无机材料学报 ›› 2023, Vol. 38 ›› Issue (1): 32-42.DOI: 10.15541/jim20220384 CSTR: 32189.14.10.15541/jim20220384
所属专题: 【信息功能】敏感陶瓷(202506)
• 专栏:抗疫生物材料(特邀编辑: 杨勇) • 上一篇 下一篇
刘瑶1,2( ), 尤勋海1,3, 赵冰1,3, 罗晓莹4(
), 尤勋海1,3, 赵冰1,3, 罗晓莹4( ), 陈星1,2,3(
), 陈星1,2,3( )
)
收稿日期:2022-07-04
修回日期:2022-08-18
出版日期:2023-01-20
网络出版日期:2022-09-15
通讯作者:
陈 星, 教授. E-mail: xingchen@hfut.edu.cn;作者简介:刘 瑶(1993-), 女, 博士研究生. E-mail: 18691965261@163.com
基金资助:
LIU Yao1,2( ), YOU Xunhai1,3, ZHAO Bing1,3, LUO Xiaoying4(
), YOU Xunhai1,3, ZHAO Bing1,3, LUO Xiaoying4( ), CHEN Xing1,2,3(
), CHEN Xing1,2,3( )
)
Received:2022-07-04
Revised:2022-08-18
Published:2023-01-20
Online:2022-09-15
Contact:
CHEN Xing, professor. E-mail: xingchen@hfut.edu.cn;About author:LIU Yao (1993-), female, PhD candidate. E-mail: 18691965261@163.com
Supported by:摘要:
新冠疫情暴发对全球公共卫生构成了巨大威胁, 病毒的快速、准确诊断对新冠疫情防控具有至关重要的作用。近年来, 以纳米材料为基础的电化学传感技术在快速、高灵敏度/高特异性分子诊断方面显示出巨大的潜力。本文简要介绍了新型冠状病毒(SARS-CoV-2)的结构特征及常规检测方法, 总结了电化学生物检测相关传感特点和机制。在此基础上, 详细评述了金纳米材料、氧化物纳米材料、碳基纳米材料等为基础的电化学传感器用于快速、准确检测新冠病毒的研究进展。最后, 展望了基于电化学传感技术在未来生物分子诊断中的应用。
中图分类号:
刘瑶, 尤勋海, 赵冰, 罗晓莹, 陈星. 功能纳米材料应用于电化学新冠病毒生物传感器的研究进展[J]. 无机材料学报, 2023, 38(1): 32-42.
LIU Yao, YOU Xunhai, ZHAO Bing, LUO Xiaoying, CHEN Xing. Functional Nanomaterials for Electrochemical SRAS-CoV-2 Biosensors: a Review[J]. Journal of Inorganic Materials, 2023, 38(1): 32-42.
| Detection method | Time/h | Advantage | Disadvantage |
|---|---|---|---|
| Reverse transcrition-polymerase chain reaction (RT-PCR) | 4-6 | High sensitivity and reliability Low cost Versatility in sample types | Special instruments Complicated operation Time-consuming |
| Enzyme linked immunosorbent assay (ELISA) | 1-3 | Simple operation Low price Fast detection | Low specificity Suitability only for the late stage of the disease |
