无机材料学报 ›› 2023, Vol. 38 ›› Issue (8): 938-946.DOI: 10.15541/jim20220741 CSTR: 32189.14.10.15541/jim20220741
所属专题: 【能源环境】储能电池(202506)
王新玲1( ), 周娜1, 田亚文1, 周明冉1, 韩静茹1, 申远升1, 胡执一1,2, 李昱1,2(
), 周娜1, 田亚文1, 周明冉1, 韩静茹1, 申远升1, 胡执一1,2, 李昱1,2( )
)
收稿日期:2022-12-07
修回日期:2023-03-27
出版日期:2023-04-11
网络出版日期:2023-04-11
通讯作者:
李 昱, 教授. E-mail: yu.li@whut.edu.cn作者简介:王新玲(1997-), 女, 硕士研究生. E-mail: wangxinling_whut@163.com
基金资助:
WANG Xinling1( ), ZHOU Na1, TIAN Yawen1, ZHOU Mingran1, HAN Jingru1, SHEN Yuansheng1, HU Zhiyi1,2, LI Yu1,2(
), ZHOU Na1, TIAN Yawen1, ZHOU Mingran1, HAN Jingru1, SHEN Yuansheng1, HU Zhiyi1,2, LI Yu1,2( )
)
Received:2022-12-07
Revised:2023-03-27
Published:2023-04-11
Online:2023-04-11
Contact:
LI Yu, professor. E-mail: yu.li@whut.edu.cnAbout author:WANG Xinling (1997-), female, Master candidate. E-mail: wangxinling_whut@163.com
Supported by:摘要:
锂硫电池(LSBs)因能量密度高、原料储量丰富、环境友好等优点引起了广泛关注。然而, 多硫化物的穿梭效应、反应过程中较大的体积膨胀以及硫较差的电子电导率等缺点极大地限制了其发展。本研究设计了一种SnS2纳米颗粒与ZIF-8衍生的花状二维多孔碳纳米片/硫复合材料(ZCN-SnS2-S), 并研究了其作为锂硫电池正极的电化学性能。其独特的二维花状多孔结构不仅有效缓解了反应过程中的体积膨胀, 而且为Li+和电子的传输提供了快速通道, 杂原子N也促进了对多硫化物的吸附作用。并且负载的极性SnS2纳米颗粒极大地增强了对多硫化物的吸附, 从而使ZCN-SnS2-S复合材料表现出优异的电化学性能。在0.2C(1C=1675 mA·g-1)电流密度下, ZCN-SnS2-S电极循环100次后仍能保持948 mAh·g-1的高可逆比容量, 容量保持率为83.7%。即使在2C的高电流密度下循环300圈, ZCN-SnS2-S电极仍具有546 mAh·g-1的可逆比容量。
