无机材料学报 ›› 2025, Vol. 40 ›› Issue (11): 1300-1308.DOI: 10.15541/jim20240532 CSTR: 32189.14.jim20240532
• 研究快报 • 上一篇
刘盼盼1( ), 姚鹏1, 刘栩孜1, 屈丽2, 曾路1, 宋兆华1, 焦毅1(
), 姚鹏1, 刘栩孜1, 屈丽2, 曾路1, 宋兆华1, 焦毅1( ), 王健礼1,2, 陈耀强1,2
), 王健礼1,2, 陈耀强1,2
收稿日期:2024-12-23
修回日期:2025-04-19
出版日期:2025-11-20
网络出版日期:2025-05-09
通讯作者:
焦 毅, 副研究员. E-mail: jiaoyiscu@163.com作者简介:刘盼盼(2000-), 男, 硕士研究生. E-mail: liupanpan@stu.scu.edu.cn
LIU Panpan1( ), YAO Peng1, LIU Xuzi1, QU Li2, ZENG Lu1, SONG Zhaohua1, JIAO Yi1(
), YAO Peng1, LIU Xuzi1, QU Li2, ZENG Lu1, SONG Zhaohua1, JIAO Yi1( ), WANG Jianli1,2, CHEN Yaoqiang1,2
), WANG Jianli1,2, CHEN Yaoqiang1,2
Received:2024-12-23
Revised:2025-04-19
Published:2025-11-20
Online:2025-05-09
Contact:
JIAO Yi, associate professor. E-mail: jiaoyiscu@163.comAbout author:LIU Panpan (2000-), male, Master candidate. E-mail: liupanpan@stu.scu.edu.cn
Supported by:摘要:
汽油机尾气碳烟颗粒严重威胁着生态环境和人类健康, 而催化型汽油颗粒物过滤器(cGPF)是一种有效的净化技术, 其核心为催化剂涂层。本研究采用不同制备方法构筑了MnOx/CeO2-ZrO2复合氧化物, 在低O2浓度下考察了其碳烟氧化性能, 旨在开发具有卓越催化活性、稳定性以及抗水性的碳烟氧化催化剂涂层。结果表明, 通过浸渍法制备的MnOx/CeO2-ZrO2(MCZ-IM)在1% O2中, 其T50(碳烟转化率达50%所需的温度)为329 ℃; 在0.5% O2中, 其T50为370 ℃。相较于高能球磨法(MCZ-HB)和共沉淀法(MCZ-CP)制备的催化剂, MCZ-IM展现出更优的综合性能。构效关系表明, 低O2浓度下碳烟氧化性能与催化剂的织构和结构性质呈弱相关, 而与活性氧物种(AOS)——超氧(O2-)和过氧(O22-)阴离子的生成及迁移密切相关, 其中涉及氧化还原性能、储释氧能力以及氧空位数量。MCZ-IM在氧物种的吸附、活化和脱附方面更具优势, 因此浸渍法被认为是提高AOS生成和迁移的更优方法。本研究不仅提供了一种适用于实际运行条件下颗粒物(PM)氧化催化剂的制备策略, 而且深入揭示了低O2浓度环境中AOS的迁移机制。
中图分类号:
刘盼盼, 姚鹏, 刘栩孜, 屈丽, 曾路, 宋兆华, 焦毅, 王健礼, 陈耀强. MnOx/CeO2-ZrO2复合氧化物的构筑及其在碳烟氧化中的应用[J]. 无机材料学报, 2025, 40(11): 1300-1308.
LIU Panpan, YAO Peng, LIU Xuzi, QU Li, ZENG Lu, SONG Zhaohua, JIAO Yi, WANG Jianli, CHEN Yaoqiang. MnOx/CeO2-ZrO2 Composite Oxides: Construction and Application in Soot Oxidation[J]. Journal of Inorganic Materials, 2025, 40(11): 1300-1308.