| Surface-enhanced Raman spectroscopy (SERS) | <1 | Simple construction Good repeatability | Specialized SERS active substrates |
| Electrochemical detection | <1 | Lower cost Simpler construction Higher specificity Relatively lower sensitivity | Lower clinical trial accuracy |
表1 SARS-CoV-2检测方法比较
Table 1 Comparison of detection methods for SARS-CoV-2 detection
| Detection method | Time/h | Advantage | Disadvantage |
|---|---|---|---|
| Reverse transcrition-polymerase chain reaction (RT-PCR) | 4-6 | High sensitivity and reliability Low cost Versatility in sample types | Special instruments Complicated operation Time-consuming |
| Enzyme linked immunosorbent assay (ELISA) | 1-3 | Simple operation Low price Fast detection | Low specificity Suitability only for the late stage of the disease |
| Surface-enhanced Raman spectroscopy (SERS) | <1 | Simple construction Good repeatability | Specialized SERS active substrates |
| Electrochemical detection | <1 | Lower cost Simpler construction Higher specificity Relatively lower sensitivity | Lower clinical trial accuracy |
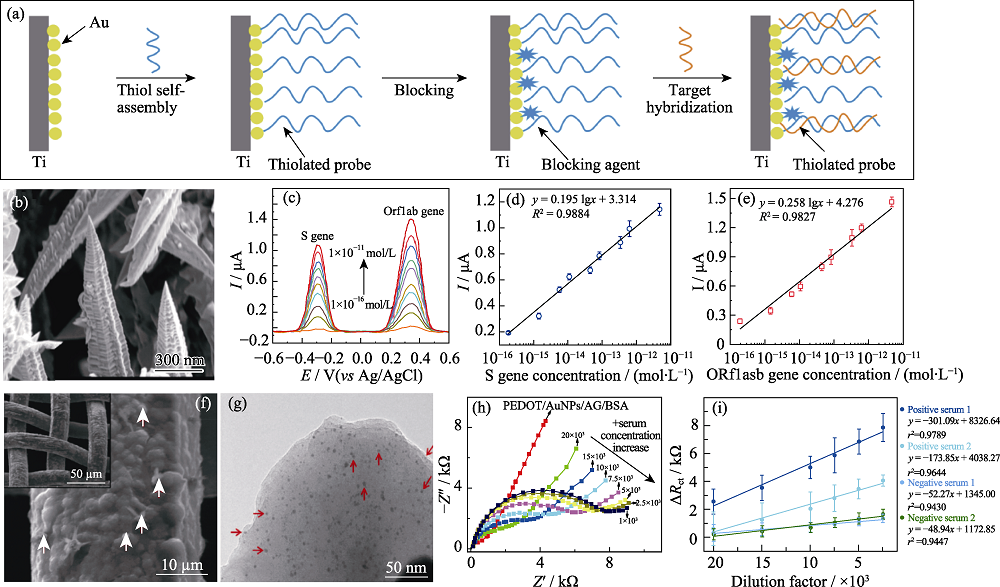
图2 基于金纳米材料构建的电化学生物传感器检测新冠病毒的应用
Fig. 2 Electrochemical biosensors based on gold nanomaterials for the detection of SARS-CoV-2 (a) Schematic diagram of probe DNA fixation and target nucleotide hybridization on gold electrode[46]; (b) SEM images of 3D gold nanoneedle structures[47];(c-e) Square wave stripping voltammetric response and corresponding calibration plots of 3D gold nanoneedle modified electrode toward S and ORF1ab genes[47]; (f) SEM and (g) TEM images of PEDOT/AuNPs/AG[48]; (h-i) Nyquist plots and corresponding calibration plots of the PEDOT/AuNPs/AG/BSA modified electrode toward different positive serum concentrations[48]