中图分类号:
王新玲, 周娜, 田亚文, 周明冉, 韩静茹, 申远升, 胡执一, 李昱. SnS2/ZIF-8衍生二维多孔氮掺杂碳纳米片复合材料的锂硫电池性能研究[J]. 无机材料学报, 2023, 38(8): 938-946.
WANG Xinling, ZHOU Na, TIAN Yawen, ZHOU Mingran, HAN Jingru, SHEN Yuansheng, HU Zhiyi, LI Yu. SnS2/ZIF-8 Derived Two-dimensional Porous Nitrogen-doped Carbon Nanosheets for Lithium-sulfur Batteries[J]. Journal of Inorganic Materials, 2023, 38(8): 938-946.

图1 (a)ZCN-SnS2复合材料合成示意图; (b, e)ZIF-8、(c, f)ZCN和(d, g)ZCN-SnS2的FESEM照片
Fig. 1 (a) Schematics of synthetic process of ZCN-SnS2, and FESEM images of (b, e) ZIF-8, (c, f) ZCN and (d, g) ZCN-SnS2 Colorful figures are available on website
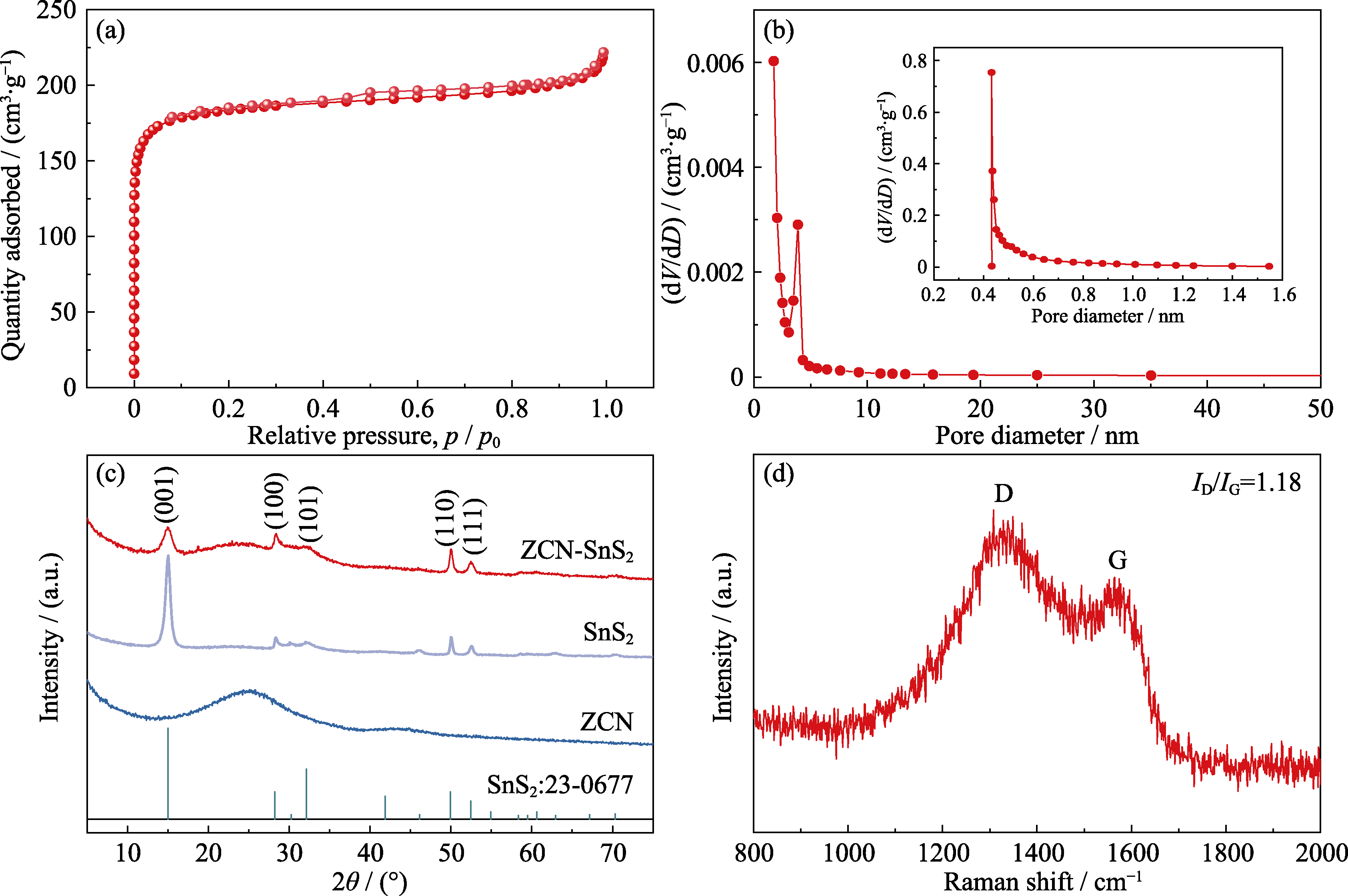
图2 ZCN样品的(a)氮气吸/脱附等温线与(b)介孔孔径分布图(插图为微孔孔径分布图); (c)ZCN-SnS2、ZCN和SnS2样品的XRD图谱; (d)ZCN样品的拉曼光谱图
Fig. 2 (a) N2 adsorption-desorption isotherm of ZCN; (b) mesoporous pore size distribution curve of ZCN with inset showing micropore pore size distribution; (c) XRD patterns of ZCN-SnS2, ZCN and SnS2; (d) Raman spectrum of ZCN
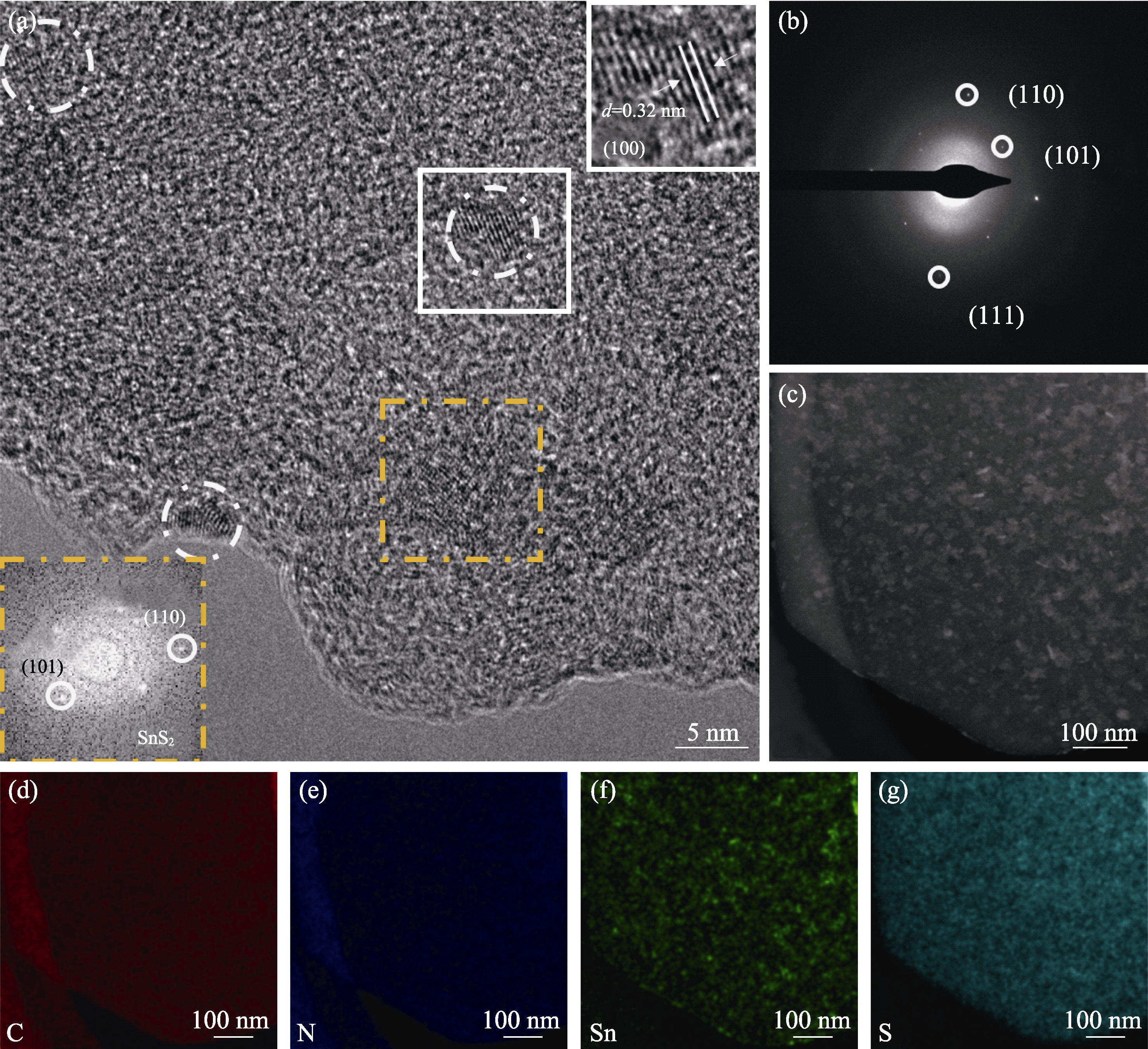
图3 ZCN-SnS2样品的(a)HRTEM, (b)SAED, (c)HAADF-STEM照片和(d~g)EDX元素分布图
Fig. 3 (a) HRTEM, (b) SAED, (c) HAADF-STEM images, and (d-g) corresponding EDX elemental mappings of ZCN-SnS2
