无机材料学报 ›› 2019, Vol. 34 ›› Issue (11): 1145-1155.DOI: 10.15541/jim20190045 CSTR: 32189.14.10.15541/jim20190045
曹逊,曹翠翠,孙光耀,金平实
收稿日期:2019-01-25
修回日期:2019-03-18
出版日期:2019-11-20
网络出版日期:2019-05-29
作者简介:曹 逊(1983-), 男, 副研究员. E-mail: cxun@mail.sic.ac.cn
基金资助:CAO Xun,CAO Cui-Cui,SUN Guang-Yao,JIN Ping-Shi
Received:2019-01-25
Revised:2019-03-18
Published:2019-11-20
Online:2019-05-29
Supported by:摘要:
白光LEDs(White Light-Emitting Diodes, WLEDs)作为一种新型的固体照明光源, 相对于已有光源(白炽灯、荧光灯等)具有发光效率高、响应速度快、寿命长等优势, 在照明和显示领域有着广阔的应用前景。目前获取WLEDs最常用的方法是蓝光LED芯片激发YAG : Ce 3+黄光荧光粉以及紫外-近紫外芯片激发三基色荧光粉(RGB混合荧光粉), 相比于以上两种方式, 单基质WLEDs荧光粉由于能克服传统RGB荧光粉颜色再吸收及配比调控的问题, 获得较高的流明效率及较高色彩还原性而受到越来越多的关注。目前关于单基质白光荧光的研究已有大量文献报道, 涉及多种材料体系, 按照发光原理的不同, 可以将其地简单分为单离子激发体系、多离子激发体系以及不依赖于稀土离子发光的其他体系等。本文综述了单基质WLEDs荧光粉的研究进展, 指出了其发展中存在的问题, 并对未来发展趋势作了展望。
中图分类号:
曹逊, 曹翠翠, 孙光耀, 金平实. 单基质白光LED荧光粉研究进展[J]. 无机材料学报, 2019, 34(11): 1145-1155.
CAO Xun, CAO Cui-Cui, SUN Guang-Yao, JIN Ping-Shi. Recent Progress of Single-phase White Light-emitting Diodes Phosphors[J]. Journal of Inorganic Materials, 2019, 34(11): 1145-1155.

图1 白光LED的发展
Fig. 1 Development of WLED (a) InGaN蓝光芯片/YAG:Ce黄色荧光粉白光LED; (b) RGB混合荧光粉白光LED; (c)单基质白光LED[4] (a) blue light emitting InGaN chips/YAG:Ce yellow light emitting phosphor WLED; (b) WLED based on red-green-blue (RGB) emitting color phosphors; (c) WLED based on single-phase phosphor[4,5,6]
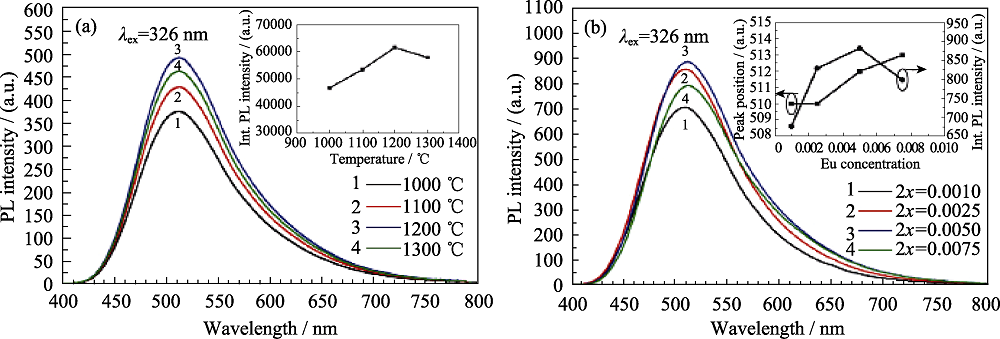
图2 不同烧结温度(a)和不同Eu2+掺杂浓度下(b)的CaSrSiO4:Eu2+发射光谱图[22]
Fig. 2 PL spectra of CaSrSiO4:Eu2+ phosphors sintered at different temperatures (a) and fabricated with different Eu2+ concentrations (b)[22]

图3 400 nm GaN芯片与Ca1-xSr1-xSiO4:2xEu2+组装成LED后的发射光谱(a)和CIE色度图(b), 及其白光LED照片(插图)[22]
Fig. 3 EL spectra (a) of the white LED fabricated by using phosphors and 400 nm GaN-based LED chips, CIE (x, y) chromaticity diagram (b) with insets showing digital images of the fabricated white LED[22]
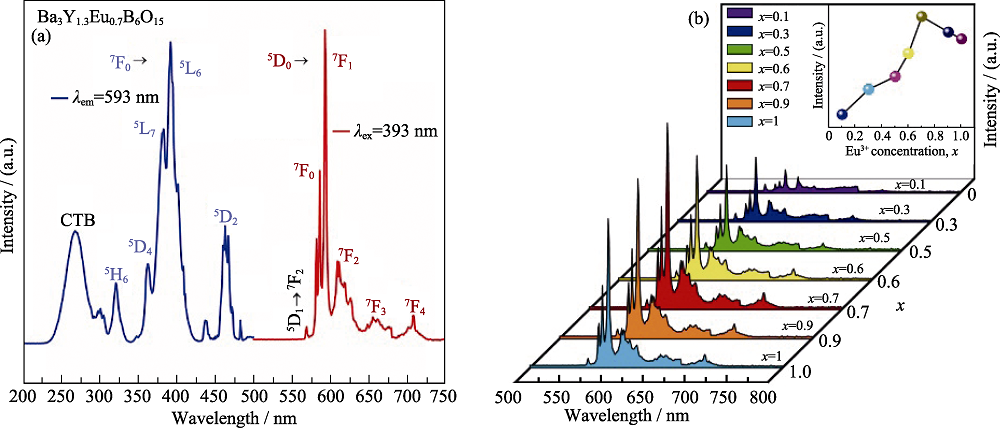