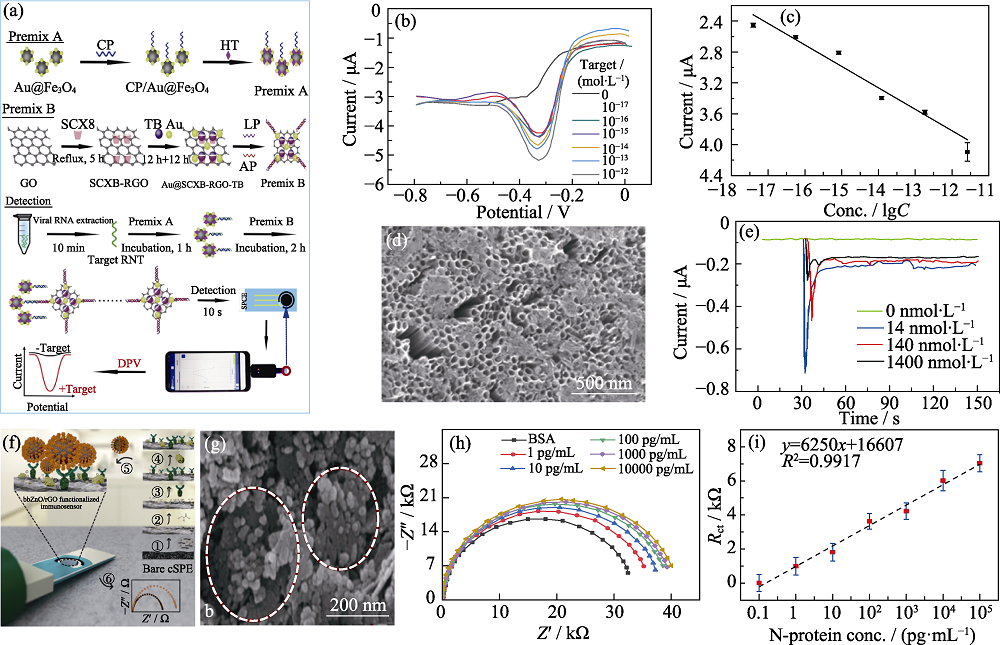
图3 基于金属氧化物纳米材料构建的电化学传感器检测SARS-CoV-2的应用
Fig. 3 Metal oxide nanomaterials used in electrochemical sensors to detect SARS-CoV-2 (a) Schematic of portable electrochemical biosensor based on probe recognition technology for the detection of SARS-CoV-2 RNA[6]; (b) DPV curves for different concentrations of artificial target for the SARS-CoV-2 biosensor[6]; (c) Resulting calibration plot for lgC vs. DPV response signals[6]; (d) SEM image of the Co-functionalized TNTs[49]; (e) Amperometry response curves of Co-TNT on SARS-CoV-2 S protein of different concentrations[49]; (f) Amperometry response curves of Co-TNT sensor upon exposure to SARS-CoV-2 S protein of different concentrations[49]; (g) FESEM image of antibodies being deposited on ZnO/rGO[5]; (h-i) Nyquist plots and corresponding calibration curve of the ZnO/rGO modified electrode towards N-protein[5] ; Colorful figures are available on website
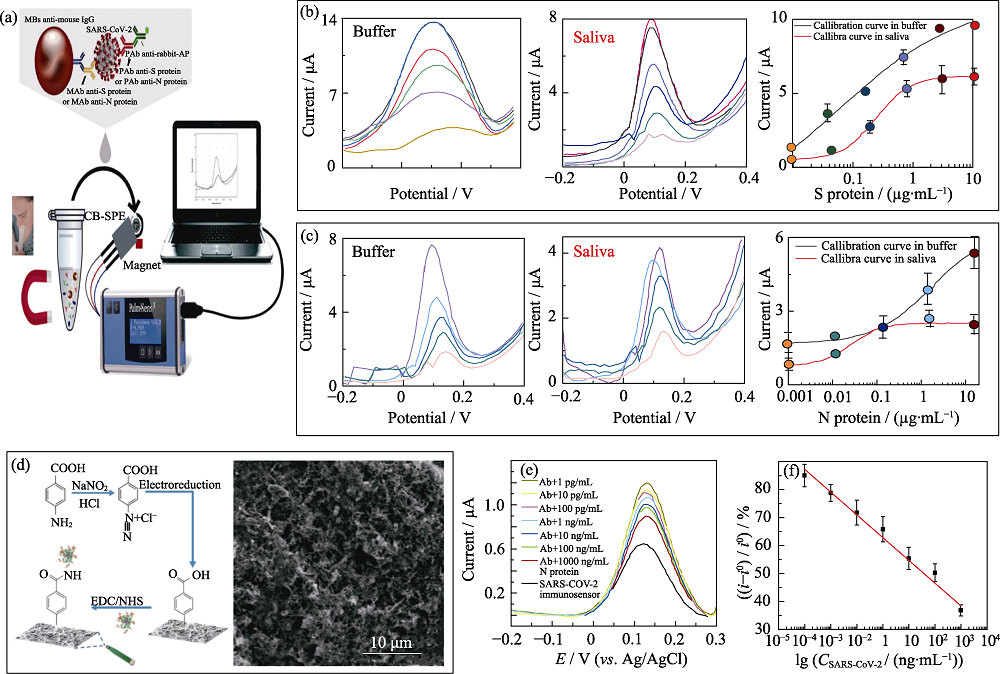
图4 基于碳纳米材料的电化学生物传感器检测SARS-CoV-2的应用
Fig. 4 Electrochemical biosensors based on carbon nanomaterials for the detection of SARS-CoV-2 (a) Schematic diagram of CBs modified SPE for SARS-CoV-2 detection[50]; (b, c) Electrochemical response signal and corresponding calibration curves of the CBs modified SPE towards S (b) and N (c) protein[50]; (d) Preparation process and SEM image of functionalized carbon nanofiber (CNF) [51]; (e, f) Square wave voltammetric respond (e) and corresponding calibration curves (f) of the the functionalized CNF modified electrode towards nucleocapsid protein at different concentrations[51]; CBs: Carbon black nanomaterials; SPE: Screen printing electrodes; EDC/NHS: 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydro/N-Hydroxy succinimide; Colorful figures are available on website