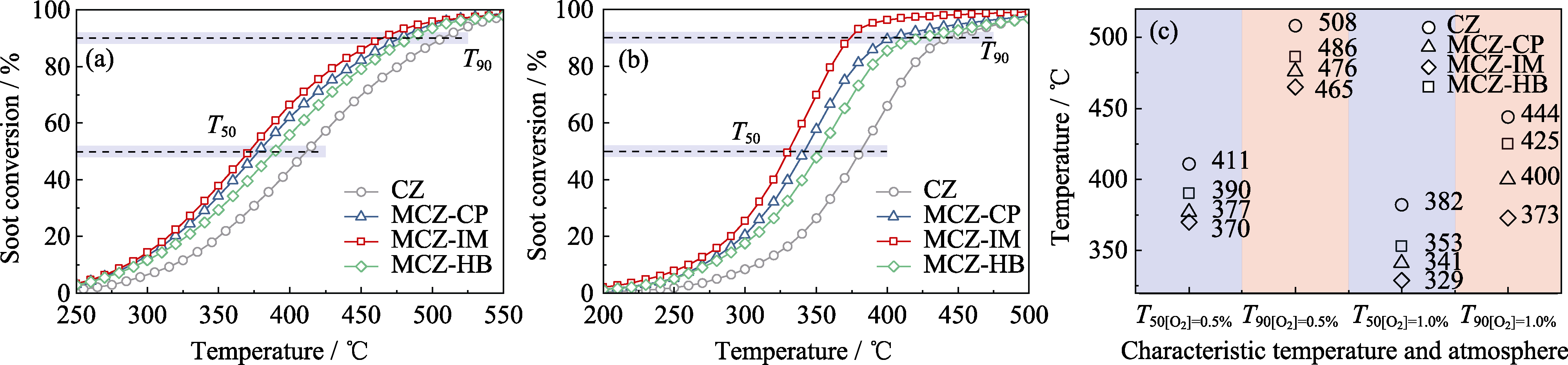
Fig. 1 Soot conversion, T50, and T90 of the catalysts under different oxygen concentrations with reaction conditions of flow rate at 500 mL/min and N2 balance (a, b) Soot conversion under (a) 0.5% and (b) 1.0% O2; (c) T50 and T90 under different oxygen concentrations
| Sample | Surface areaa /(m2•g-1) | Pore volumea /(mL•g-1) | Mean pore diametera /nm | Crystallinityb /% | Grain sizec /nm |
|---|---|---|---|---|---|
| CZ | 110.3 | 0.26 | 9.40 | 52.1 | 7.2 |
| MCZ-CP | 145.9 | 0.34 | 9.64 | 40.2 | 5.1 |
| MCZ-IM | 79.2 | 0.22 | 11.30 | 45.6 | 7.4 |
| MCZ-HB | 81.3 | 0.22 | 10.80 | 44.8 | 7.6 |
Table 1 Textural and structural properties of the prepared catalysts
| Sample | Surface areaa /(m2•g-1) | Pore volumea /(mL•g-1) | Mean pore diametera /nm | Crystallinityb /% | Grain sizec /nm |
|---|---|---|---|---|---|
| CZ | 110.3 | 0.26 | 9.40 | 52.1 | 7.2 |
| MCZ-CP | 145.9 | 0.34 | 9.64 | 40.2 | 5.1 |
| MCZ-IM | 79.2 | 0.22 | 11.30 | 45.6 | 7.4 |
| MCZ-HB | 81.3 | 0.22 | 10.80 | 44.8 | 7.6 |
| Sample | Mass fraction/% | |||
|---|---|---|---|---|
| O | Mn | Zr | Ce | |
| MCZ-CP | 24.38 | 2.98 | 22.44 | 50.20 |
| MCZ-IM | 24.13 | 7.54 | 23.14 | 45.18 |
| MCZ-HB | 21.52 | 1.03 | 24.58 | 52.87 |
Table 2 Surface elements composition of the catalysts
| Sample | Mass fraction/% | |||
|---|---|---|---|---|
| O | Mn | Zr | Ce | |
| MCZ-CP | 24.38 | 2.98 | 22.44 | 50.20 |
| MCZ-IM | 24.13 | 7.54 | 23.14 | 45.18 |
| MCZ-HB | 21.52 | 1.03 | 24.58 | 52.87 |

Fig. 6 Surface oxygen vacancies and active oxygen species of the samples (a) Raman spectra; (b-d) Ce3d (b), Mn2p (c), and O1s (d) XPS spectra; (e) DRIFTS spectra of O2 adsorption collected at 300 ℃; (f) Low-temperature EPR. Colorful figures are available on website
| Catalyst | Ce3+/Ce | Ce4+/Ce | Mn4+/Mn | (O22-+O2-)/OT | O2-/OT |
|---|---|---|---|---|---|
| CZ | 0.21 | 0.79 | — | 0.28 | 0.09 |
| MCZ-CP | 0.14 | 0.86 | 0.36 | 0.35 | 0.11 |
| MCZ-IM | 0.15 | 0.85 | 0.39 | 0.34 | 0.17 |
| MCZ-HB | 0.17 | 0.83 | 0.32 | 0.31 | 0.06 |
Table 3 Surface compositions and charge states of Ce, Mn and O species derived from XPS analysis
| Catalyst | Ce3+/Ce | Ce4+/Ce | Mn4+/Mn | (O22-+O2-)/OT | O2-/OT |
|---|---|---|---|---|---|
| CZ | 0.21 | 0.79 | — | 0.28 | 0.09 |
| MCZ-CP | 0.14 | 0.86 | 0.36 | 0.35 | 0.11 |
| MCZ-IM | 0.15 | 0.85 | 0.39 | 0.34 | 0.17 |
