无机材料学报 ›› 2025, Vol. 40 ›› Issue (11): 1201-1211.DOI: 10.15541/jim20240518 CSTR: 32189.14.jim20240518
所属专题: 【能源环境】金属有机框架材料MOF(202510)
李勇锋1,2( ), 顾玉萍2,3,4, 师广照2,3,4, 胡九林2,3,4, 雷萌2,3,4, 彭晖1,5(
), 顾玉萍2,3,4, 师广照2,3,4, 胡九林2,3,4, 雷萌2,3,4, 彭晖1,5( ), 曾宇平2,3, 李驰麟2,3,4(
), 曾宇平2,3, 李驰麟2,3,4( )
)
收稿日期:2024-12-16
修回日期:2025-03-07
出版日期:2025-11-20
网络出版日期:2025-03-24
通讯作者:
李驰麟, 研究员. E-mail: chilinli@mail.sic.ac.cn;作者简介:李勇锋(1999-), 男, 硕士研究生. E-mail: 51254700112@stu.ecnu.edu.cn
基金资助:
LI Yongfeng1,2( ), GU Yuping2,3,4, SHI Guangzhao2,3,4, HU Jiulin2,3,4, LEI Meng2,3,4, PENG Hui1,5(
), GU Yuping2,3,4, SHI Guangzhao2,3,4, HU Jiulin2,3,4, LEI Meng2,3,4, PENG Hui1,5( ), ZENG Yuping2,3, LI Chilin2,3,4(
), ZENG Yuping2,3, LI Chilin2,3,4( )
)
Received:2024-12-16
Revised:2025-03-07
Published:2025-11-20
Online:2025-03-24
Contact:
LI Chilin, professor. E-mail: chilinli@mail.sic.ac.cn;About author:LI Yongfeng (1999-), male, Master candidate. E-mail: 51254700112@stu.ecnu.edu.cn
Supported by:摘要:
Li1.3Al0.3Ti1.7(PO4)3(LATP)是一种NASICON型固态电解质, 其离子电导率高、化学稳定性优良、剪切模量(40~60 GPa)高。然而含四价钛离子的LATP在与锂金属负极接触时和循环过程中容易发生还原反应, 进而导致电解质结构降解, 并在电解质中引入电子电导。为了提高LATP的化学和电化学稳定性, 本研究对LATP固态电解质表面进行了普鲁士蓝(PB)界面层的改性, 优化了电解质与负极之间的接触。引入具有丰富开框架锂离子扩散通道的PB作为电解质-负极界面的混合导电改性层, 具有如下优点。(1)锂化后PB层的本征电导率得到提升, 可以加速界面层电子向负极的均化传输。(2)锂化过程中PB中间层的亲锂性增强, 从而使电化学过程中LATP与锂金属之间的界面接触更加紧密。(3)锂化后PB仍然保持三维骨架结构, 有利于实现界面处锂离子通量的均质化效应, 从而提升锂沉积/剥离过程的稳定性。(4)具有金属有机框架(MOF)结构的PB可以保证循环过程中界面的机械稳定性, 并减小锂负极的体积变化。(5)PB结构在锂化后不发生坍塌, 不易出现相分离, 不会形成额外的相边界或相缝隙, 有利于集成锂离子流和电子流。(6)更为独特的是, PB的氧化还原电位高于界面两侧的锂金属和LATP, 有利于在Li-LATP之间形成能垒, 阻隔电子传输, 防止LATP的还原降解。改进后的固态电池具有良好的循环稳定性和动力学性能。在0.025 mA·cm-2电流密度下, PB改性的Li/Li对称固态电池可稳定循环800 h。PB改性的Li/LiFePO4固态电池在0.025 mA·cm-2电流密度下循环160圈后, 容量仍接近200 mAh·g-1。改性的Li/FeF3固态电池在0.025 mA·cm-2电流密度下可以保持高的库仑效率, 表明PB改性对电化学循环过程中产生的体积变化具有较好的容忍性。
中图分类号:
李勇锋, 顾玉萍, 师广照, 胡九林, 雷萌, 彭晖, 曾宇平, 李驰麟. NASICON型陶瓷固态电池的电化学电位界面调控[J]. 无机材料学报, 2025, 40(11): 1201-1211.
LI Yongfeng, GU Yuping, SHI Guangzhao, HU Jiulin, LEI Meng, PENG Hui, ZENG Yuping, LI Chilin. Interface Regulation of Electrochemical Potential in NASICON-type Ceramic Solid-state Batteries[J]. Journal of Inorganic Materials, 2025, 40(11): 1201-1211.