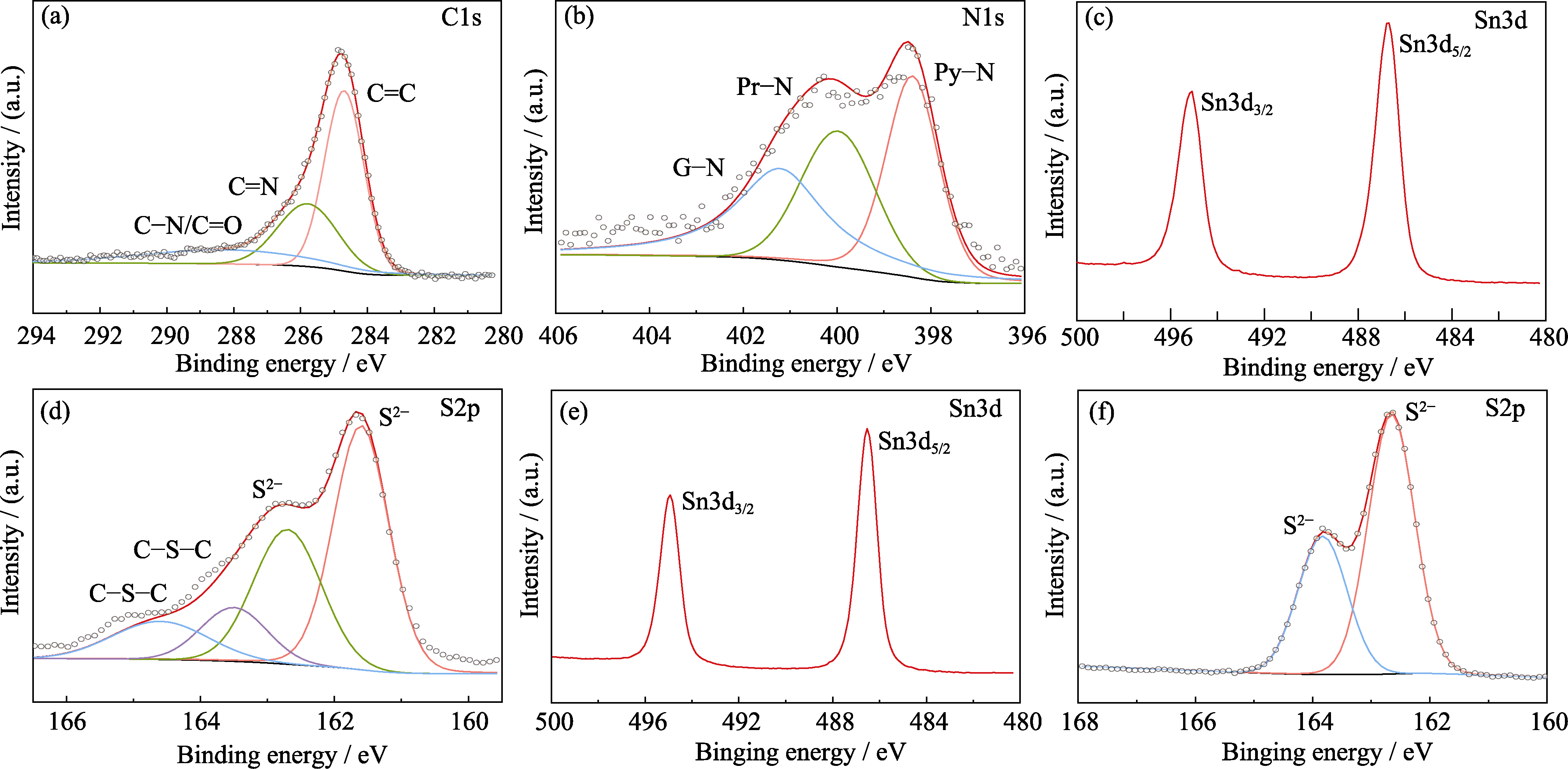
图4 ZCN-SnS2的高分辨(a)C1s, (b)N1s, (c)Sn3d, (d)S2p XPS光谱图; SnS2的高分辨(e)Sn3d, (f)S2p XPS光谱图
Fig. 4 (a) C1s, (b) N1s, (c) Sn3d, (d) S2p XPS spectra of ZCN-SnS2, and (e) Sn3d, (f) S2p XPS spectra of SnS2
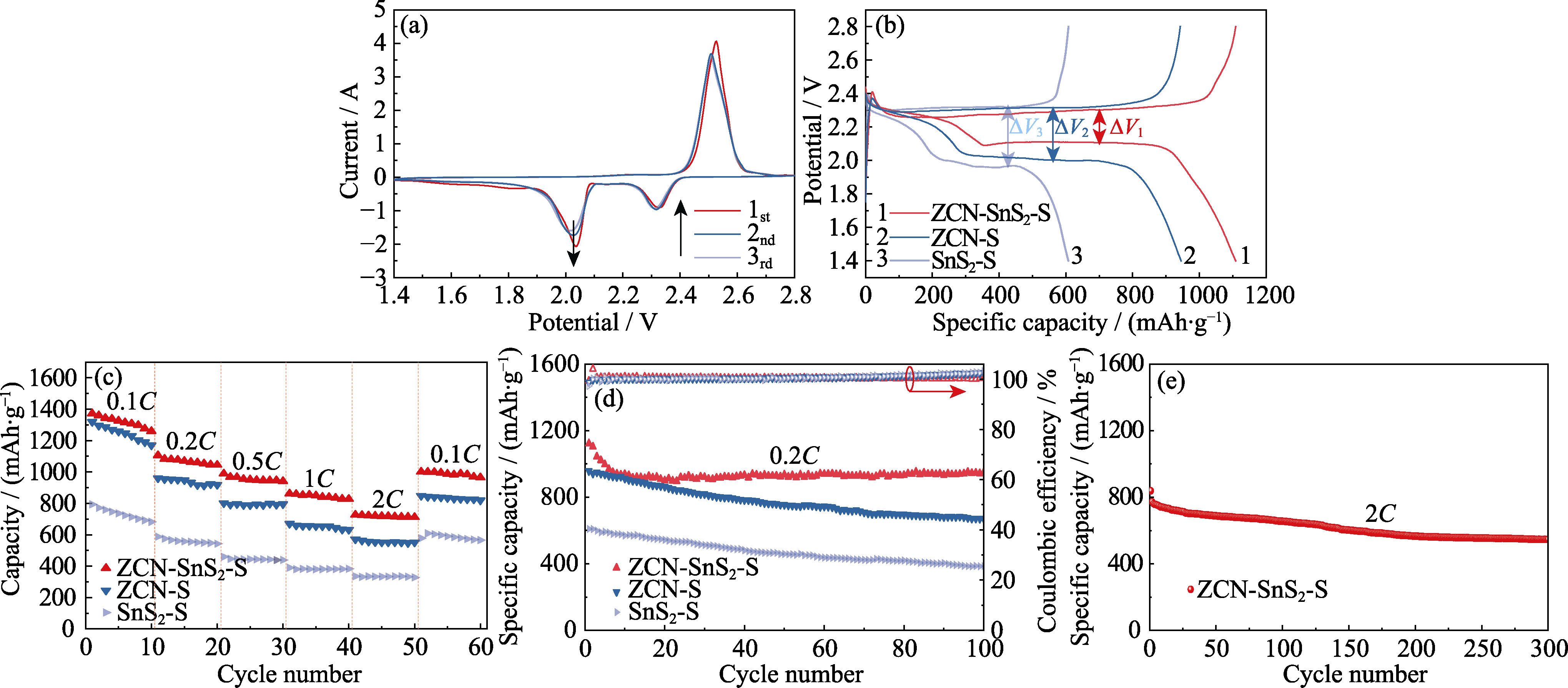
图5 (a)以ZCN-SnS2-S为电极的锂硫电池的CV曲线; 以ZCN-SnS2-S、ZCN-S、SnS2-S为电极的锂硫电池的(b)充放电曲线, (c)倍率性能和(d)0.2C下的循环性能; (e)ZCN-SnS2-S电极在2C下的循环性能
Fig. 5 (a) CV curves of Li-S battery with ZCN-SnS2-S electrode; (b) Charge-discharge curves, (c) rate performances and (d) cycling performances at 0.2C of Li-S batteries with ZCN-SnS2-S, ZCN-S and SnS2-S electrodes; (e) Cycling performance of ZCN-SnS2-S at 2C
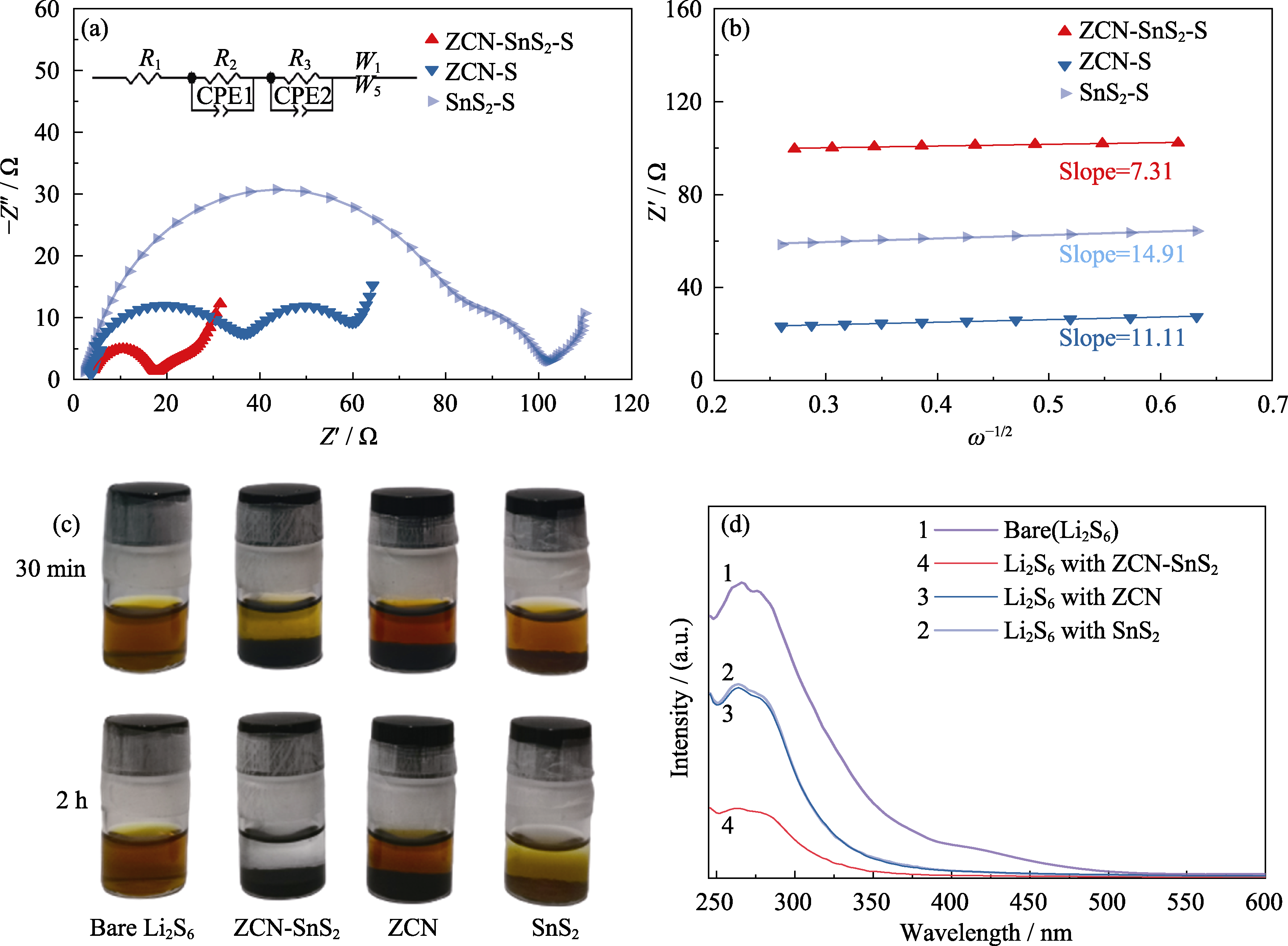
图6 ZCN-SnS2-S、ZCN-S和SnS2-S电极的(a)阻抗谱图与(b)在较低的角频率下, 阻抗的实部和倒数指数(-1/2)之间的线性关系图; ZCN-SnS2、ZCN和SnS2样品的(c)Li2S6吸附实验照片与(d)UV-Vis吸收光谱图
Fig. 6 (a) EIS plots and (b) linear relationships between the real parts of the impedance and the reciprocal exponentials (-1/2) at the lower angular frequency of ZCN-SnS2-S, ZCN-S and SnS2-S, respectively; (c) Li2S6 adsorption plots and (d) UV-Vis absorption spectra of ZCN-SnS2, ZCN and SnS2
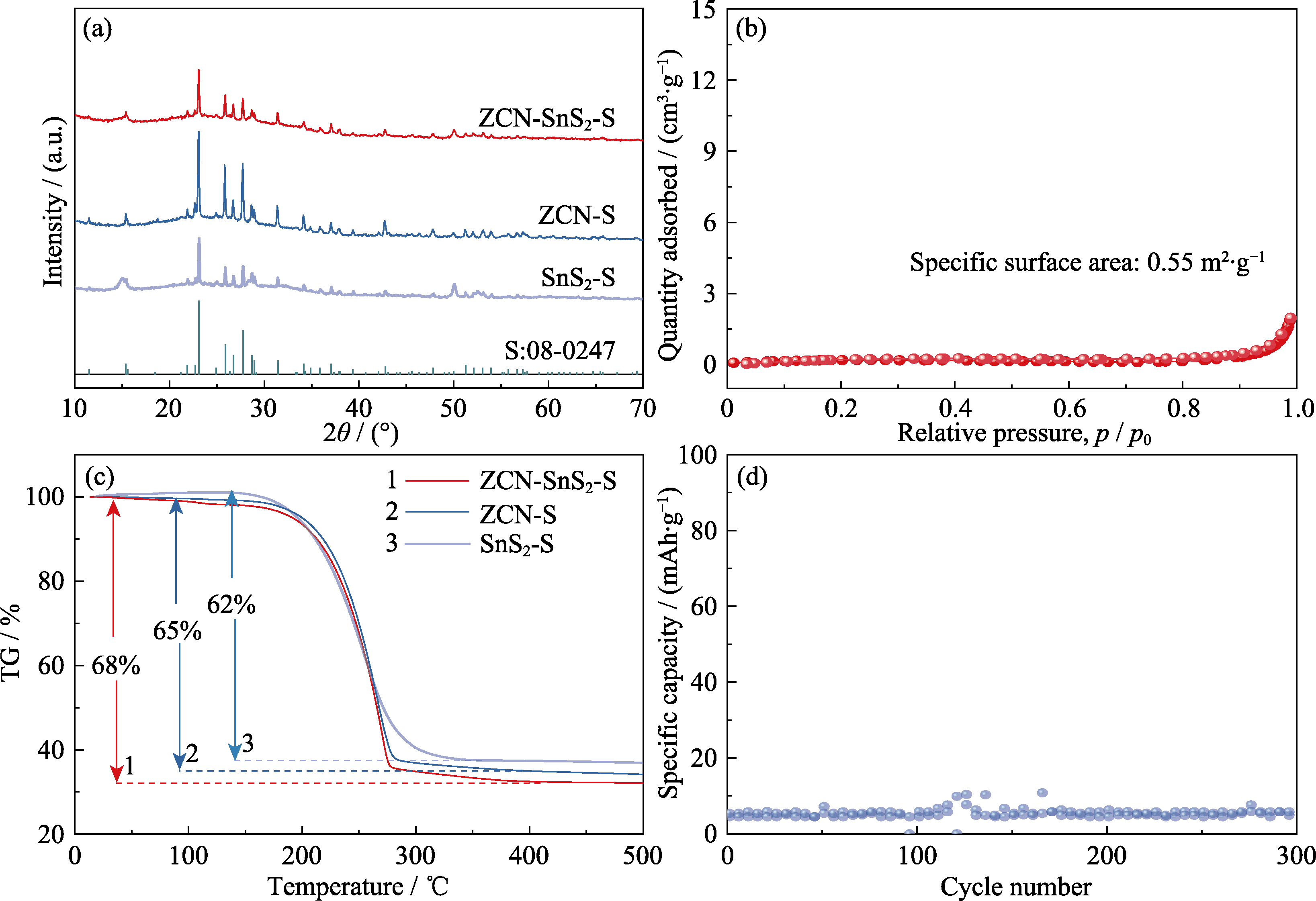
图S5 (a)ZCN-SnS2-S、ZCN-S、SnS2-S样品的XRD图谱, (b)ZCN-SnS2-S样品的氮气吸/脱附等温线, (c)ZCN-SnS2-S、ZCN-S、SnS2-S样品的TGA曲线, (d)SnS2样品在1C下的循环性能。