| MCZ-HB | 0.17 | 0.83 | 0.32 | 0.31 | 0.06 |
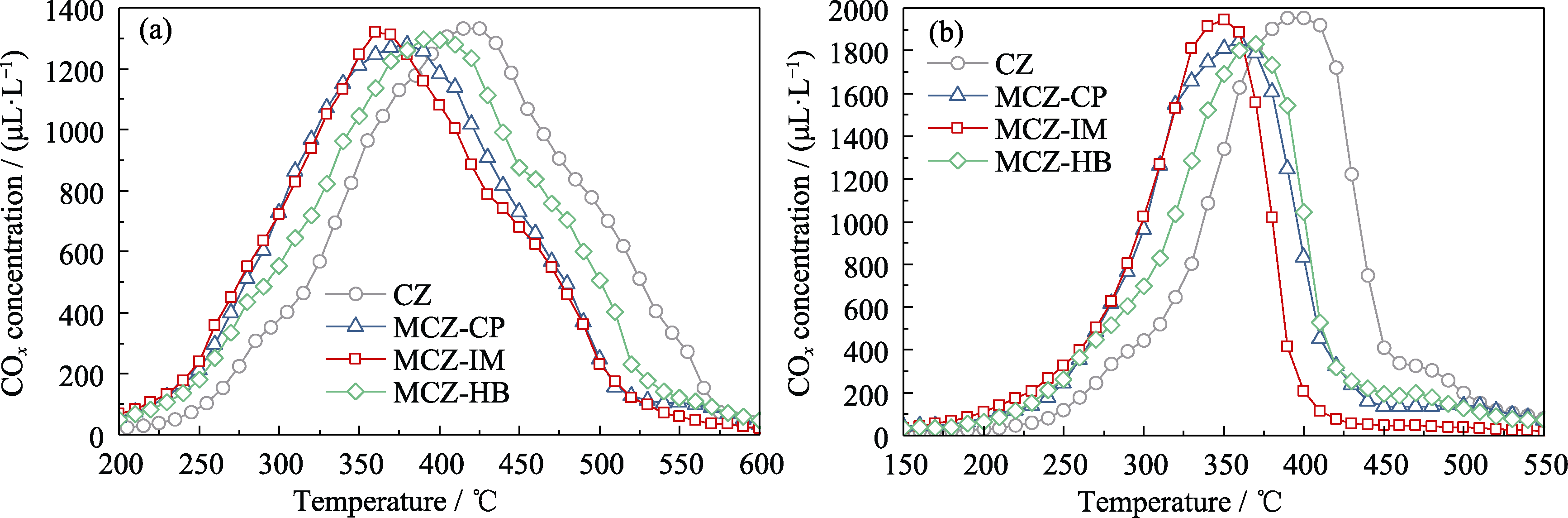
Fig. S1 Generated COx concentration of soot oxidation over the catalysts Reaction conditions: flow rate at 500 mL/min and N2 balance; [O2]: (a) 0.5% and (b) 1.0%
| Catalyst | Method | Reaction condition (gas and flow rate) | Heating rate/ (℃•min-1) | Catalyst and soot mass ratio | Contact mode | T50/Tmax/ ℃ | Ref. |
|---|---|---|---|---|---|---|---|
| MCZ-IM | Incipient wetness impregnation | 1% O2 | 5 | 10 : 1 | Tight | 329 | This work |
| 0.5% O2 (500 mL/min) | 370 | ||||||
| M10-CZ | Co-precipitation | 0.5% O2 (500 mL/min) | 5 | 10 : 1 | Loose | 520 | [S1] |
| Mn2O3 | Flame spray pyrolysis | 1% O2 + 2% H2O (500 mL/min) | 3.3 | 15 : 1 | Tight | 321 | [S4] |
| 10LM-CZ | Co-precipitation and citric acid complexation impregnation | 1% O2 (100 mL/min) | 5 | 10 : 1 | Tight | 362 | [S5] |
| 0.57Mn-CeO2 | Nitrate aerosol pyrolysis | 10% O2 (100 mL/min) | 10 | 4 : 1 | Tight | 355 | [S6] |
| 5 Mn-CP | Solution combustion synthesis | Air (100 mL/min) | 10 | 10 : 1 | Tight | 365 | [S7] |
| CM5 | EDTA-Citrate | Air (100 mL/min) | 10 | 10 : 1 | Tight | 360 | [S8] |
| Ce0.5Mn0.5O2 | Sol-gel | 12% O2 (100 mL/min) | 15 | 4 : 1 | Tight | 383 | [S9] |
| CMO_st | Solvothermal | - | 10 | 19 : 1 | Tight | 442 | [S10] |
| CM | Co-precipitation | Air (100 mL/min) | 10 | 4 : 1 | Tight | 396 | [S11] |
| CMC | Co-precipitation | Air (100 mL/min) | - | 4 : 1 | Tight | 363 | [S12] |
| Ce0.9Mn0.1 | Solid-phase grinding | 10% O2 (50 mL/min) | 10 | 10 : 1 | Tight | 389 | [S13] |
| Mn-Fib Ce | Plasma-assisted deposition | 18% O2 + 0.1%NO (20 mL/min) | 5 | 20 : 1 | Tight | 384 | [S14] |
Table S1 Comparison of catalytic activity with other studies
| Catalyst | Method | Reaction condition (gas and flow rate) | Heating rate/ (℃•min-1) | Catalyst and soot mass ratio | Contact mode | T50/Tmax/ ℃ | Ref. |
|---|---|---|---|---|---|---|---|
| MCZ-IM | Incipient wetness impregnation | 1% O2 | 5 | 10 : 1 | Tight | 329 | This work |
| 0.5% O2 (500 mL/min) | 370 | ||||||
| M10-CZ | Co-precipitation | 0.5% O2 (500 mL/min) | 5 | 10 : 1 | Loose | 520 | [S1] |
| Mn2O3 | Flame spray pyrolysis | 1% O2 + 2% H2O (500 mL/min) | 3.3 | 15 : 1 | Tight | 321 | [S4] |
| 10LM-CZ | Co-precipitation and citric acid complexation impregnation | 1% O2 (100 mL/min) | 5 | 10 : 1 | Tight | 362 | [S5] |