图4 (a)合成的Ba3Y1.3Eu0.7B6O15荧光体(λem= 593 nm和λex= 393 nm)在室温下的PLE和PL光谱, (b)Ba3Y2-xEuxB6O15(x = 0.1, 0.3, 0.5, 0.6, 0.7, 0.9和1.0)荧光粉的PL光谱, 及其PL强度(593 nm)随Eu3 +浓度的变化(插图)[23]
Fig. 4 (a) PLE and PL spectra of as-synthesized Ba3Y1.3Eu0.7B6O15 phosphors (λem=?593?nm and λex=?393?nm) at room temperature, (b) PL spectra of Ba3Y2-xEuxB6O15 (x?=?0.1, 0.3, 0.5, 0.6, 0.7, 0.9 and 1.0) phosphors with the inset illustrating the variation of the PL intensity (593?nm) on the concentration of Eu3+[23]
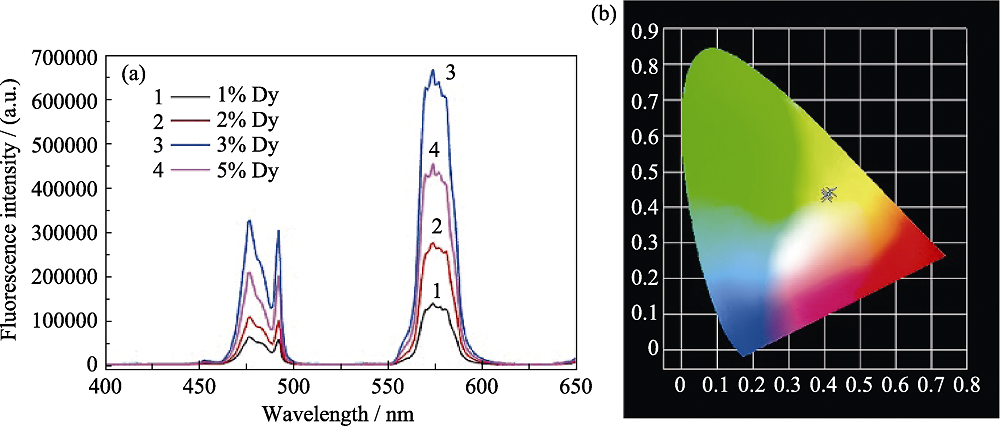
图5 具有不同Dy3 +掺杂浓度的CYAO磷光体的发射光谱(a)和CIE色度图(b)[24]
Fig. 5 Emission spectra (a) and CIE chromaticity diagram (b) of CYAO phosphors with different Dy3+ doping concentrations[24]
| Phosphor | UV/nm | Emission | CIE(x, y) | Ref. |
|---|---|---|---|---|
| BaSrMg(PO4)2: Eu2+ | 385 | 460 nm, 550 nm | (0.29, 0.35) | [25] |
| Sr3MgSi2O8: Eu2+ | 375 | 470 nm, 570 nm | (0.32, 0.33) | [26] |
| LaOF: Eu3+ | 274 | All the emissions from Eu3+ | (0.29, 0.34) | [27] |
| NaYF4: Eu3+ | 397 | 5DJ-7FJ’ (J,J’=0,1,2,3,4) | (0.29, 0.33) | [28] |
| CaIn2O4: Eu3+ | 397 | - | (0.32, 0.32) | [29] |
| BaY2ZnO5: Dy3+ | 355/351 | 489 nm, 579 nm | (0.32, 0.39) | [30] |
表1 单离子掺杂单基质白光荧光材料总结
Table 1 Summary of single activator ion doped systems for single-phase white-emitting phosphors
| Phosphor | UV/nm | Emission | CIE(x, y) | Ref. |
|---|---|---|---|---|
| BaSrMg(PO4)2: Eu2+ | 385 | 460 nm, 550 nm | (0.29, 0.35) | [25] |
| Sr3MgSi2O8: Eu2+ | 375 | 470 nm, 570 nm | (0.32, 0.33) | [26] |
| LaOF: Eu3+ | 274 | All the emissions from Eu3+ | (0.29, 0.34) | [27] |
| NaYF4: Eu3+ | 397 | 5DJ-7FJ’ (J,J’=0,1,2,3,4) | (0.29, 0.33) | [28] |
| CaIn2O4: Eu3+ | 397 | - | (0.32, 0.32) | [29] |
| BaY2ZnO5: Dy3+ | 355/351 | 489 nm, 579 nm | (0.32, 0.39) | [30] |
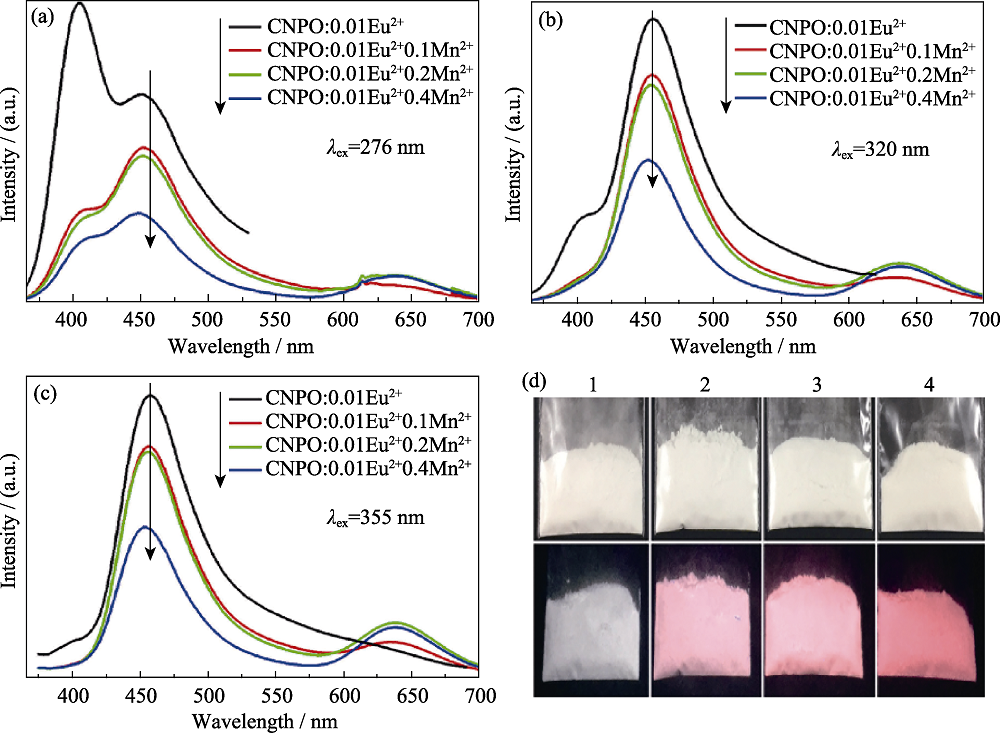
图8 CNPO:0.01Eu2+, nMn2+(n = 0, 0.1, 0.2, 0.4) 的发射光谱, 激发波长分别为276 (a), 320 (b)和355 nm (c); (d) 365 nm 紫外灯照射下的荧光粉(下面一行)、日光下的荧光粉(上面一行)的照片, 其中的1, 2, 3, 4分别对应n=0, 0.1, 0.2, 0.4[33]