Fig. S5 (a) XRD patterns of ZCN-SnS2-S, ZCN-S, SnS2-S, (b) nitrogen adsorption-desorption isotherm of ZCN-SnS2-S, (c) TGA curves of ZCN-SnS2-S, ZCN-S and SnS2-S, and (d) cycling performance of SnS2 at 1C
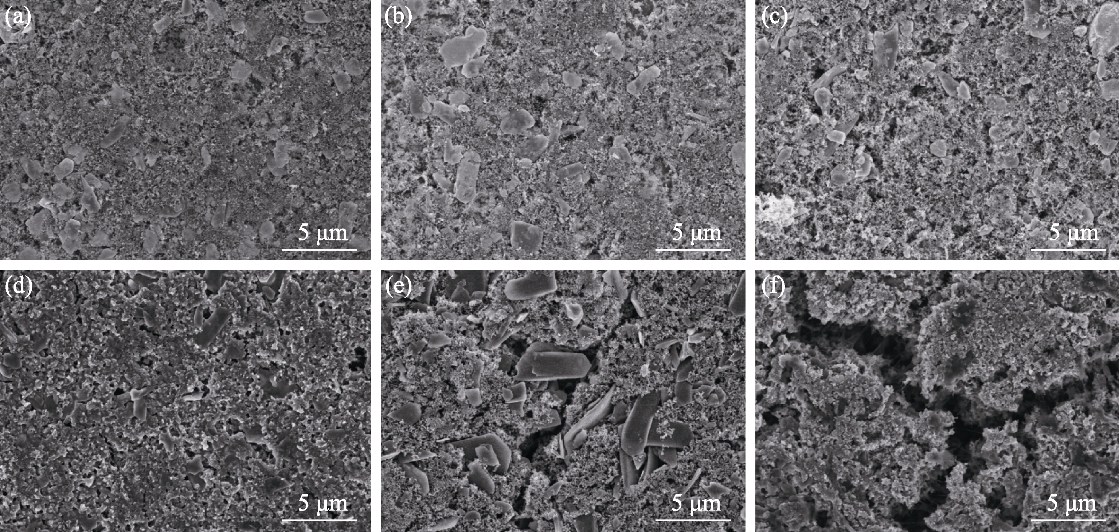
图S7 0.5C电流密度下循环150圈前后电极材料的FESEM照片
Fig. S7 FESEM images of electrode materials before and after 150 cycles at 0.5C current density (a, d) ZCN-SnS2-S; (b, e) ZCN-S; (c, f) SnS2-S
| Host materials | Current rate | Cycle number | Reversible capacity/(mAh·g-1) | Ref. |
|---|---|---|---|---|
| ZCN-SnS2-S | 0.2C | 100 | 948 | This work |
| ZCN-SnS2-S | 2.0C | 300 | 546 | This work |
| SnS2/CNTs/S | 0.1C | 100 | 1002.3 | [ |
| NG/SnS2/TiO2-S | 0.2C | 100 | 739 | [ |
| SnS2@N-CNFs | 0.2C | 150 | 889 | [ |
| NHCS-SnS2/S | 1.0C | 200 | 634 | [ |
| PCN-SnS2-S | 2.0C | 150 | 650 | [ |
表S1 基于SnS2复合材料的电化学性能比较
Table S1 Comparison of electrochemical properties of SnS2 composites
| Host materials | Current rate | Cycle number | Reversible capacity/(mAh·g-1) | Ref. |
|---|---|---|---|---|
| ZCN-SnS2-S | 0.2C | 100 | 948 | This work |
| ZCN-SnS2-S | 2.0C | 300 | 546 | This work |
| SnS2/CNTs/S | 0.1C | 100 | 1002.3 | [ |
| NG/SnS2/TiO2-S | 0.2C | 100 | 739 | [ |