| 0.57Mn-CeO2 | Nitrate aerosol pyrolysis | 10% O2 (100 mL/min) | 10 | 4 : 1 | Tight | 355 | [S6] |
| 5 Mn-CP | Solution combustion synthesis | Air (100 mL/min) | 10 | 10 : 1 | Tight | 365 | [S7] |
| CM5 | EDTA-Citrate | Air (100 mL/min) | 10 | 10 : 1 | Tight | 360 | [S8] |
| Ce0.5Mn0.5O2 | Sol-gel | 12% O2 (100 mL/min) | 15 | 4 : 1 | Tight | 383 | [S9] |
| CMO_st | Solvothermal | - | 10 | 19 : 1 | Tight | 442 | [S10] |
| CM | Co-precipitation | Air (100 mL/min) | 10 | 4 : 1 | Tight | 396 | [S11] |
| CMC | Co-precipitation | Air (100 mL/min) | - | 4 : 1 | Tight | 363 | [S12] |
| Ce0.9Mn0.1 | Solid-phase grinding | 10% O2 (50 mL/min) | 10 | 10 : 1 | Tight | 389 | [S13] |
| Mn-Fib Ce | Plasma-assisted deposition | 18% O2 + 0.1%NO (20 mL/min) | 5 | 20 : 1 | Tight | 384 | [S14] |
| Sample | Element | Atomic fraction/% | Mass fraction/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Average | Area 1 | Area 2 | Area 3 | Average | ||
| MCZ-CP | O | 62.48 | 66.22 | 69.82 | 66.17 | 21.55 | 24.14 | 27.45 | 24.38 |
| Mn | 2.49 | 2.85 | 2.92 | 2.75 | 2.52 | 3.06 | 3.37 | 2.98 | |
| Zr | 15.70 | 11.73 | 10.37 | 12.60 | 26.47 | 20.91 | 19.94 | 22.44 | |
| Ce | 19.33 | 19.20 | 16.89 | 18.47 | 49.45 | 51.90 | 49.24 | 50.20 | |
| MCZ-IM | O | 60.16 | 63.95 | 68.43 | 64.18 | 21.21 | 24.43 | 26.76 | 24.13 |
| Mn | 6.91 | 8.73 | 4.92 | 6.86 | 7.16 | 9.81 | 5.66 | 7.54 | |
| Zr | 16.25 | 12.07 | 9.86 | 12.73 | 28.02 | 22.54 | 18.86 | 23.14 | |
| Ce | 16.68 | 15.25 | 16.80 | 16.24 | 43.61 | 43.22 | 48.72 | 45.18 | |
| MCZ-HB | O | 61.05 | 65.04 | 63.64 | 63.24 | 20.63 | 22.36 | 21.58 | 21.52 |
| Mn | 0.89 | 0.91 | 1.30 | 1.03 | 0.88 | 0.92 | 1.30 | 1.03 | |
| Zr | 19.84 | 11.62 | 12.91 | 14.79 | 32.79 | 19.54 | 21.41 | 24.58 | |
| Ce | 18.23 | 22.43 | 22.15 | 20.94 | 45.70 | 57.19 | 55.71 | 52.87 | |
Table S2 Surface elements composition of the prepared catalysts
| Sample | Element | Atomic fraction/% | Mass fraction/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Area 1 | Area 2 | Area 3 | Average | Area 1 | Area 2 | Area 3 | Average | ||
| MCZ-CP | O | 62.48 | 66.22 | 69.82 | 66.17 | 21.55 | 24.14 | 27.45 | 24.38 |
| Mn | 2.49 | 2.85 | 2.92 | 2.75 | 2.52 | 3.06 | 3.37 | 2.98 | |
| Zr | 15.70 | 11.73 | 10.37 | 12.60 | 26.47 | 20.91 | 19.94 | 22.44 | |
| Ce | 19.33 | 19.20 | 16.89 | 18.47 | 49.45 | 51.90 | 49.24 | 50.20 | |
