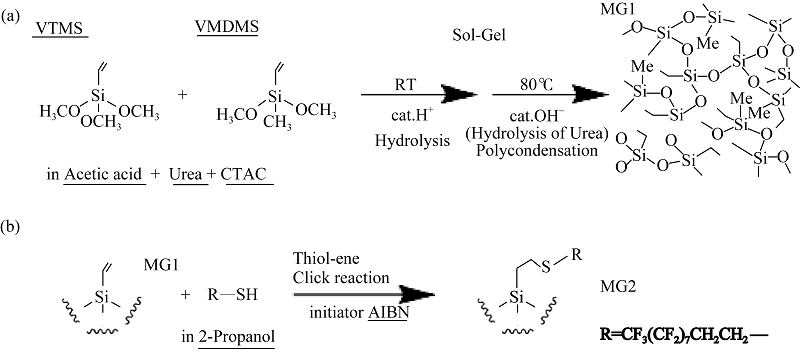
图6 超双疏气凝胶制备流程和反应原理示意图[55]
Fig. 6 Synthetic approach of superamphiphobic aerogels[55] (a) The synthesis for the VTMS+VMDMS marshmallow-like gel (MG1); (b) Synthetic approach for the superamphiphobic aerogels (MG2)
| Precursors | Strength/ MPa | Elastic modulus/ MPa | Density/ (g·cm-3) | Contact angle/(°) | Thermal conductivity/ (W·m-1·K-1) | Thermostability /℃ |
|---|---|---|---|---|---|---|
| TMOS | 0.031,compressed, crushed[ | - | 0.12[ | Hydrophilic[ | 0.01~0.03[ | 600[ |
| MTMS | >9 MPa, compressed 80%, recovered[ | 0.034~0.062 [ | 0.04~0.1[ | 158~164[ | - | 257[ |
| TMOS+PTES a | - | - | 0.15[ | 132[ | - | 520[ |
| TMOS+PTES b | - | - | 0.11[ | 121[ | - | 520[ |
| TEOS+MTMS a | - | - | 0.107[ | 120[ | - | 435[ |
| TEOS+PTES a | - | - | 0.33[ | 129[ | - | 520[ |
| TEOS +PTES b | - | - | 0.14[ | 115[ | - | 520[ |
| TEOS+HMDZ b | - | - | 0.09[ | 146[ | 0.08[ | 330[ |
| MTMS+ DMDMS | ~0.1, compression, recovered | - | - | hydrophobicity | - | 320[ |
| VTMS+ VMDMS | ~0.07, compressed 80%, recovered | - | 0.122 | 150, superamphiphobic | - | 170[ |
| MTMS+ GPTMS | ~0.17, compressed 35%, recovered | 0.46 | 0.104 | - | 0.0388 | -[ |
| MPTMS+ VTMS | compression, recovered | 0.117 | 0.085 | - | 0.047 | -[ |
表2 不同先驱体制备的氧化硅基气凝胶性能参数比较
Table 2 Comparison of performance parameters of silica aerogels prepared by different precursors
| Precursors | Strength/ MPa | Elastic modulus/ MPa | Density/ (g·cm-3) | Contact angle/(°) | Thermal conductivity/ (W·m-1·K-1) | Thermostability /℃ |
|---|---|---|---|---|---|---|
| TMOS | 0.031,compressed, crushed[ | - | 0.12[ | Hydrophilic[ | 0.01~0.03[ | 600[ |
| MTMS | >9 MPa, compressed 80%, recovered[ | 0.034~0.062 [ | 0.04~0.1[ | 158~164[ | - | 257[ |
| TMOS+PTES a | - | - | 0.15[ | 132[ | - | 520[ |
| TMOS+PTES b | - | - | 0.11[ | 121[ | - | 520[ |
| TEOS+MTMS a | - | - | 0.107[ | 120[ | - | 435[ |
| TEOS+PTES a | - | - | 0.33[ | 129[ | - | 520[ |
| TEOS +PTES b | - | - | 0.14[ | 115[ | - | 520[ |
| TEOS+HMDZ b | - | - | 0.09[ | 146[ | 0.08[ | 330[ |
| MTMS+ DMDMS | ~0.1, compression, recovered | - | - | hydrophobicity | - | 320[ |
| VTMS+ VMDMS | ~0.07, compressed 80%, recovered | - | 0.122 | 150, superamphiphobic | - | 170[ |
| MTMS+ GPTMS | ~0.17, compressed 35%, recovered | 0.46 | 0.104 | - | 0.0388 | -[ |
| MPTMS+ VTMS | compression, recovered | 0.117 | 0.085 | - | 0.047 | -[ |
| [1] | RAO A V, BHAGAT S D, HIRASHIMA H, et al.Synthesis of flexible silica aerogels using methyltrimethoxysilane (MTMS) precursor.Journal of Colloid and Interface Science, 2006, 300(1): 279-285. |
| [2] | RANDALL J P, MEADOR M A B, JANA S C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Applied Materials & Interfaces, 2011, 3(3): 613-626. |
| [3] | KANAMORI K, NAKANISHI K.Controlled pore formation in organotrialkoxysilane-derived hybrids: from aerogels to hierarchically porous monoliths.Chemical Society Reviews, 2011, 40(2): 754-770. |
| [4] | MALEKI H, DURÃES L, PORTUGAL A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies.Journal of Non-Crystalline Solids, 2014, 385(2): 55-74. |
| [5] | RAO A V, PAJONK G M, BHAGAT S D, et al.Comparative studies on the surface chemical modification of silica aerogels based on various organosilane compounds of the type RnSiX4-n.Journal of Non-Crystalline Solids, 2004, 350(12): 216-223. |
| [6] | HAYASE G, KANAMORI K, NAKANISHI K.New flexible aerogels and xerogels derived from methyltrimethoxysilane/ dimethyldimethoxysilane co-precursors.Journal of Materials Chemistry, 2011, 21(43): 17077-17079. |