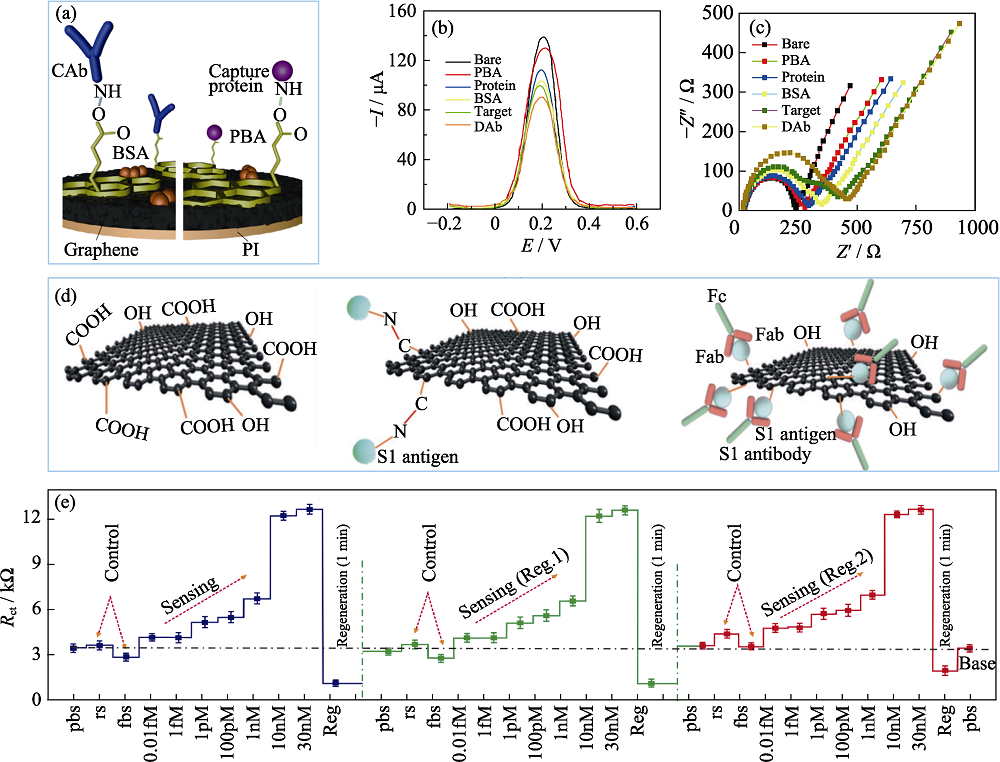
图5 石墨烯纳米复合材料在新冠病毒检测中的应用
Fig. 5 Graphene nanocomposites used in electrochemical sensors to detect SARS-CoV-2 (a) Schematic diagram of functionalized graphene connected to the corresponding bioreceptors by covalent bonds[52]; (b, c) DPV respond (b) and Nyquist diagram (c) of the electrode at different steps[52]; (d) Surface modification process of reduced graphene oxide nanosheets by carboxyl functionalization[55]; (e) Continuous detection of neo-coronavirus S protein after sensor regeneration[55]. CAb: Capture antibody; DAb: Detector antibody; PI: Polyimide; BSA: Bovine serum albumin: PBA: 1-Pyrenebutyric acid; Fc: Fragment crystallizable; Fab: Fragment of antigen binding; M: mol/L; Colorful figures are available on website
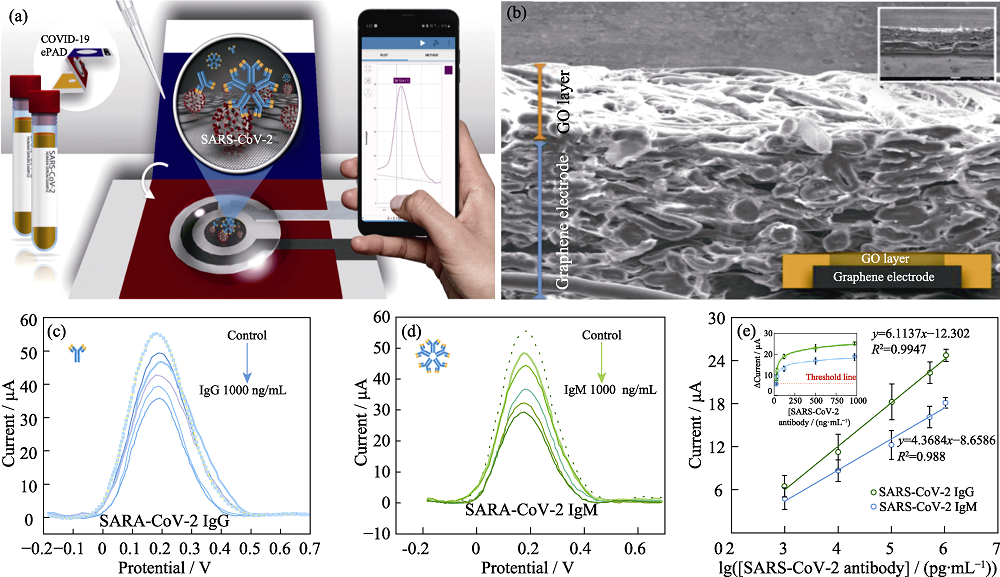
图6 纸基电化学传感器检测SARS-CoV-2的抗体[59]
Fig. 6 Paper-based electrochemical biosensor for diagnosing COVID-19[59] (a) Schematic illustration of the detection procedure of COVID-19; (b) SEM image of the corresponding cross-sectional of GO modified paper; (c, d) Square wave stripping voltammetric responses of SARS-CoV-2 IgG (c) and IgM (d) at different concentrations; (e) linear relationship between Δ current vs logarithmic concentration of SARS-CoV-2 IgG and IgM and their corresponding relationships between Δ current and concentration of SARS-CoV-2 IgG and IgM; Colorful figures are available on website
| Material | Method | Detecting object | Limit of detection | Ref. |
|---|---|---|---|---|
| AuNPs | i-t | RNA or cDNA | N/A | [ |
| Gold nanoneedle | SWV | S gene Orf1ab gene | 5.0×10-18 g·μL-1 6.8×10-18 g·μL-1 | [ |
| AuNPs/PEDOT | EIS | Positive and negative serum sample | N/A | [ |
| Au@Fe3O4/rGO | DPV | RNA | 3×10-18 mol·L-1 | [ |