| MCZ-IM | O | 60.16 | 63.95 | 68.43 | 64.18 | 21.21 | 24.43 | 26.76 | 24.13 |
| Mn | 6.91 | 8.73 | 4.92 | 6.86 | 7.16 | 9.81 | 5.66 | 7.54 | |
| Zr | 16.25 | 12.07 | 9.86 | 12.73 | 28.02 | 22.54 | 18.86 | 23.14 | |
| Ce | 16.68 | 15.25 | 16.80 | 16.24 | 43.61 | 43.22 | 48.72 | 45.18 | |
| MCZ-HB | O | 61.05 | 65.04 | 63.64 | 63.24 | 20.63 | 22.36 | 21.58 | 21.52 |
| Mn | 0.89 | 0.91 | 1.30 | 1.03 | 0.88 | 0.92 | 1.30 | 1.03 | |
| Zr | 19.84 | 11.62 | 12.91 | 14.79 | 32.79 | 19.54 | 21.41 | 24.58 | |
| Ce | 18.23 | 22.43 | 22.15 | 20.94 | 45.70 | 57.19 | 55.71 | 52.87 | |
| [1] | LIU S, FANG Z, WU X. CeO2-based materials for gasoline soot combustion: reaction mechanism and catalyst design. Journal of the Chinese Society of Rare Earths, 2022, 40(3): 351. |
| [2] |
LIU S, WU X D, TANG J, et al. An exploration of soot oxidation over CeO2-ZrO2 nanocubes: do more surface oxygen vacancies benefit the reaction? Catalysis Today, 2017, 281: 454.
DOI URL |
| [3] |
MATARRESE R. Catalytic materials for gasoline particulate filters soot oxidation. Catalysts, 2021, 11(8): 890.
DOI URL |
| [4] |
ZHAO M J, DENG J L, LIU J, et al. Roles of surface-active oxygen species on 3DOM cobalt-based spinel catalysts MxCo3-xO4 (M=Zn and Ni) for NOx-assisted soot oxidation. ACS Catalysis, 2019, 9(8): 7548.
DOI URL |
| [5] |
WANG X, JIN B F, FENG R X, et al. A robust core-shell silver soot oxidation catalyst driven by Co3O4: effect of tandem oxygen delivery and Co3O4-CeO2 synergy. Applied Catalysis B: Environmental, 2019, 250: 132.
DOI URL |
| [6] |
LI Y F, QIN T, WEI Y C, et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nature Communications, 2023, 14: 7149.
DOI PMID |
| [7] |
YU D, WANG L Y, ZHANG C L, et al. Alkali metals and cerium-modified La-co-based perovskite catalysts: facile synthesis, excellent catalytic performance, and reaction mechanisms for soot combustion. ACS Catalysis, 2022, 12(24): 15056.
DOI URL |
| [8] |
LIU S R, LUO S T, WU X D, et al. Application of silica-alumina as hydrothermally stable supports for Pt catalysts for acid-assisted soot oxidation. Rare Metals, 2023, 42(5): 1614.
DOI |
| [9] |
YU X H, REN Y, YU D, et al. Hierarchical porous K-OMS-2/ 3DOM-m Ti0.7Si0.3O2 catalysts for soot combustion: easy preparation, high catalytic activity, and good resistance to H2O and SO2. ACS Catalysis, 2021, 11(9): 5554.