| SnS2@N-CNFs | 0.2C | 150 | 889 | [ |
| NHCS-SnS2/S | 1.0C | 200 | 634 | [ |
| PCN-SnS2-S | 2.0C | 150 | 650 | [ |
| [1] |
SUN Y K. Direction for development of next-generation lithium-ion batteries. ACS Energy Letters, 2017, 2(12): 2694.
DOI URL |
| [2] |
SU N, HAN J, GUO Y, et al. ZIF-8-derived three-dimensional silicon-carbon network composite for high-performance lithium- ion batteries. Journal of Inorganic Materials, 2022, 37(9): 1016.
DOI URL |
| [3] | ZHANG Q, HUANG Q, HAO S M, et al. Polymers in lithium- sulfur batteries. Advanced Science, 2022, 9(2): 2103798. |
| [4] | ZHANG Z, FANG Z, XIANG Y, et al. Cellulose-based material in lithium-sulfur batteries: a review. Carbohydrate Polymers, 2021, 255: 117469. |
| [5] |
WU P, SUN M H, YU Y, et al. Physical and chemical dual-confinement of polysulfides within hierarchically meso- microporous nitrogen-doped carbon nanocages for advanced Li-S batteries. RSC Advances, 2017, 7(68): 42627.
DOI URL |
| [6] |
HUA W, YANG Z, NIE H, et al. Polysulfide-scission reagents for the suppression of the shuttle effect in lithium-sulfur batteries. ACS Nano, 2017, 11(2): 2209.
DOI URL |
| [7] |