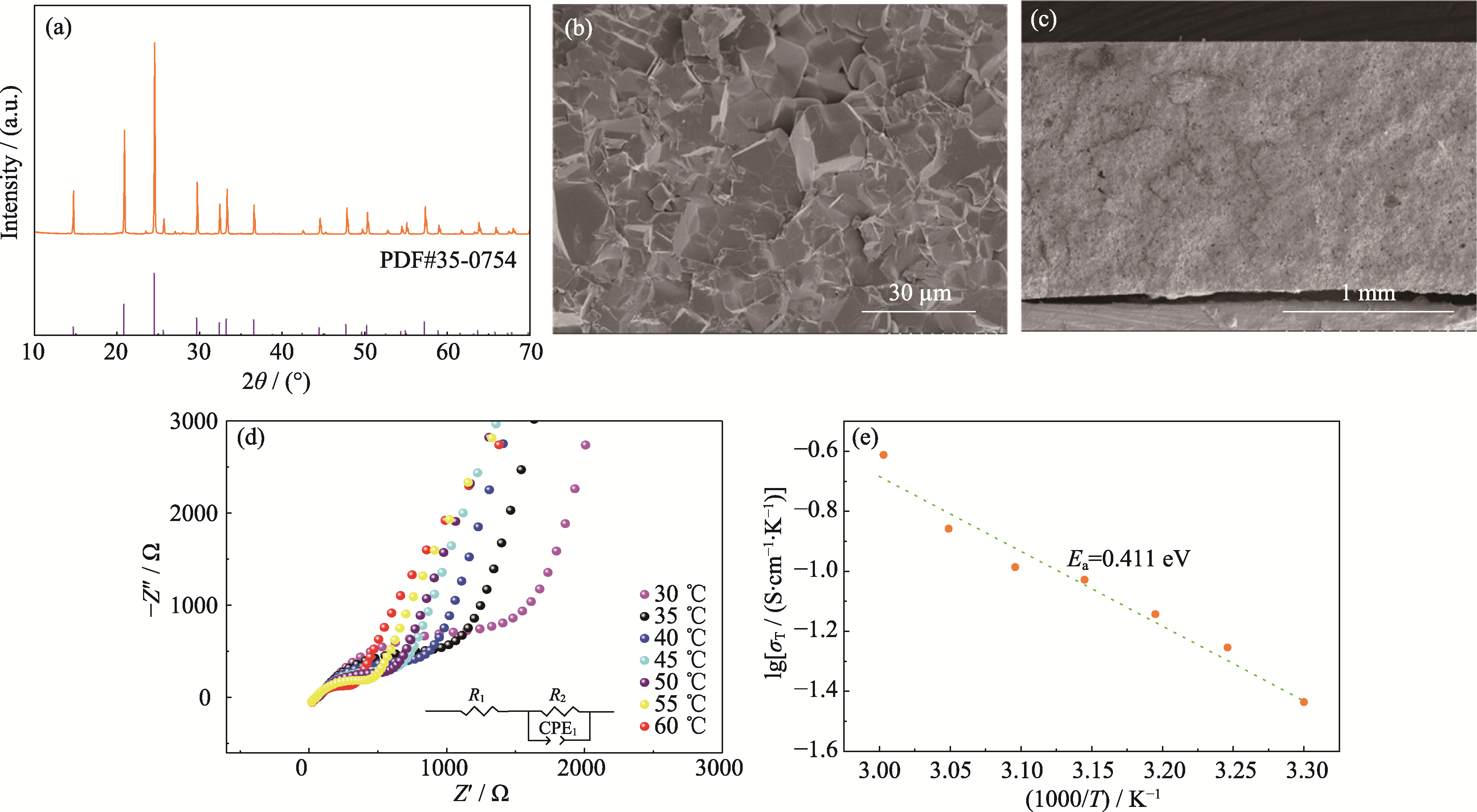
图1 LATP的基本特性
Fig. 1 Basic characteristics of LATP (a) XRD pattern of LATP; (b, c) Cross-sectional SEM images of LATP electrolyte; (d) AC impedance spectra of LATP at different temperatures and corresponding equivalent circuit; (e) Arrhenius plots based on LATP ionic conductivities
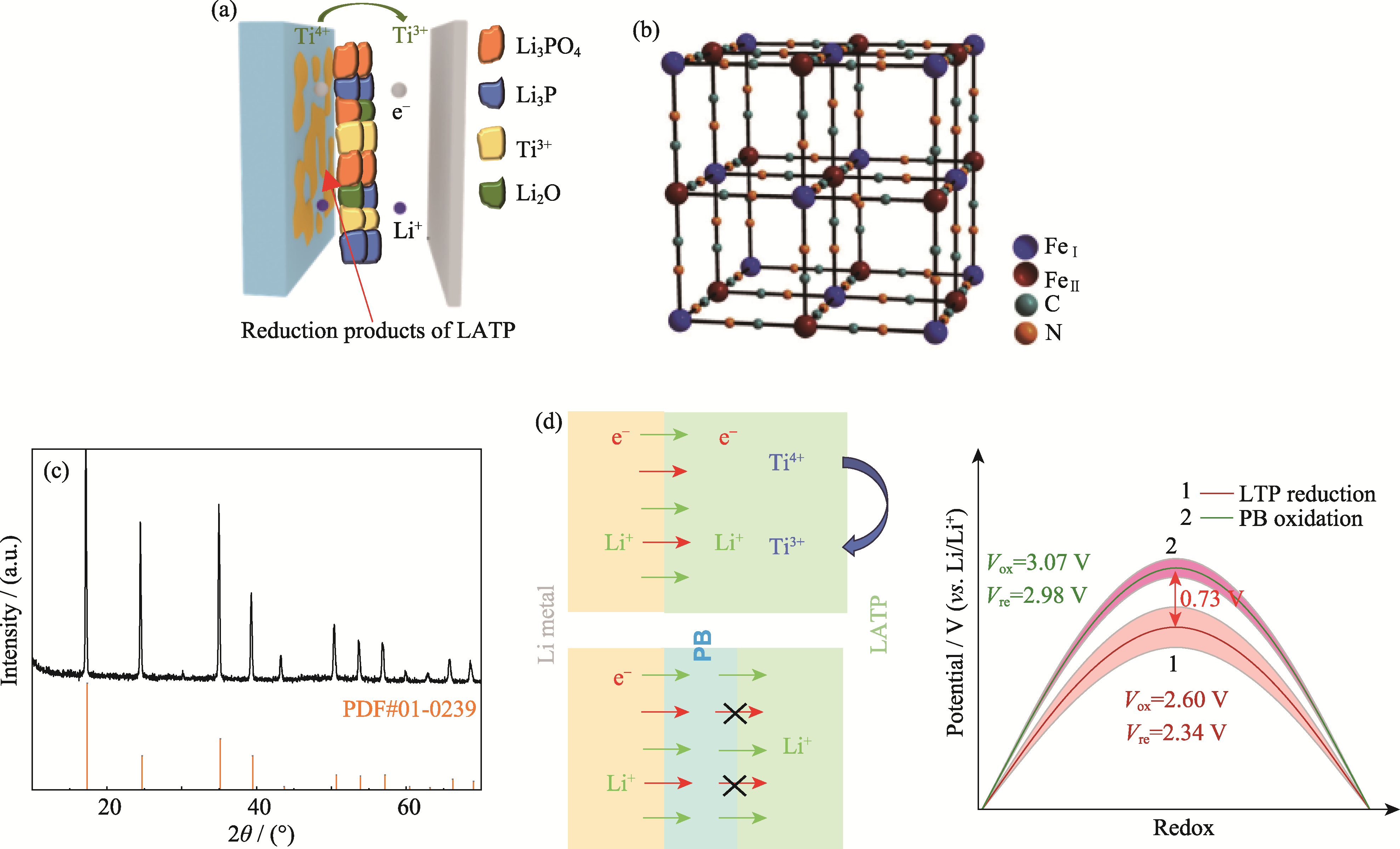
图2 PB基本特征及其改性机理
Fig. 2 Basic characteristics of PB and its modification mechanism (a) Schematic diagram of LATP interface reaction and corresponding products; (b) Schematic diagram of PB structure; (c) XRD pattern of PB; (d) Modulation mechanism of PB interface on Li-ion and electron flux
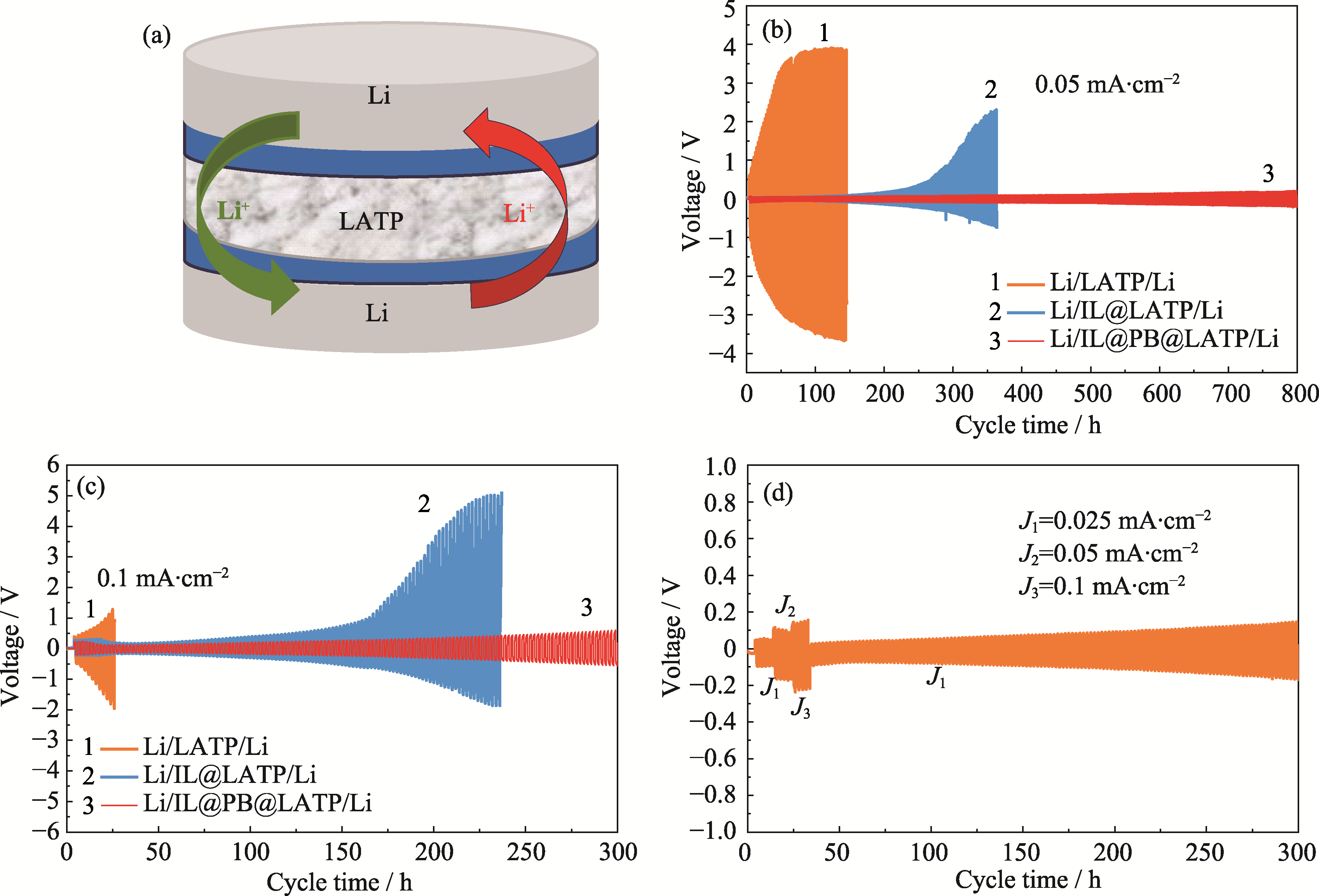
图3 对称电池的结构示意图及其电化学性能