DOI URL |
| [10] |
ZHU C X, DU S C, WANG S B, et al. PGM-free metal oxide nanoarray forests for water-promoted low-temperature soot oxidation. Applied Catalysis B: Environmental, 2024, 341: 123336.
DOI URL |
| [11] |
JIN B F, ZHAO B H, LIU S, et al. SmMn2O5 catalysts modified with silver for soot oxidation: dispersion of silver and distortion of mullite. Applied Catalysis B: Environmental, 2020, 273: 119058.
DOI URL |
| [12] |
LIU J X, YANG Z, ZHAI Y J, et al. High performance of PrMnO3 perovskite catalysts for low-temperature soot oxidation. Separation and Purification Technology, 2025, 354: 129227.
DOI URL |
| [13] |
YANG W N, WANG S M, LI K Z, et al. Highly selective α-Mn2O3 catalyst for cGPF soot oxidation: surface activated oxygen enhancement via selective dissolution. Chemical Engineering Journal, 2019, 364: 448.
DOI URL |
| [14] |
ZHAO Z, MA J, LI M, et al. Model Ag/CeO2 catalysts for soot combustion: roles of silver species and catalyst stability. Chemical Engineering Journal, 2022, 430: 132802.
DOI URL |
| [15] |
AWAD O I, MA X, KAMIL M, et al. Particulate emissions from gasoline direct injection engines: a review of how current emission regulations are being met by automobile manufacturers. Science of the Total Environment, 2020, 718: 137302.
DOI URL |
| [16] |
WANG X C, CHEN W H, HUANG Y H, et al. Advances in soot particles from gasoline direct injection engines: a focus on physical and chemical characterisation. Chemosphere, 2023, 311: 137181.
DOI URL |
| [17] |
KONTSES A, TRIANTAFYLLOPOULOS G, NTZIACHRISTOS L, et al. Particle number (PN) emissions from gasoline, diesel, LPG, CNG and hybrid-electric light-duty vehicles under real-world driving conditions. Atmospheric Environment, 2020, 222: 117126.
DOI URL |
| [18] |
NOSSOVA L, CARAVAGGIO G. Effect of dopants on soot oxidation over doped Ag/ZrO2 catalysts for catalyzed gasoline particulate filter. Catalysis Communications, 2023, 182: 106744.
DOI URL |
| [19] |
HERNÁNDEZ W Y, LOPEZ-GONZALEZ D, NTAIS S, et al. Silver-modified manganite and ferrite perovskites for catalyzed gasoline particulate filters. Applied Catalysis B: Environmental, 2018, 226: 202.
DOI URL |
| [20] |
YAO P, HUANG Y, JIAO Y, et al. Soot oxidation over Pt-loaded CeO2-ZrO2 catalysts under gasoline exhaust conditions: soot-catalyst contact efficiency and Pt chemical state. Fuel, 2023, 334: 126782.
DOI URL |
| [21] |
KUBO H, OHSHIMA Y, KATO S, et al. The effect of supported metal Species on soot oxidation over PGM/CeO2-ZrO2. Bulletin of the Chemical Society of Japan, 2024, 97(10): uoae092.
DOI URL |
| [22] |
LUO J B, ZHU X B, ZHONG Z W, et al. Enhanced catalytic soot oxidation over co-based metal oxides: effects of transition metal doping. Molecules, 2023, 29(1): 41.
DOI URL |
| [23] |
ZHANG P, MEI X L, ZHAO X C, et al. Boosting catalytic purification of soot particles over double perovskite-type La2-xKxNiCoO6 catalysts with an ordered macroporous structure. Environmental Science & Technology, 2021, 55(16): 11245.
DOI URL |
| [24] |
XIONG J X, ZHANG B J, LIANG Z F, et al. Highly reactive peroxide species promoted soot oxidation over an ordered macroporous Ce0.8Zr0.2O2 integrated catalyzed diesel particulate filter. Environmental Science & Technology, 2024, 58(18): 8096.
DOI URL |
| [25] |
HE J S, YAO P, QIU J, et al. Enhancement effect of oxygen mobility over Ce0.5Zr0.5O2 catalysts doped by multivalent metal oxides for soot combustion. Fuel, 2021, 286: 119359.
DOI URL |
| [26] |
DENG J, LI S S, XIONG L, et al. Preparation of nanostructured CeO2-ZrO2-based materials with stabilized surface area and their catalysis in soot oxidation. Applied Surface Science, 2020, 505: 144301.
DOI URL |
| [27] |
WANG L M, ZHAO N R, YIN X Y, et al. Highlights on the key roles of interfaces between CeO2-based oxide and perovskite (LaMnO3/LaFeO3) in creating active oxygen species for soot oxidation. Fuel, 2024, 356: 129444.