| Co-TiO2 nanotubes | i-t | RBD | 7×10-10 mol·L-1 | [ |
| ZnO/rGO | EIS | N protein antigens | 2×10-14 g·mL-1 | [ |
| Carbon black nanomaterial | LSV | S protein N protein | 1.9×10-8 g·mL-1 8×10-9 g·mL-1 | [ |
| Laser-engraved graphene | LSV | N-protein, S1-IgM S1-IgG C-reactive protein | N/A | [ |
| AuNPs/rGO | EIS | S1 protein RBD antibodies | 2.8×10-15 mol·L-1 1.69×10-14 mol·L-1 | [ |
| SiO2@UiO-66 | EIS | S protein | 1×10-13 g·mL-1 | [ |
| GO | SWV | IgG IgM | 9.6×10-10 g·mL-1 1.4×10-10 g·mL-1 | [ |
| Au@Pt/MIL-5(Al) | DPV | N-protein | 8.33×10-12 g·mL-1 | [ |
表2 不同纳米材料构建的电化学传感器检测SARS-CoV-2的性能对比
Table 2 Comparison of SARS-CoV-2 detection performance of electrochemical sensors constructed from different nanomaterials
| Material | Method | Detecting object | Limit of detection | Ref. |
|---|---|---|---|---|
| AuNPs | i-t | RNA or cDNA | N/A | [ |
| Gold nanoneedle | SWV | S gene Orf1ab gene | 5.0×10-18 g·μL-1 6.8×10-18 g·μL-1 | [ |
| AuNPs/PEDOT | EIS | Positive and negative serum sample | N/A | [ |
| Au@Fe3O4/rGO | DPV | RNA | 3×10-18 mol·L-1 | [ |
| Co-TiO2 nanotubes | i-t | RBD | 7×10-10 mol·L-1 | [ |
| ZnO/rGO | EIS | N protein antigens | 2×10-14 g·mL-1 | [ |
| Carbon black nanomaterial | LSV | S protein N protein | 1.9×10-8 g·mL-1 8×10-9 g·mL-1 | [ |
| Laser-engraved graphene | LSV | N-protein, S1-IgM S1-IgG C-reactive protein | N/A | [ |
| AuNPs/rGO | EIS | S1 protein RBD antibodies | 2.8×10-15 mol·L-1 1.69×10-14 mol·L-1 | [ |
| SiO2@UiO-66 | EIS | S protein | 1×10-13 g·mL-1 | [ |
| GO | SWV | IgG IgM | 9.6×10-10 g·mL-1 1.4×10-10 g·mL-1 | [ |
| Au@Pt/MIL-5(Al) | DPV | N-protein | 8.33×10-12 g·mL-1 | [ |
| [1] |
CHU D K W, PAN Y, CHENG S M S, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clinical Chemistry, 2020, 66(4): 549.