Fig. 3 Schematic and electrochemical performance of symmetric cells (a) Schematic diagram of assembly of Li-Li symmetric cell; (b, c) Li plating and stripping voltage curves of symmetric cells of Li/LATP/Li, Li/IL@LATP/Li, and Li/IL@PB@LATP/Li at current densities of (b) 0.05 and (c) 0.1 mA·cm-2; (d) Li plating and stripping voltage curves of symmetric cell of Li/IL@PB@LATP/Li at different current densities
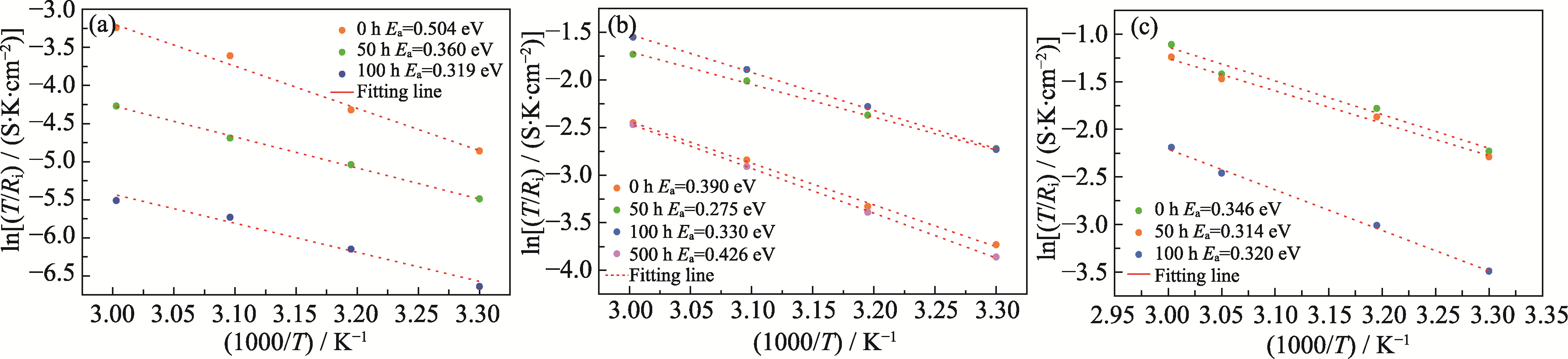
图4 基于不同温度下对称电池(a) Li/LATP/Li、(b) Li/IL@LATP/Li和(c) Li/IL@PB@LATP/Li循环不同时间后界面电阻的阿伦尼乌斯曲线
Fig. 4 Arrhenius curves based on interface resistances at different temperatures for symmetric cells of (a) Li/LATP/Li, (b) Li/IL@LATP/Li, and (c) Li/IL@PB@LATP/Li after different cycling time
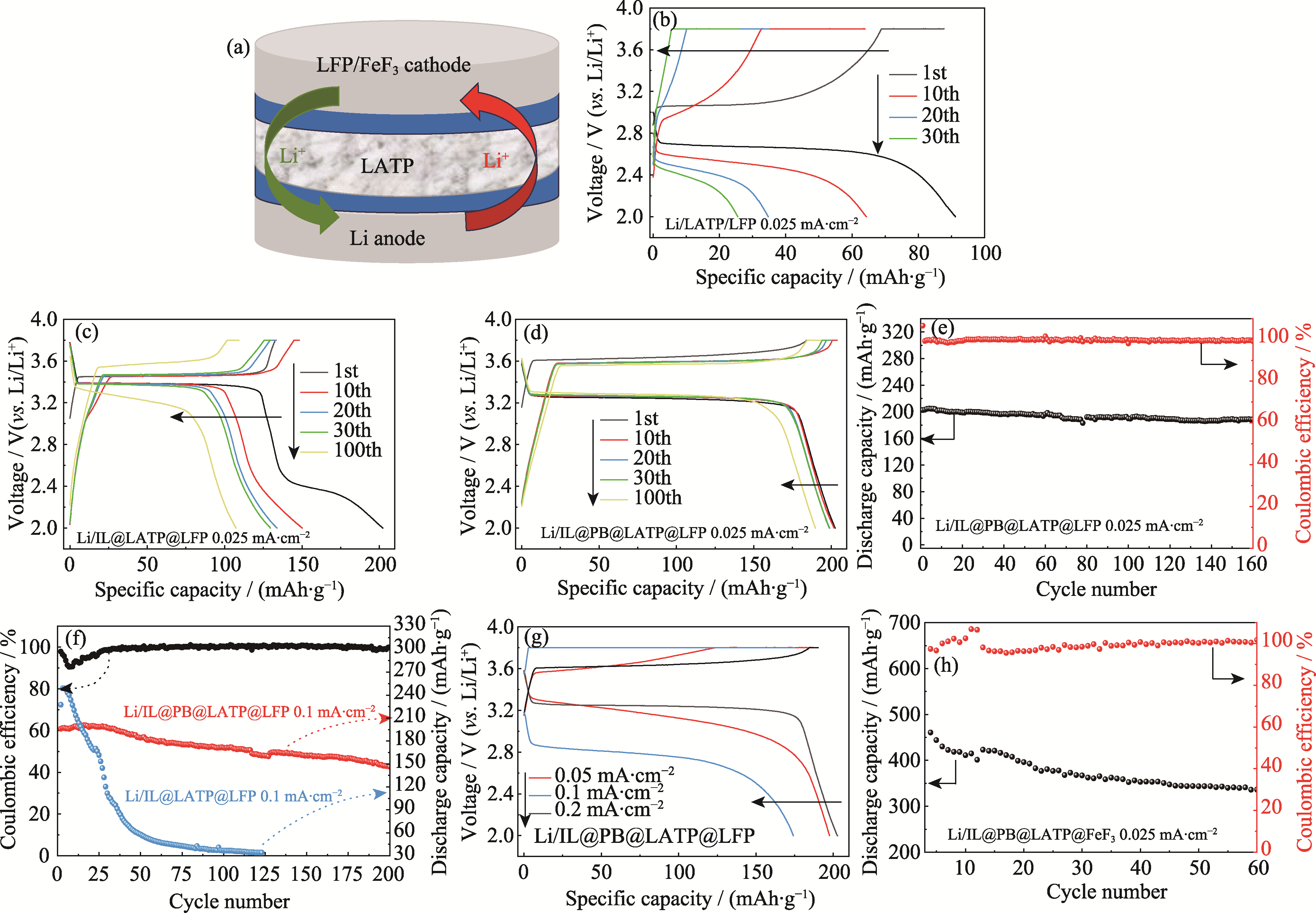