DOI URL |
| [28] |
ZHENG C L, MAO D J, XU Z Y, et al. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation. Journal of Catalysis, 2022, 411: 122.
DOI URL |
| [29] |
XIONG L, YAO P, LIU S, et al. Soot oxidation over CeO2-ZrO2 based catalysts: the influence of external surface and low- temperature reducibility. Molecular Catalysis, 2019, 467: 16.
DOI URL |
| [30] |
MISHRA U K, CHANDEL V S, SINGH O P, et al. Synthesis of CeO2 and Zr-doped CeO2 (Ce1-xZrxO2) catalyst by green synthesis for soot oxidation activity. Arabian Journal for Science and Engineering, 2023, 48(1): 771.
DOI |
| [31] |
LIU S, WU X D, WENG D, et al. Ceria-based catalysts for soot oxidation: a review. Journal of Rare Earths, 2015, 33(6): 567.
DOI URL |
| [32] |
LI Y F, QIN T, XIONG J, et al. Mn-modified near-surface atomic structure of CeO2 nanorods for promoting catalytic oxidation of auto-exhaust carbon particles. Chemical Engineering Science, 2023, 282: 119309.
DOI URL |
| [33] |
ZHAO H, ZHOU X X, WANG M, et al. Highly active MnOx-CeO2 catalyst for diesel soot combustion. RSC Advances, 2017, 7(6): 3233.
DOI URL |
| [34] |
ALINEZHADCHAMAZKETI A, KHODADADI A A, MORTAZAVI Y, et al. Catalytic evaluation of promoted CeO2-ZrO2 by transition, alkali, and alkaline-earth metal oxides for diesel soot oxidation. Journal of Environmental Sciences, 2013, 25(12): 2498.
DOI URL |
| [35] | XING L L, YANG Y X, CAO C M, et al. Decorating CeO2 nanoparticles on Mn2O3 nanosheets to improve catalytic soot combustion. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16544. |
| [36] |
YANG Y, FANG J, MENG Z W, et al. Catalytic activity and influence factors of Mn-Ce mixed oxides by hydrothermal method on diesel soot combustion. Molecular Catalysis, 2022, 524: 112334.
DOI URL |
| [37] |
SUN Y, FANG S Y, XU J C, et al. Unveiling the surface chemical reactions during multi-phase catalytic oxidation of soot on nanoengineering/interfacing/doping-prepared Mn-CeO2 catalysts using TG-MS and operando DRIFTS-MS. Langmuir, 2023, 39(44): 15773.
DOI URL |
| [38] |
ZHAO H, LI H C, PAN Z F, et al. Design of CeMnCu ternary mixed oxides as soot combustion catalysts based on optimized Ce/Mn and Mn/Cu ratios in binary mixed oxides. Applied Catalysis B: Environmental, 2020, 268: 118422.
DOI URL |
| [39] |
LI S S, DENG J, WANG J L, et al. Effects of thermal treatment conditions on redox properties of ceria-zirconia materials. Journal of Rare Earths, 2023, 41(12): 1969.
DOI URL |
| [40] |
YAO P, HE J S, JIANG X, et al. Factors determining gasoline soot abatement over CeO2-ZrO2-MnOx catalysts under low oxygen concentration condition. Journal of the Energy Institute, 2020, 93(2): 774.
DOI URL |
| [41] |
ZHANG H L, WANG J L, ZHANG Y H, et al. A study on H2-TPR of Pt/Ce0.27Zr0.73O2 and Pt/Ce0.27Zr0.70La0.03Ox for soot oxidation. Applied Surface Science, 2016, 377: 48.
DOI URL |
| [42] | WANG S N, WANG J L, HUA W B, et al. Designed synthesis of Zr-based ceria-zirconia-neodymia composite with high thermal stability and its enhanced catalytic performance for Rh-only three- way catalyst. Catalysis Science & Technology, 2016, 6(20): 7437. |
| [43] |
HE H, LIN X T, LI S J, et al. The key surface species and oxygen vacancies in MnOx(0.4)-CeO2 toward repeated soot oxidation. Applied Catalysis B: Environmental, 2018, 223: 134.
DOI URL |
| [44] |
WANG H L, LUO S T, ZHANG M S, et al. Roles of oxygen vacancy and Ox- in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts. Journal of Catalysis, 2018, 368: 365.
DOI URL |
| [45] |
XIONG J, WU Q Q, MEI X L, et al. Fabrication of spinel-type PdxCo3-xO4 binary active sites on 3D ordered meso-macroporous Ce-Zr-O2 with enhanced activity for catalytic soot oxidation. ACS Catalysis, 2018, 8(9): 7915.