ZHANG Y, LIU X, WU L, et al. A flexible, hierarchically porous PANI/MnO2 network with fast channels and an extraordinary chemical process for stable fast-charging lithium-sulfur batteries. Journal of Materials Chemistry A, 2020, 8(5): 2741.
DOI URL |
| [8] |
HAN F, YUE J, FAN X, et al. High-performance all-solid-state lithium-sulfur battery enabled by a mixed-conductive Li2S nanocomposite. Nano Letters, 2016, 16(7): 4521.
DOI URL |
| [9] | YU Y, YAN M, DONG W D, et al. Optimizing inner voids in yolk- shell TiO2 nanostructure for high-performance and ultralong-life lithium-sulfur batteries. Chemical Engineering Journal, 2021, 417: 129241. |
| [10] |
TANG W, ZHANG Y, ZHONG W, et al. A labyrinth-like network electrode design for lithium-sulfur batteries. Nanoscale, 2019, 11(31): 14648.
DOI URL |
| [11] |
YAN M, ZHANG Y, LI Y, et al. Manganese dioxide nanosheet functionalized sulfur@PEDOT core-shell nanospheres for advanced lithium-sulfur batteries. Journal of Materials Chemistry A, 2016, 4(24): 9403.
DOI URL |
| [12] |
LI C, LIU R, XIAO Y, et al. Recent progress of separators in lithium-sulfur batteries. Energy Storage Materials, 2021, 40: 439.
DOI URL |
| [13] |
CUI J, LI Z, LI J, et al. An atomic-confined-space separator for high performance lithium-sulfur batteries. Journal of Materials Chemistry A, 2020, 8(4): 1896.