Fig. 8 PL spectra of the CNPO:0.01Eu2+, nMn2+(n = 0, 0.1, 0.2, and 0.4) under the excitations at 276 (a), 320 (b), and 355 nm (c), respectively; The photos of the phosphors (d) excited by 365 nm UV lamp (bottom row), and photos obtained in daylight environment (upper row). The photos 1-4 correspond to n=0, 0.1, 0.2, and 0.4, respectively[33]
| Representative examples | Excitation/nm | Emission | CIE(x,y) | Ref. |
|---|---|---|---|---|
| Ca9Gd(PO4)7: Eu2+, Mn2+ | 380 | Eu2+: blue-greenish emission band (peaking at 494 nm) + Mn2+: red emission band (peaking at 652 nm) | (0.326, 0.328) | [34] |
| CaAl2Si2O8: Eu2+, Mn2+ | 354 | Eu2+: a broad band centered at 425 nm + Mn2+: a broad band centered at 568 nm | (0.33, 0.31) | [35] |
| MgY4Si3O13: Ce3+, Mn2+ | 328 | Ce3+: an asymmetric broad band peaking at 455 nm + Mn2+: orange-red emission band at 587 nm | (0.36, 0.26) | [36] |
| Ca3Sc2Si3O12: Ce3+, Mn2+, Y3+ | 450 | Ce3+: a green emission band peaked at 505 nm + Mn2+: a yellow band at around 574 nm and a red band at around 680 nm | (0.30, 0.33) | [37] |
| Sr2SiO4: Ce3+, Eu2+ | 354 | Ce3+: an asymmetric blue emission + Eu2+: a broad band covering the blue-green to yellow region | - | [38] |
| Sr3B2O6: Ce3+, Eu2+ | 351 | Ce3+: a broad asymmetric blue emission band centering at 434 nm + Eu2+: a broad yellow orange emission band centering at 574 nm | (0.31, 0.24) | [39] |
| Ca4Y6(SiO4)6O: Ce3+, Tb3+ | 352 | Ce3+: a blue band centered at 421 nm + Tb3+: characteristic emission lines ranging from 470 to 650 nm with yellow-greenish emission | (0.278, 0.353) | [40] |
| Ca2Al2SiO7: Ce3+, Tb3+ | 352 | Ce3+: a blue band centered at 419 nm + Tb3+: characteristic emission lines ranging from 470 to 650 nm with yellow-greenish emission | (0.316, 0.336) | [41] |
| Sr2Al2SiO7: Ce3+, Dy3+ | 335 | Ce3+: a blue emission band at 408 nm + Dy3+: the emission bands at 491 nm and 573 nm | - | [42] |