| [7] | GURAV J L, RAO A V, BANGI U K H. Hydrophobic and low density silica aerogels dried at ambient pressure using TEOS precursor. Journal of Alloys and Compounds, 2009, 471(1): 296-302. |
| [8] | DONATTI D A, VOLLET D R.Study of the hydrolysis of TEOS-TMOS mixtures under ultrasound stimulation.Journal of Non-Crystalline Solids, 1996, 204(3): 301-304. |
| [9] | VOLLET D R, NUNES L M, DONATTI D A, et al.Structural characteristics of silica sonogels prepared with different proportions of TEOS and TMOS.Journal of Non-Crystalline Solids, 2008, 354(14): 1467-1474. |
| [10] | LATTHE S S, DHERE S L, KAPPENSTEIN C, et al.Sliding behavior of water drops on Sol-Gel derived hydrophobic silica films. Applied Surface Science, 2010, 256(10): 3259-3264. |
| [11] | MAHADIK D B, RAO A V, RAO A P, et al.Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. Journal of Colloid and Interface Science, 2011, 356(1): 298-302. |
| [12] | MAHADIK D B, RAO A V, KUMAR R S. et al. Reduction of processing time by mechanical shaking of the ambient pressure dried TEOS based silica aerogel granules.Journal of Porous Materials, 2012, 19(1): 87-94. |
| [13] | GURAV J L, RAO A V, NADARGI D Y.Study of thermal conductivity and effect of humidity on HMDZ modified TEOS based aerogel dried at ambient pressure.Journal of Sol-Gel Science and Technology, 2009, 50(3): 275-280. |
| [14] | GURAV J L, NADARGI D Y, RAO A V.Effect of mixed catalysts system on TEOS-based silica aerogels dried at ambient pressure.Applied Surface Science, 2008, 255(5): 3019-3027. |
| [15] | GURAV J L, RAO A V, NADARGI D Y, et al.Ambient pressure dried TEOS-based silica aerogels: good absorbents of organic liquids.Journal of Materials Science, 2010, 45(2): 503-510. |
| [16] | PARALE V G, MAHADIK D B, MAHADIK S A, et al.Wettability study of surface modified silica aerogels with different silylating agents.Journal of Sol-Gel Science and Technology, 2012, 63(3): 573-579. |
| [17] | SHEWALE P M, RAO A V, GURAV J L, et al.Synthesis and characterization of low density and hydrophobic silica aerogels dried at ambient pressure using sodium silicate precursor.Journal of Porous Materials, 2009, 16(1): 101-108. |
| [18] | SHEWALE P M, RAO A V, RAO A P.Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels.Applied Surface Science, 2008, 254(19): 6902-6907. |
| [19] | RAO A P, RAO A V.Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents.Journal of Materials Science, 2010, 45(1): 51-63. |
| [20] | RAO A P, RAO A V, PAJONK G M.Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents.Applied Surface Science, 2007, 253(14): 6032-6040. |
| [21] | BANGI U K H, RAO A P, HIRASHIMA H, et al. Physico-chemical properties of ambiently dried sodium silicate based aerogels catalyzed with various acids.Journal of Sol-Gel Science and Technology, 2009, 50(1): 87-97. |
| [22] | GURAV J L, RAO A V, RAO A P, et al.Physical properties of sodium silicate based silica aerogels prepared by single step Sol-Gel process dried at ambient pressure.Journal of Alloys and Compounds, 2009, 476(1): 397-402. |
| [23] | LATTHE S S, NADARGI D Y, RAO A V.TMOS based water repellent silica thin films by co-precursor method using TMES as a hydrophobic agent.Applied Surface Science, 2009, 255(6): 3600-3604. |
| [24] | LATTHE S S, IMAI H, GANESAN V, et al.Porous superhydrophobic silica films by Sol-Gel process.Microporous and Mesoporous Materials, 2010, 130(1): 115-121. |