DOI PMID |
| [2] |
OROOJI Y, SOHRABI H, HEMMAT N, et al. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nano-Micro Letters, 2020, 13(1): 18.
DOI URL |
| [3] | SAMSON R, NAVALE G R, DHARNE M S, et al. Biosensors: frontiers in rapid detection of COVID-19. Biotech, 2020, 10(9): 385. |
| [4] |
ALAFEEF M, DIGHE K, MOITRA P, et al. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano, 2020, 14(12): 17028.
DOI PMID |
| [5] |
HAGHAYEGH F, SALAHANDISH R, HASSANI M, et al. Highly stable buffer-based zinc oxide/reduced graphene oxide nanosurface chemistry for rapid immunosensing of SARS-CoV-2 antigens. ACS Appl. Mater. Interfaces, 2022, 14(8): 10844.
DOI URL |
| [6] |
ZHAO H, LIU F, XIE W, et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sensors and Actuators B Chemical, 2021, 327: 128899.
DOI URL |
| [7] |
FALSEY A R, WALSH E E. Novel coronavirus and severe acute respiratory syndrome. Lancet, 2003, 361(9366): 1312.
DOI PMID |
| [8] |
ZAKI A M, VANBOHEEMEN S, BESTEBROER T M, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine, 2012, 367: 1814.
DOI URL |
| [9] |
ZHU N, ZHANG D, WANG W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 2020, 382(8): 727.
DOI URL |
| [10] |
YAO H, SONG Y, CHEN Y, et al. Molecular architecture of the SARS-CoV-2 virus. Cell, 2020, 183(3): 730.
DOI PMID |
| [11] |
CHOUDHRY N, ZHAO X, XU D, et al. Chinese therapeutic strategy for fighting COVID-19 and potential small-molecule inhibitors against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Journal of Medicinal Chemistry, 2020, 63(22): 13205.
DOI URL |
| [12] |
THOMS M, BUSCHAUER R, AMEISMEIER M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science, 2020, 369(6508): 1249.
DOI PMID |
| [13] |
FENG W, NEWBIGGING A M, LE C, et al. Molecular diagnosis of COVID-19: challenges and research needs. Analytical Chemistry, 2020, 92: 10196.
DOI PMID |
| [14] |
XIE C B, JIANG L X, HUANG G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. International Journal of Infectious Diseases, 2020, 93: 264.
DOI PMID |
| [15] |
SADIGHBAYAN D, HASANZADEH M, GHAFAR-ZADEH E. Biosensing based on field-effect transistors (FET): recent progress and challenges. Trac-Trends in Analytical Chemistry, 2020, 133: 116067.
DOI URL |
| [16] | LIU W, LIU L, KOU G, et al. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. Journal of Clinical Microbiology, 2020, 58(6): e0461. |
| [17] |
PENG Y, LIN C, LI Y, et al. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter, 2022, 5(2): 694.
DOI URL |
| [18] |
SITJAR J, LIAO J D, LEE H, et al. Challenges of SERS technology as a non-nucleic acid or -antigen detection method for SARS-CoV-2 virus and its variants. Biosensors & Bioelectronics, 2021, 181: 113153.
DOI URL |
| [19] |
YANG Y, PENG Y, LIN C, et al. Human ACE2-functionalized gold "virus-trap" nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Letters, 2021, 13(1): 109.
DOI PMID |
| [20] |
CHAIBUN T, PUENPA J, NGAMDEE T, et al. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nature Communications, 2021, 12(1): 802.