图5 Li//LFP和Li//FeF3全电池的结构示意图和不同条件下的电化学性能
Fig. 5 Schematic diagram of structure and electrochemical performance of Li//LFP and Li//FeF3 full cells under different conditions (a) Schematic diagram of Li//LFP or Li//FeF3 full cell; (b-d) Charging-discharging curves of (b) Li/LATP/LFP, (c) Li/IL@LATP/LFP, and (d) Li/IL@PB@LATP/LFP full cells at different cycle stages under a current density of 0.025 mA·cm-2; (e) Cycling performance of Li/IL@PB@LATP/LFP full cell at a current density of 0.025 mA·cm-2; (f) Cycling performance of Li/IL@LATP/LFP and Li/IL@PB@LATP/LFP full cells at a current density of 0.1 mA·cm-2, and Coulombic efficiency of Li/IL@PB@LATP/LFP; (g) Initial charging-discharging curves of Li/IL@PB@LATP/LFP cell at different current densities; (h) Cycling performance of Li/IL@PB@LATP/FeF3 full cell at 0.025 mA·cm-2
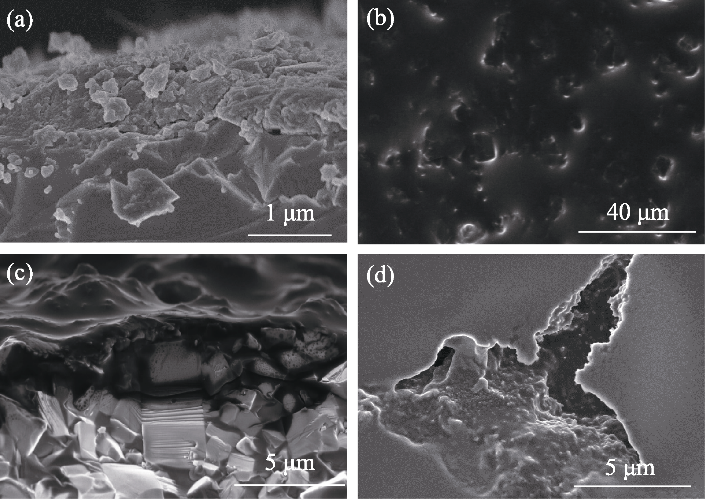
图6 不同条件下PB@LATP的SEM照片
Fig. 6 SEM images of PB@LATP under different conditions (a) Cross-sectional SEM image of PB-modified solid electrolyte LATP; (b) Surface and (c) cross-sectional SEM images of LATP for Li/IL@PB@LATP/LFP full cell after 50 cycles at a current density of 0.05 mA·cm-2; (d) SEM image of LATP surface from Li/IL@LATP/LFP full cell after 50 cycles at a current density of 0.05 mA·cm-2
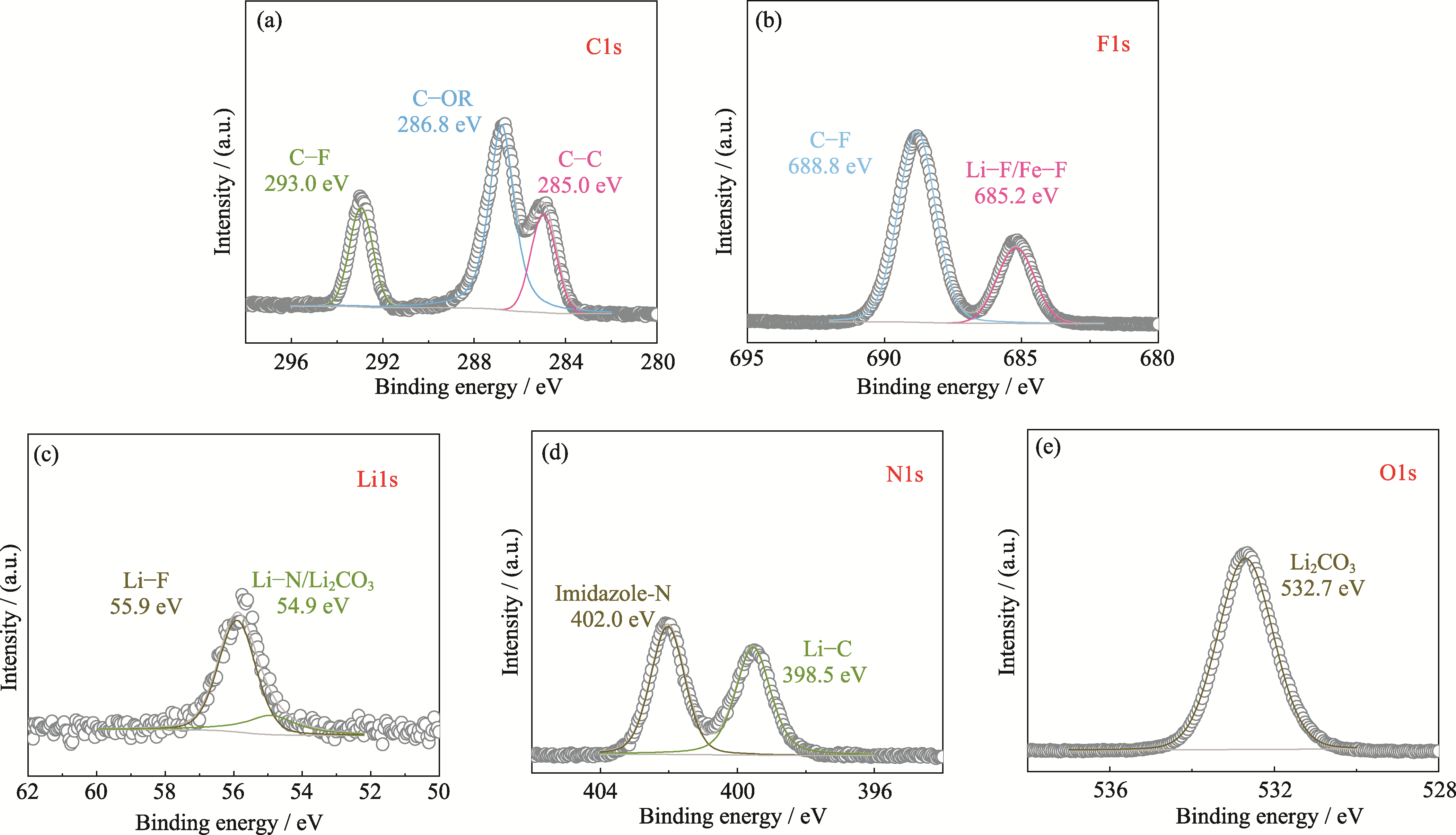
图7 Li/IL@PB@LATP/Li对称电池在0.05 mA·cm-2电流密度下循环50圈后LATP表面的XPS谱图
Fig. 7 XPS spectra of LATP surface of symmetric cell Li/IL@PB@LATP/Li after 50 cycles at 0.05 mA·cm-2 (a) C1s; (b) F1s; (c) Li1s; (d) N1s; (e) O1s