| [25] | GANBAVLE V V, BANGI U K H, LATTHE S S, et al. Self-cleaning silica coatings on glass by single step Sol-Gel route.Surface & Coatings Technology, 2011, 205(23): 5338-5344. |
| [26] | PARALE V G, MAHADIK D B, KAVALE M S, et al.Sol-Gel preparation of PTMS modified hydrophobic and transparent silica coatings.Journal of Porous Materials, 2013, 20(4): 733-739. |
| [27] | LATTHE S S, IMAI H, GANESAN V, et al.Ultrahydrophobic silica films by Sol-Gel process.Journal of Porous Materials, 2010, 17(5): 565-571. |
| [28] | DHERE S L, LATTHE S S, KAPPENSTEIN C, et al.Transparent water repellent silica films by Sol-Gel process.Applied Surface Science, 2010, 256(11): 3624-3629. |
| [29] | LATTHE S S, IMAI H, GANESAN V, et al.Superhydrophobic silica films by Sol-Gel co-precursor method.Applied Surface Science, 2009, 256(1): 217-222. |
| [30] | PARALE V G, MAHADIK D B, MAHADIK S A, et al.OTES modified transparent dip coated silica coatings.Ceramics International, 2013, 39(1): 835-840. |
| [31] | GUO P, ZHAI S, XIAO Z, et al.One-step fabrication of highly stable, superhydrophobic composites from controllable and low-cost PMHS/TEOS sols for efficient oil cleanup.Journal of Colloid and Interface Science, 2015, 446(5): 155-162. |
| [32] | ZHANG X, WU Y, HE S, et al.Structural characterization of sol-gel composites using TEOS/MEMO as precursors.Surface & Coatings Technology, 2007, 201(12): 6051-6058. |
| [33] | MAHADIK S A, KAVALE M S, MUKHERJEE S K, et al.Transparent superhydrophobic silica coatings on glass by Sol-Gel method.Applied Surface Science, 2010, 257(2): 333-339. |
| [34] | RAO A V, PAJONK G M.Effect of methyltrimethoxysilane as a co-precursor on the optical properties of silica aerogels.Journal of Non-Crystalline Solids, 2001, 285(1): 202-209. |
| [35] | RAO A V, KULKARNI M M, AMALNERKAR D P, et al.Surface chemical modification of silica aerogels using various alkyl- alkoxy/chloro silanes. Applied Surface Science, 2003, 206(1): 262-270. |
| [36] | RAO A V, KULKARNI M M.Hydrophobic properties of TMOS-TMES-based silica aerogels.Materials Research Bulletin, 2002, 37(9): 1667-1677. |
| [37] | RAO A V, Wagh P B.Preparation and characterization of hydrophobic silica aerogels.Materials Chemistry and Physics, 1998, 53(1): 13-18. |
| [38] | RAO A V, HARANATH D. Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous and Mesoporous Materials, 1999, 30(2): 267-273. |
| [39] | BHAGAT S D, RAO A V.Surface chemical modification of TEOS based silica aerogels synthesized by two step (acid-base) Sol-Gel process.Applied Surface Science, 2006, 252(12): 4289-4297. |
| [40] | NADARGI D Y, KALESH R R, RAO A V.Rapid reduction in gelation time and impregnation of hydrophobic property in the tetraethoxysilane (TEOS) based silica aerogels using NH4F catalyzed single step Sol-Gel process.Journal of Alloys and Compounds, 2009, 480(2): 689-695. |
| [41] | HEGDE N D, RAO A V.Organic modification of TEOS based silica aerogels using hexadecyltrimethoxysilane as a hydrophobic reagent.Applied Surface Science, 2006, 253(3): 1566-1572. |
| [42] | RAO A V, HEGDE N D, Shewale P M.Imperviousness of the hydrophobic silica aerogels against various solvents and acids.Applied Surface Science, 2007, 253(9): 4137-4141. |
| [43] | HUANG S L, CHIN W K, YANG W P.Structural characteristics and properties of silica_poly(2-hydroxyethyl methacrylate) (PHEMA) nanocomposites prepared by mixing colloidal silica or tetraethyloxysilane (TEOS) with PHEMA.Polymer, 2005, 46(6): 1865-1877. |