DOI PMID |
| [21] |
KUDR J, MICHALEK P, ILIEVA L, et al. COVID-19: a challenge for electrochemical biosensors. TrAC Trends in Analytical Chemistry, 2021, 136: 116192.
DOI URL |
| [22] |
TRAN V V, TRAN N H T, HWANG H S, et al. Development strategies of conducting polymer-based electrochemical biosensors for virus biomarkers: potential for rapid COVID-19 detection. Biosensors & Bioelectronics, 2021, 182: 113192.
DOI URL |
| [23] |
EJAZI S A, GHOSH S, ALI N. Antibody detection assays for COVID-19 diagnosis: an early overview. Immunology and Cell Biology, 2020, 99(1): 21.
DOI URL |
| [24] | MATHEW D, GILES J R, BAXTER A E, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science, 2020, 369(6508): 8511. |
| [25] |
ONG D, FRAGKOU P C, SCHWEITZER V A, et al. How to interpret and use COVID-19 serology and immunology tests. Clinical Microbiology and Infection, 2021, 27(7): 981.
DOI URL |
| [26] |
KIMMEL D W, LEBLANC G, MESCHIEVITZ M E, et al. Electrochemical sensors and biosensors. Analytical Chemistry, 2012, 84(2): 685.
DOI PMID |
| [27] | FREW J E, HILL H A. Electrochemical biosensors. Analytical Chemistry, 2010, 39(5): 1747. |
| [28] | BALKOURANI G, BROUZGOU A, ARCHONTI M, et al. Emerging materials for the electrochemical detection of COVID-19. Journal of Electroanalytical Chemistry, 2021, 893: 115285. |
| [29] |
ANTIOCHIA R. Developments in biosensors for CoV detection and future trends. Biosensors and Bioelectronics, 2020, 173: 112777.
DOI URL |
| [30] |
ERDEN P E, KILIÇ E. A review of enzymatic uric acid biosensors based onamperometric detection. Talanta, 2013, 107: 312.
DOI URL |
| [31] |
BRETT C M A, OLIVEIRA B A M. Electrochemical sensing in solution-origins, applications and future perspectives. Journal of Solid State Electrochemistry, 2011, 15(7/8): 1487.
DOI URL |
| [32] |
GUTH U, VONAU W, ZOSEL J. Recent developments in electrochemical sensor application and technology-a review. Measurement Science and Technology, 2009, 20(4): 042002.
DOI URL |
| [33] |
KARIMI-MALEH H, OROOJI Y, KARIMI F, et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosensors & Bioelectronics, 2021, 184: 113252.
DOI URL |
| [34] | CHAROENKITAMORN K, TUE PT, CHIKAE M, et al. Gold nanoparticle-labeled electrochemical immunoassay using open circuit potential for human chorionic gonadotropin detection. Electroanalysis, 2018, 30(8): 1766. |
| [35] |
RASHED M Z, KOPECHEK J A, PRIDDY M C, et al. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance- based detector. Biosensors & Bioelectronics, 2021, 171: 112709.
DOI URL |
| [36] |
LASSERRE P, BALANSETHUPATHY B, VEZZA V J, et al. SARS-CoV-2 aptasensors based on electrochemical impedance spectroscopy and low-cost gold electrode substrates. Analytical Chemistry, 2022, 94(4): 2126.
DOI PMID |
| [37] |
XU H, ZHENG J, LIANG H, et al. Electrochemical sensor for cancer cell detection using calix 8 arene/polydopamine/phosphorene nanocomposite based on host-guest recognition. Sensors and Actuators B-Chemical, 2020, 317: 128193.
DOI URL |
| [38] |
SEO G, LEE G, MI J K, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14: 5135.
DOI PMID |
| [39] |
MOKHTARZADEH A, EIVAZZADEH-KEIHAN R, PASHAZADEH P, et al. Nanomaterial-based biosensors for detection of pathogenic virus. Trends in Analytical Chemistry, 2017, 97: 445.
DOI URL |
| [40] |
YUAN F, XIA Y, LU Q, et al. Recent advances in inorganic functional nanomaterials based flexible electrochemical sensors. Talanta, 2022, 244: 123419.