DOI URL |
| [14] | BAEK M, SHIN H, CHAR K, et al. New high donor electrolyte for lithium-sulfur batteries. Advanced Materials, 2020, 32(52): 2005022. |
| [15] |
CHOUDHURY S, SAHA T, NASKAR K, et al. A highly stretchable gel-polymer electrolyte for lithium-sulfur batteries. Polymer, 2017, 112: 447.
DOI URL |
| [16] | HE B, RAO Z, CHENG Z, et al. Rationally design a sulfur cathode with solid-phase conversion mechanism for high cycle-stable Li-S Batteries. Advanced Energy Materials, 2021, 11(14): 2003690. |
| [17] | WANG Z, SHEN J, LIU J, et al. Self-supported and flexible sulfur cathode enabled via synergistic confinement for high-energy-density lithium-sulfur batteries. Advanced Materials, 2019, 31(33): 1902228. |
| [18] |
WU P, CHEN L H, XIAO S S, et al. Insight into the positive effect of porous hierarchy in S/C cathodes on the electrochemical performance of Li-S batteries. Nanoscale, 2018, 10(25): 11861.
DOI URL |
| [19] |
ZHANG Y, GAO Z, SONG N, et al. Graphene and its derivatives in lithium-sulfur batteries. Materials Today Energy, 2018, 9: 319.
DOI URL |
| [20] |
LI C, YU J, XUE S L, et al. Wood-inspired multi-channel tubular graphene network for high-performance lithium-sulfur batteries. Carbon, 2018, 139: 522.
DOI URL |
| [21] |
YANG W, ZHAO H, CHEN L, et al. Ferrous sulfide-assisted hollow carbon spheres as sulfur host for advanced lithium-sulfur batteries. Chemical Engineering Journal, 2017, 326: 1040.
DOI URL |
| [22] |
ZHE R, ZHU T, WEI X, et al. Graphene oxide wrapped hollow mesoporous carbon spheres as a dynamically bipolar host for lithium-sulfur batteries. Journal of Materials Chemistry A, 2022, 10(45): 24422.
DOI URL |
| [23] | CHEN H, DONG W D, XIA F J, et al. Hollow nitrogen-doped carbon/sulfur@MnO2 nanocomposite with structural and chemical dual-encapsulation for lithium-sulfur battery. Chemical Engineering Journal, 2020, 381: 122746. |
| [24] |
LI C, XI Z, DONG S, et al. CNTs/MOFs-derived carbon/ Al2(OH)2.76F3.24/S cathodes for high-performance lithium-sulfur batteries. Energy Storage Materials, 2018, 12: 341.
DOI URL |
| [25] |
DENG T, MEN X L, JIAO X C, et al. CNTs decorated Cu-BTC with catalytic effect for high-stability lithium-sulfur batteries. Ceramics International, 2022, 48(3): 4352.
DOI URL |
| [26] | AN Y, TIAN Y, LI Y, et al. Heteroatom-doped 3D porous carbon architectures for highly stable aqueous zinc metal batteries and non-aqueous lithium metal batteries. Chemical Engineering Journal, 2020, 400: 125843. |
| [27] | FAN L, ZHUANG H L, ZHANG K, et al. Chloride-reinforced carbon nanofiber host as effective polysulfide traps in lithium-sulfur batteries. Advanced Science, 2016, 3(12): 1600175. |
| [28] |
CHEN Y, XU P, LIU Q, et al. Cobalt embedded in porous carbon fiber membranes for high-performance lithium-sulfur batteries. Carbon, 2022, 187: 187.
DOI URL |
| [29] |
ZHENG Y, ZHENG S, XUE H, et al. Metal-organic frameworks for lithium-sulfur batteries. Journal of Materials Chemistry A, 2019, 7(8): 3469.
DOI URL |
| [30] | CHEN K, SUN Z, FANG R, et al. Metal-organic frameworks (MOFs)-derived nitrogen-doped porous carbon anchored on graphene with multifunctional effects for lithium-sulfur batteries. Advanced Functional Materials, 2018, 28(38): 1707592. |
| [31] |
ZHANG N, YANG Y, FENG X, et al. Sulfur encapsulation by MOF-derived CoS2 embedded in carbon hosts for high-performance Li-S batteries. Journal of Materials Chemistry A, 2019, 7(37): 21128.
DOI URL |
| [32] |
SHAO Q, LU P, XU L, et al. Rational design of MoS2 nanosheets decorated on mesoporous hollow carbon spheres as a dual- functional accelerator in sulfur cathode for advanced pouch-type Li-S batteries. Journal of Energy Chemistry, 2020, 51: 262.
DOI URL |
| [33] |
WANG H E, YIN K, ZHAO X, et al. Coherent TiO2/BaTiO3 heterostructure as a functional reservoir and promoter for polysulfide intermediates. Chemical Communications, 2018, 54(86): 12250.
DOI URL |
| [34] |
DONG W, WANG D, LI X, et al. Bronze TiO2 as a cathode host for lithium-sulfur batteries. Journal of Energy Chemistry, 2020, 48: 259.
DOI URL |
| [35] | GAO X, YANG X, LI M, et al. Cobalt-doped SnS2 with dual active centers of synergistic absorption-catalysis effect for high-S loading Li-S batteries. Advanced Functional Materials, 2019, 29(8): 1806724. |
| [36] |
ZHOU N, DONG W D, ZHANG Y J, et al. Embedding tin disulfide nanoparticles in two-dimensional porous carbon nanosheet interlayers for fast-charging lithium-sulfur batteries. Science China Materials, 2021, 64(11): 2697.
DOI |
| [37] |
LI X, GUO G, QIN N, et al. SnS2/TiO2 nanohybrids chemically bonded on nitrogen-doped graphene for lithium-sulfur batteries: synergy of vacancy defects and heterostructures. Nanoscale, 2018, 10(33): 15505.
DOI URL |
| [38] | JIANG Y, LIU H, TAN X, et al. Monoclinic ZIF-8 nanosheet- derived 2D carbon nanosheets as sulfur immobilizer for high-performance lithium sulfur batteries. ACS Applied Materials & Interfaces, 2017, 9(30): 25239. |
| [39] |
WU L, LI Y, FU Z, et al. Hierarchically structured porous materials: synthesis strategies and applications in energy storage. National Science Review, 2020, 7: 1667.