| [44] | LI J, CAO J, YANG M, et al.‘Seeded’ growth of silica aerogel by tetraethoxysilane and trimethylchlorosilane co-precursor method.Journal of Non-Crystalline Solids, 2013, 362(1): 216-221. |
| [45] | LI J, CAO J, HUO L, et al.One-step synthesis of hydrophobic silica aerogel via in situ surface modification.Materials Letters, 2012, 87(11): 146-149. |
| [46] | LI Z, CHENG X, HE SONG, et al.Preparation of ambient pressure dried MTMS/TEOS co-precursor silica aerogel by adjusting NH4OH concentration.Materials Letters, 2014, 129(8): 12-15. |
| [47] | ZHOU B, SHEN J, WU Y, et al.Hydrophobic silica aerogels derived from polyethoxydisiloxane and perfluoroalkylsilane.Materials Science and Engineering C, 2007, 27(5-8): 1291-1294. |
| [48] | AL-OWEINI R, EL-RASSY H.Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R″Si(OR′)3 precursors.Journal of Molecular Structure, 2009, 919(1/2/3): 140-145. |
| [49] | AL-OWEINI R, EL-RASSY H.Surface characterization by nitrogen adsorption of silica aerogels synthesized from various Si(OR)4 and R″Si(OR′)3 precursors.Applied Surface Science, 2010, 257(1): 276-281. |
| [50] | KANAMORI K, AIZAWA M, NAKANISHI K, et al.New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties.Advanced Materilals, 2007, 19(12): 1589-1593. |
| [51] | KANAMORI K, AIZAWA M, NAKANISHI K, et al.Elastic organic- inorganic hybrid aerogels and xerogels.Journal of Sol-Gel Science and Technology, 2008, 48(1): 172-181. |
| [52] | KANAMORI K, NAKANISHI K, HANADA T.Sol-Gel synthesis, porous structure, and mechanical property polymethylsilsesquioxane aerogels.Journal of the Ceramic Society of Japan, 2009, 117(12): 1333-1338. |
| [53] | KANAMORI K, KODERA Y, HAYASE G, et al.Transition from transparent aerogels to hierarchically porous monoliths in polymethylsilsesquioxane Sol-Gel system.Journal of Colloid and Interface Science, 2011, 357(2): 336-344. |
| [54] | HAYASE G, KANAMORI K, FUKUCHI M, et al.Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water.Angewandte Chemie, 2013, 125(7): 2040-2043. |
| [55] | HAYASE G, KANAMORI K, HASEGAWA G, et al.A superamphiphobic macroporous silicone monolith with marshmallow- like flexibility. Angewandte Chemie International Edition, 2013, 52(41): 1-5. |
| [56] | ARAVIND P R, NIEMEYER P, RATKE L.Novel flexible aerogels derived from methyltrimethoxysilane/3-(2,3-epoxypropoxy) propyltrimethoxysilane co-precursor.Microporous and Mesoporous Materials, 2013, 181(11): 111-115. |
| [57] | WANG Z, DAI Z, WU J J, et al.Vacuum-dried robust bridged silsesquioxane aerogels.Advanced Materilals, 2013, 25(32): 4494-4497. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈义, 邱海鹏, 陈明伟, 徐昊, 崔恒. SiC/SiC复合材料基体硼改性方法及其力学性能研究[J]. 无机材料学报, 2025, 40(5): 504-510. |
| [8] | 崔宁, 张玉新, 王鲁杰, 李彤阳, 于源, 汤华国, 乔竹辉. (TiVNbMoW)Cx高熵陶瓷的单相形成过程与碳空位调控[J]. 无机材料学报, 2025, 40(5): 511-520. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 李紫薇, 弓伟露, 崔海峰, 叶丽, 韩伟健, 赵彤. 前驱体法制备(Zr, Hf, Nb, Ta, W)C-SiC复相陶瓷及性能研究[J]. 无机材料学报, 2025, 40(3): 271-280. |
| [13] | 高晨光, 孙晓亮, 陈君, 李达鑫, 陈庆庆, 贾德昌, 周玉. 基于湿法纺丝技术的SiBCN-rGO陶瓷纤维的组织结构、力学和吸波性能[J]. 无机材料学报, 2025, 40(3): 290-296. |
| [14] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [15] | 穆浩洁, 张源江, 喻彬, 付秀梅, 周世斌, 李晓东. ZrO2掺杂Y2O3-MgO纳米复相陶瓷的制备及性能研究[J]. 无机材料学报, 2025, 40(3): 281-289. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||