DOI URL |
| [41] |
ZHONG C, YANG B, JIANG X, et al. Current progress of nanomaterials in molecularly imprinted electrochemical sensing. Critical Reviews in Analytical Chemistry, 2018, 48(1): 15.
DOI PMID |
| [42] |
CHOI H K, LEE M J, SANG N L, et al. Noble metal nanomaterial-based biosensors for electrochemical and optical detection of viruses causing respiratory illnesses. Frontiers in Chemistry, 2021, 9: 672739.
DOI URL |
| [43] |
REZAEI B, BOROUJENI MK, ENSAFI A A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosensors & Bioelectronics, 2015, 66: 490.
DOI URL |
| [44] |
XIAO T, HUANG J, WANG D, et al. Au and Au-based nanomaterials: synthesis and recent progress in electrochemical sensor applications. Talanta, 2020, 206: 120210.
DOI URL |
| [45] |
JANS H, HUO Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chemical Society Reviews, 2012, 41(7): 2849.
DOI PMID |
| [46] |
TRIPATHY S, SINGH S G. Label-free electrochemical detection of DNA hybridization: a method for COVID-19 diagnosis. Transactions of the Indian National Academy of Engineering, 2020, 5(2): 205.
DOI URL |
| [47] |
KASHEFI-KHEYRABADI L, NGUYEN H V, GO A, et al. Rapid, multiplexed, and nucleic acid amplification-free detection of SARS-CoV-2 RNA using an electrochemical biosensor. Biosensors & Bioelectronics, 2021, 195: 113649.
DOI URL |
| [48] |
LORENZEN A L, DOS SANTOS A M, DOS SANTOS L P, et al. PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies. Electrochimica Acta, 2022, 404: 139757.
DOI URL |
| [49] |
VADLAMANI B S, UPPAL T, VERMA S C, et al. Functionalized TiO2 nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors, 2020, 20(20): 5871.
DOI URL |
| [50] |
ARDUINI F, CINTI S, MAZZARACCHIO V, et al. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio) sensor design. Biosensors and Bioelectronics, 2020, 156: 112033.
DOI URL |
| [51] |
EISSA S, ZOUROB M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Analytical Chemistry, 93(3): 1826.
DOI URL |
| [52] |
TORRENTE-RODRÍGUEZ R, LUKAS H, TU J, et al. SARS-CoV-2 rapidplex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter, 2020, 3: 1981.
DOI URL |
| [53] |
LIV L, OBAN G, NAKIBOLU N, et al. A rapid, ultrasensitive voltammetric biosensor for determining SARS-CoV-2 spike protein in real samples. Biosensors & Bioelectronics, 2021, 192: 113497.
DOI URL |
| [54] |
HASHEMI S A, BEHBAHAN N, BAHRANI S, et al. Ultra-sensitive viral glycoprotein detection nanosystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosensors & Bioelectronics, 2021, 171: 112731.
DOI URL |
| [55] | ALI MA, HU C, JAHAN S, et al. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Advanced Materials, 2021, 33(7): 2006647. |
| [56] | WITT S, ROGIEN A, WERNER D, et al. Boron doped diamond thin films for the electrochemical detection of SARS-CoV-2 S1 protein. Diamond and Related Materials, 2021, 4: 108542. |
| [57] |
MEHMANDOUST M, GUMUS Z P, SOYLAK M, et al. Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta, 2022, 240: 123211.
DOI URL |
| [58] |
TIAN J, LIANG Z, HU O, et al. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochimica Acta, 2021, 387: 138533.
DOI URL |
| [59] |
YAKOH A, PIMPITAK U, RENGPIPAT S, et al. Paper-based electrochemical biosensor for diagnosing COVID-19: detection of SARS-CoV-2 antibodies and antigen. Biosensors & Bioelectronics, 2020, 176(14): 112912.
DOI URL |
| [60] |
RAZIQ A, KIDAKOVA A, BOROZNJAK R, et al. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosensors & Bioelectronics, 2021, 178: 113029.
DOI URL |
| [61] |
TORRENTE R, LUKAS H, Tu J, et al. SARS-CoV-2 rapidPlex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter, 2020, 3: 1981.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈曦, 袁媛, 谭业强, 刘昌胜. 无机非金属生物材料发展战略研究[J]. 无机材料学报, 2025, 40(5): 449-456. |
| [8] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [9] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [10] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [11] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [12] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [13] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [14] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [15] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||