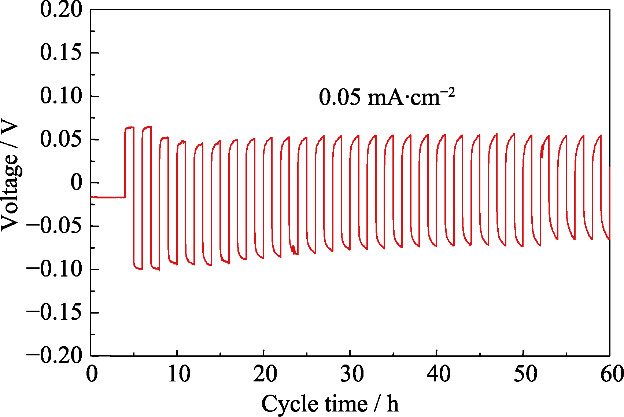
图S1 对称电池Li/IL@PB@LATP/Li在0.05 mA·cm-2电流密度下的锂金属沉积和剥离循环的电压曲线
Fig. S1 Li plating and stripping voltage curves of symmetric cell of Li/IL@PB@LATP/Li at a current density of 0.05 mA·cm-2
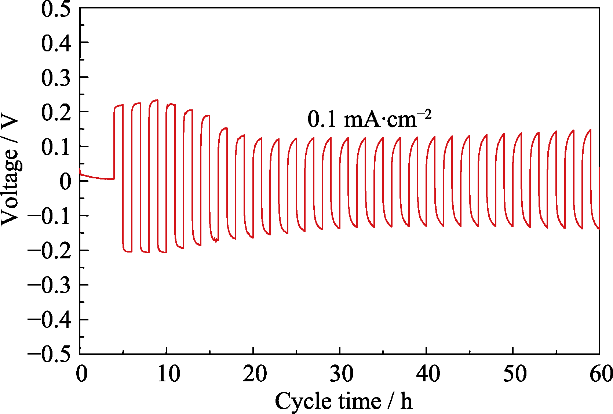
图S2 Li/IL@PB@LATP/Li对称电池在0.1 mA·cm-2电流密度下的锂金属沉积和剥离循环的电压曲线
Fig. S2 Li plating and stripping voltage curves of symmetric cell of Li/IL@PB@LATP/Li at a current density of 0.1 mA·cm-2
| [1] |
LIU B, ZHANG J G, XU W. Advancing lithium metal batteries. Joule, 2018, 2(5): 833.
DOI URL |
| [2] |
LI C, CHEN K, ZHOU X, et al. Electrochemically driven conversion reaction in fluoride electrodes for energy storage devices. npj Computational Materials, 2018, 4: 22.