DOI URL |
| [40] |
YUAN H, ZHANG W, WANG J G, et al. Facilitation of sulfur evolution reaction by pyridinic nitrogen doped carbon nanoflakes for highly-stable lithium-sulfur batteries. Energy Storage Materials, 2018, 10: 1.
DOI URL |
| [41] |
YAN M, DONG W, LIU F, et al. Unprecedented strong and reversible atomic orbital hybridization enables a highly stable Li-S battery. National Science Review, 2022, 9(7): nwac078.
DOI URL |
| [42] | YAN M, WANG Z Y, YU G W, et al. Adsorption-catalysis- conversion of polysulfides in sandwiched ultrathin Ni(OH)2-PANI for stable lithium-sulfur batteries. Small, 2022, 18(25): 2201822. |
| [43] | LIU D, ZHANG C, ZHOU G, et al. Catalytic effects in lithium- sulfur batteries: promoted sulfur transformation and reduced shuttle effect. Advanced Science, 2018, 5(1): 1700270. |
| [44] |
LIU N, HUO K, MCDOWELL M T, et al. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Scientific Reports, 2013, 3: 1919.
DOI |
| [45] |
JING W, ZU J, ZOU K, et al. Tin disulfide embedded on porous carbon spheres for accelerating polysulfide conversion kinetics toward lithium-sulfur batteries. Journal of Colloid and Interface Science, 2022, 635: 32.
DOI URL |
| [46] | YANG J L, ZHAO S X, ZENG X T, et al. Catalytic interfaces- enriched hybrid hollow spheres sulfur host for advanced Li-S batteries. Advanced Materials Interfaces, 2019, 7(1): 1901420. |
| [47] | YAN M, CHEN H, YU Y, et al. 3D ferroconcrete-like aminated carbon nanotubes network anchoring sulfur for advanced lithium- sulfur battery. Advanced Energy Materials, 2018, 8(25): 1801066. |
| [48] | FAN L, LI X, SONG X, et al. Promising dual-doped graphene aerogel/SnS2 nanocrystal building high performance sodium ion batteries. ACS Applied Materials & Interfaces, 2018, 10(3): 2637. |
| [49] |
ZHANG J, YOU C, WANG J, et al. Synergistic catalytic effect of ion tunnels with polar dopants to boost the electrochemical kinetics for high-performance sulfur cathodes. ChemElectroChem, 2019, 6(19): 5051.
DOI URL |
| [50] |
WU J, CHEN B, LIU Q, et al. Preparing a composite including SnS2, carbon nanotubes and S and using as cathode material of lithium-sulfur battery. Scripta Materialia, 2020, 177: 208.
DOI URL |
| [1] | 李婷婷, 张阳, 陈加航, 闵宇霖, 王久林. 锂硫电池S@pPAN正极用柔性黏结剂研究[J]. 无机材料学报, 2022, 37(2): 182-188. |
| [2] | 李高然, 李红阳, 曾海波. 硼基材料在锂硫电池中的研究进展[J]. 无机材料学报, 2022, 37(2): 152-162. |
| [3] | 金高尧, 何海传, 吴杰, 张梦源, 李亚娟, 刘又年. 锂硫电池正极用钴掺杂空心多孔碳载体材料[J]. 无机材料学报, 2021, 36(2): 203-209. |
| [4] | 汤嘉伟, 王永邦, 马成, 杨海潇, 王际童, 乔文明, 凌立成. 甲基萘沥青基有序中孔炭的制备及电化学性能[J]. 无机材料学报, 2021, 36(10): 1031-1038. |
| [5] | 蒋浩,吴淏,侯成义,李耀刚,肖茹,张青红,王宏志. 切割方向对桦木衍生的取向微通道生物质炭锂硫电池隔膜性能的影响[J]. 无机材料学报, 2020, 35(6): 717-723. |
| [6] | 王佳宁, 靳俊, 温兆银. α-MoC1-x纳米晶富集碳球修饰隔膜对锂硫电池性能的影响[J]. 无机材料学报, 2020, 35(5): 532-540. |
| [7] | 山巍,傅正钱,张发强,马名生,刘志甫,李永祥. SnS2纳米片的制备及其对NO2气体的检测[J]. 无机材料学报, 2020, 35(4): 497-504. |
| [8] | 李亚东, 李伟平, 王琴, 郑道光, 王建新. 碳纤维支撑柔性碳硫复合电极的制备、物性及电池性能研究[J]. 无机材料学报, 2019, 34(4): 373-378. |
| [9] | 王宇晖, 靳 俊, 郭战胜, 温兆银. 锂硫电池循环过程中变形演化的直接观测[J]. 无机材料学报, 2017, 32(3): 247-251. |
| [10] | 柴二亚, 潘俊安, 袁国龙, 程豪, 安峰, 谢淑红. 聚苯胺包覆蛋白石页岩/硫复合材料的制备及其电化学性能[J]. 无机材料学报, 2017, 32(11): 1165-1170. |
| [11] | 杨书廷, 闫 崇, 曹朝霞, 史梦姣, 李艳蕾, 尹艳红. 以荷叶制备多级孔碳硫复合正极材料及性能研究[J]. 无机材料学报, 2016, 31(2): 135-140. |
| [12] | 马国强, 温兆银, 王清松, 靳 俊, 吴相伟, 张敬超. CeO2纳米晶的添加对锂硫电池电化学性能的影响[J]. 无机材料学报, 2015, 30(9): 913-918. |
| [13] | 陈飞彪, 王英男, 吴伯荣, 熊云奎, 廖维林, 吴 锋, 孙 喆. 锂硫电池石墨烯/硫复合正极材料的制备及其电化学性能[J]. 无机材料学报, 2014, 29(6): 627-632. |
| [14] | 胡菁菁, 李国然, 高学平. 锂/硫电池的研究现状、问题及挑战[J]. 无机材料学报, 2013, 28(11): 1181-1186. |
| [15] | 陈 龙, 刘景东, 张诗群. 负载ZnS的介孔炭复合硫正极材料的制备及性能研究[J]. 无机材料学报, 2013, 28(10): 1127-1131. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||