DOI URL |
| [46] |
KHATUN R, PAL R S, SHOEB M A, et al. Generation of active oxygen species by CO2 dissociation over defect-rich Ni-Pt/CeO2 catalyst for boosting methane activation in low-temperature dry reforming: experimental and theoretical study. Applied Catalysis B: Environmental, 2024, 340: 123243.
DOI URL |
| [47] |
LIN X T, LI S J, HE H, et al. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Applied Catalysis B: Environmental, 2018, 223: 91.
DOI URL |
| [48] |
ZHANG Z R, KANG R N, YI X K, et al. Migration roles of different oxygen species over Cu/CeO2 for propane and soot combustion. Separation and Purification Technology, 2024, 349: 127820.
DOI URL |
| [49] |
XU J W, ZHANG Y, XU X L, et al. Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: the effect of phase structures, superoxide anions, and alkalinity on the reactivity. ACS Catalysis, 2019, 9(5): 4030.
DOI URL |
| [50] |
SUBBOTINA I R, BARSUKOV D V. Direct evidence of the key role of UV-formed peroxide species in photocatalytic gas-solid oxidation in air on anatase TiO2 particles. Physical Chemistry Chemical Physics, 2020, 22(4): 2200.
DOI URL |
| [51] | ZHANG M Y, DUAN X L, GAO Y, et al. Tuning oxygen vacancies in oxides by configurational entropy. ACS Applied Materials & Interfaces, 2023, 15(39): 45774. |
| [52] | MAO H F, XU M L, LI S J, et al. Accelerating surface lattice oxygen activation of Pt/TiO2-x by modulating the interface electron interaction for efficient photocatalytic toluene oxidation. ACS ES&T Engineering, 2023, 3(11): 1851. |
| [53] |
LOU D M, SONG G F, XU K W, et al. The oxidation performance of a carbon soot catalyst based on the Pt-Pd synergy effect. Energies, 2024, 17(7): 1737.
DOI URL |
| [1] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [2] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [3] | 李建军, 陈芳明, 张梨梨, 王磊, 张丽亭, 陈慧雯, 薛长国, 徐良骥. CoFe2O4/MgAl-LDH催化剂活化过氧一硫酸盐促进抗生素降解[J]. 无机材料学报, 2025, 40(4): 440-448. |
| [4] | 信震宇, 郭瑞华, 乌仁托亚, 王艳, 安胜利, 张国芳, 关丽丽. Pt-Fe/GO纳米催化剂的制备及其电催化乙醇氧化性能研究[J]. 无机材料学报, 2025, 40(4): 379-387. |
| [5] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [6] | 李薛茹, 马哲杰, 郭宇杰, 李平. 载体特性对Pt/C催化剂上离聚物覆盖度及氧还原性能的影响[J]. 无机材料学报, 2025, 40(12): 1395-1404. |
| [7] | 赵丽娟, 谭哲, 张晓光, 蒋国赛, 陶然, 潘德安. 废加氢催化剂颗粒分级数值模拟研究[J]. 无机材料学报, 2025, 40(12): 1387-1394. |
| [8] | 刘会来, 李志豪, 孔德峰, 陈星. 酞菁铁/MXene复合阴极的制备及电芬顿降解磺胺间二甲氧嘧啶[J]. 无机材料学报, 2025, 40(1): 61-69. |
| [9] | 李娜, 曹锐霄, 魏进, 周晗, 肖红梅. 铁基正仲氢转化催化剂的影响因素[J]. 无机材料学报, 2025, 40(1): 47-52. |
| [10] | 连敏丽, 苏佳欣, 黄鸿杨, 嵇玉寅, 邓海帆, 张彤, 陈崇启, 李达林. Ni-Mg-Al类水滑石衍生镍基催化剂的制备及其氨分解性能[J]. 无机材料学报, 2025, 40(1): 53-60. |
| [11] | 刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46. |
| [12] | 靳宇翔, 宋二红, 朱永福. 3d过渡金属单原子掺杂石墨烯缺陷电催化还原CO2的第一性原理研究[J]. 无机材料学报, 2024, 39(7): 845-852. |
| [13] | 叶梓滨, 邹高昌, 吴琪雯, 颜晓敏, 周明扬, 刘江. 阳极支撑型锥管串接式直接碳固体氧化物燃料电池组的制备及性能[J]. 无机材料学报, 2024, 39(7): 819-827. |
| [14] | 张文宇, 郭瑞华, 岳全鑫, 黄雅荣, 张国芳, 关丽丽. 高熵磷化物双功能催化剂的制备及高效电解水性能[J]. 无机材料学报, 2024, 39(11): 1265-1274. |
| [15] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||