DOI |
| [3] |
YU Y, LAI C, LEI M, et al. Dual strategies of mild C-F scissoring fluorination and local high-concentration electrolyte to enable reversible Li-Fe-F conversion batteries. Materials Horizons, 2024, 11: 2169.
DOI URL |
| [4] |
GU Y, HU J, LEI M, et al. Electrochemical activation of ordered mesoporous solid electrolyte interphases to enable ultra-stable lithium metal batteries. Advanced Energy Materials, 2024, 14(4): 2302174.
DOI URL |
| [5] | WU H, HU J, YU S, et al. Heterostructure conductive interface and melt-penetration-bonding process to afford all-solid-state Li-FeF3 garnet batteries with high cathode loading. Energy & Environmental Science, 2025, 18(2): 923. |
| [6] |
LEE Y, FUJIKI S, JUNG C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nature Energy, 2020, 5: 299.
DOI |
| [7] |
NIE X, LEI M, HU J, et al. Boosting Li-ion conductivity of fluoride solid electrolyte by low-temperature molten salt ablation and particle boundary doping. ACS Nano, 2024, 18(43): 30099.
DOI PMID |
| [8] |
HU J, LEI M, ZHU C, et al. Highly conductive doped fluoride solid electrolytes with solidified ionic liquid to enable reversible FeF3conversion solid state batteries. Advanced Functional Materials, 2024, 34(41): 2314044.
DOI URL |
| [9] |
XIAO Y, WANG Y, BO S, et al. Understanding interface stability in solid-state batteries. Nature Reviews Materials, 2020, 5: 105.
DOI |
| [10] | YU Q, HU J, NIE X, et al. Liquid metal mediated heterostructure fluoride solid electrolytes of high conductivity and air stability for sustainable Na metal batteries. ACS Nano, 2024, 18(7): 5790. |
| [11] |
CHEN R, LI Q, YU X, et al. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chemical Reviews, 2020, 120(14): 6820.
DOI PMID |
| [12] |
YU Q, XU Y, HU J, et al. Heterostructured fluoride-based solid electrolytes engineered by grain boundary softening and bonding for sustainable Na metal batteries. Energy Storage Materials, 2024, 73: 103795.
DOI URL |
| [13] |
LIU Y, LEI M, LAI C, et al. Enable high reversibility of Fe/Cu based fluoride conversion batteries via interfacial gas release and detergency of garnet electrolytes. Materials Today, 2022, 61: 65.
DOI URL |
| [14] |
LIU Y, MENG J, LEI M, et al. Alloyable viscous fluid for interface welding of garnet electrolyte to enable highly reversible fluoride conversion solid state batteries. Advanced Functional Materials, 2023, 33(4): 2208013.
DOI URL |
| [15] |
ZHANG Y, MENG J, CHEN K, et al. Garnet-based solid-state lithium fluoride conversion batteries benefiting from eutectic interlayer of superior wettability. ACS Energy Letters, 2020, 5(4): 1167.
DOI URL |
| [16] | ZHANG Y, MENG J, CHEN K, et al. Behind the candelabra: a facile flame vapor deposition method for interfacial engineering of garnet electrolyte to enable ultralong cycling solid-state Li-FeF3 conversion batteries. ACS Applied Materials & Interfaces, 2020, 12(30): 33729. |
| [17] |
ZENG Y, ZHANG Y, HU J, et al. Garnet-based solid state batteries benefitting from anionic/electronic mixed conductive interface constructed by lithiation of porous FeS2. APL Energy, 2024, 2: 026104.
DOI URL |
| [18] |
LEI M, WU X, LIU Y, et al. Polymer electrolytes reinforced by 2D fluorinated filler for all-solid-state Li-Fe-F conversion-type lithium metal batteries. Nano Research, 2023, 16: 8469.
DOI |
| [19] |
AONO H, SUGIMOTO E, SADAOKA Y, et al. Ionic conductivity of the lithium titanium phosphate (Li1+XMXTi2-X(PO4)3, M=Al, Sc, Y and La) systems. Journal of the Electrochemical Society, 1989, 136(2): 590.
DOI |
| [20] | ZHANG Z, SHAO Y, LOTSCH B V, et al. New horizons for inorganic solid state ion conductors. Energy & Environmental Science, 2018, 11: 1945. |
| [21] |
ZHANG B, TAN R, YANG L, et al. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Materials, 2018, 10: 139.
DOI URL |
| [22] |
YANG Q, Li C. Li metal batteries and solid state batteries benefiting from halogen-based strategies. Energy Storage Materials, 2018, 14: 100.
DOI URL |
| [23] |
HARTMANN P, LEICHTWEISS T, BUSCHE M R, et al. Degradation of NASICON-type materials in contact with lithium metal: formation of mixed conducting interphases (MCI) on solid electrolytes. The Journal of Physical Chemistry C, 2013, 117(41): 21064.
DOI URL |
| [24] |
WANG S, XU H, LI W, et al. Interfacial chemistry in solid-state batteries: formation of interphase and its consequences. Journal of the American Chemical Society, 2018, 140(1): 250.
DOI PMID |
| [25] |
ZHENG F, SONG Z, LI H, et al. Distinct functional Janus interfaces for dendrite-free Li1.3Al0.3Ti1.7(PO4)3-based lithium metal batteries. Electrochimica Acta, 2022, 436: 141395.
DOI URL |
| [26] |
HUANG C, LI Z, DUAN S, et al. Improving the stability of NASICON-type electrolyte with Li metal anode by interfacial modification. Journal of Power Sources, 2022, 536: 231491.
DOI URL |
| [27] |
GU Y, HU J, LAI C, et al. NASICON-based solid state Li-fluoride conversion batteries enabled by constructing a fluorine-rich trap for Ti4+. Advanced Energy Materials, 2023, 13(12): 2203679.
DOI URL |
| [28] |
LEI M, FAN S, YU Y, et al. NASICON-based solid state Li-Fe-F conversion batteries enabled by multi-interface-compatible sericin protein buffer layer. Energy Storage Materials, 2022, 47: 551.
DOI URL |
| [29] |
WU C, HU J, YANG Q, et al. Open framework perovskite derivate SEI with fluorinated heterogeneous nanodomains for practical Li-metal pouch cells. Nano Energy, 2023, 113: 108523.
DOI URL |
| [30] |
PAOLELLA A, FAURE C, TIMOSHEVSKII V, et al. A review on hexacyanoferrate-based materials for energy storage and smart windows: challenges and perspectives. Journal of Materials Chemistry A, 2017, 5: 18919.
DOI URL |
| [31] |
ZHANG B, ZHANG Y, LEI M, et al. Lithiation-induced conductivity modulation in Prussian blue interlayer for stable Li/garnet solid-state batteries. Applied Physics Letters, 2023, 122(3): 033901.
DOI URL |
| [32] |
CHEN K, QIU W, LEI M, et al. Manipulating cation-anion coordination in fire-retardant electrolytes to enable high-areal- capacity fluoride conversion batteries. Matter, 2024, 7(11): 3907.
DOI URL |
| [33] | CHEN K, LEI M, YAO Z, et al. Construction of solid-liquid fluorine transport channel to enable highly reversible conversion cathodes. Science Advances, 2021, 7(45): abj1491. |
| [34] |
WU X, ZHENG Y, LI W, et al. Solid electrolytes reinforced by infinite coordination polymer nano-network for dendrite-free lithium metal batteries. Energy Storage Materials, 2023, 41: 436.
DOI URL |
| [35] |
HU J, LAI C, CHEN K, et al. Dual fluorination of polymer electrolyte and conversion-type cathode for high-capacity all-solid- state lithium metal batteries. Nature Communications, 2022, 13: 7914.
DOI |
| [1] | 文伸豪, 彭德招, 林喆与, 郭霞, 黄培鑫, 章志珍. 基于LLZTO电解质的固态锂金属电池负极界面调控[J]. 无机材料学报, 2025, 40(9): 1013-1021. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 王琨鹏, 刘兆林, 林存生, 王治宇. 基于低含水量普鲁士蓝正极的准固态钠离子电池[J]. 无机材料学报, 2024, 39(9): 1005-1012. |
| [4] | 于业帆, 徐玲, 倪忠斌, 施冬健, 陈明清. 普鲁士蓝/生物炭材料的制备及其氨氮吸附机理[J]. 无机材料学报, 2023, 38(2): 205-212. |
| [5] | 谭淑雨, 刘晓宁, 毕志杰, 万勇, 郭向欣. 正极包覆与界面修饰: 双策略改善聚氧化乙烯固态电解质对高电压正极稳定性[J]. 无机材料学报, 2023, 38(12): 1466-1474. |
| [6] | 杜邱静, 刘天智, 陈菊锋, 陈航榕. 普鲁士蓝基HClO荧光纳米探针及其对肿瘤细胞的特异性检测[J]. 无机材料学报, 2023, 38(1): 55-61. |
| [7] | 张家强, 邹馨蕾, 王能泽, 贾春阳. 两步电沉积法制备Zn-Fe PBA薄膜及其在电致变色器件中的性能研究[J]. 无机材料学报, 2022, 37(9): 961-968. |
| [8] | 夏求应, 孙硕, 昝峰, 徐璟, 夏晖. 非晶LiSiON薄膜电解质的全固态薄膜锂电池性能[J]. 无机材料学报, 2022, 37(2): 230-236. |
| [9] | 李栋, 雷超, 赖华, 刘小林, 姚文俐, 梁彤祥, 钟盛文. 全固态锂离子电池正极与石榴石型固体电解质界面的研究进展[J]. 无机材料学报, 2019, 34(7): 694-702. |
| [10] | 李勇, 何玮鑫, 郑芯月, 于胜兰, 李海同, 黎弘毅, 张蓉, 王雨. 水系钠离子电池普鲁士蓝正极材料的制备与电化学性能研究[J]. 无机材料学报, 2019, 34(4): 365-372. |
| [11] | 杜付明, 赵宁, 方锐, 崔忠慧, 李忆秋, 郭向欣. 电子导电剂对石榴石基固态锂电池循环性能的影响[J]. 无机材料学报, 2018, 33(4): 462-468. |
| [12] | 卢东亮, 代广周, 姚英邦, 陶涛, 梁波, 鲁圣国. 煅烧温度对Li0.33La0.56TiO3固态离子电容器性能的影响[J]. 无机材料学报, 2018, 33(10): 1077-1082. |
| [13] | 徐顺建, 罗玉峰, 钟 炜, 肖宗湖, 罗永平, 欧 惠 . 纤维状纸炭对电极构造的准固态染料敏化太阳电池[J]. 无机材料学报, 2015, 30(1): 29-34. |
| [14] | 章志珍, 施思齐, 胡勇胜, 陈立泉. 溶胶-凝胶法制备钠离子固态电解质Na3Zr2Si2PO12及其电导性能研究[J]. 无机材料学报, 2013, 28(11): 1255-1260. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||