| 12CaO·7Al2O3: Ce3+, Dy3+ | 362 | Ce3+: a broad band centered at 430 nm + Dy3+: two narrow bands centered at 476 nm and 576 nm | (0.324, 0.323) | [43] |
表2 多离子掺杂单基质白光荧光材料
Table 2 Summary of representative multi-ion doped single-phased white-emitting phosphors
| Representative examples | Excitation/nm | Emission | CIE(x,y) | Ref. |
|---|---|---|---|---|
| Ca9Gd(PO4)7: Eu2+, Mn2+ | 380 | Eu2+: blue-greenish emission band (peaking at 494 nm) + Mn2+: red emission band (peaking at 652 nm) | (0.326, 0.328) | [34] |
| CaAl2Si2O8: Eu2+, Mn2+ | 354 | Eu2+: a broad band centered at 425 nm + Mn2+: a broad band centered at 568 nm | (0.33, 0.31) | [35] |
| MgY4Si3O13: Ce3+, Mn2+ | 328 | Ce3+: an asymmetric broad band peaking at 455 nm + Mn2+: orange-red emission band at 587 nm | (0.36, 0.26) | [36] |
| Ca3Sc2Si3O12: Ce3+, Mn2+, Y3+ | 450 | Ce3+: a green emission band peaked at 505 nm + Mn2+: a yellow band at around 574 nm and a red band at around 680 nm | (0.30, 0.33) | [37] |
| Sr2SiO4: Ce3+, Eu2+ | 354 | Ce3+: an asymmetric blue emission + Eu2+: a broad band covering the blue-green to yellow region | - | [38] |
| Sr3B2O6: Ce3+, Eu2+ | 351 | Ce3+: a broad asymmetric blue emission band centering at 434 nm + Eu2+: a broad yellow orange emission band centering at 574 nm | (0.31, 0.24) | [39] |
| Ca4Y6(SiO4)6O: Ce3+, Tb3+ | 352 | Ce3+: a blue band centered at 421 nm + Tb3+: characteristic emission lines ranging from 470 to 650 nm with yellow-greenish emission | (0.278, 0.353) | [40] |
| Ca2Al2SiO7: Ce3+, Tb3+ | 352 | Ce3+: a blue band centered at 419 nm + Tb3+: characteristic emission lines ranging from 470 to 650 nm with yellow-greenish emission | (0.316, 0.336) | [41] |
| Sr2Al2SiO7: Ce3+, Dy3+ | 335 | Ce3+: a blue emission band at 408 nm + Dy3+: the emission bands at 491 nm and 573 nm | - | [42] |
| 12CaO·7Al2O3: Ce3+, Dy3+ | 362 | Ce3+: a broad band centered at 430 nm + Dy3+: two narrow bands centered at 476 nm and 576 nm | (0.324, 0.323) | [43] |
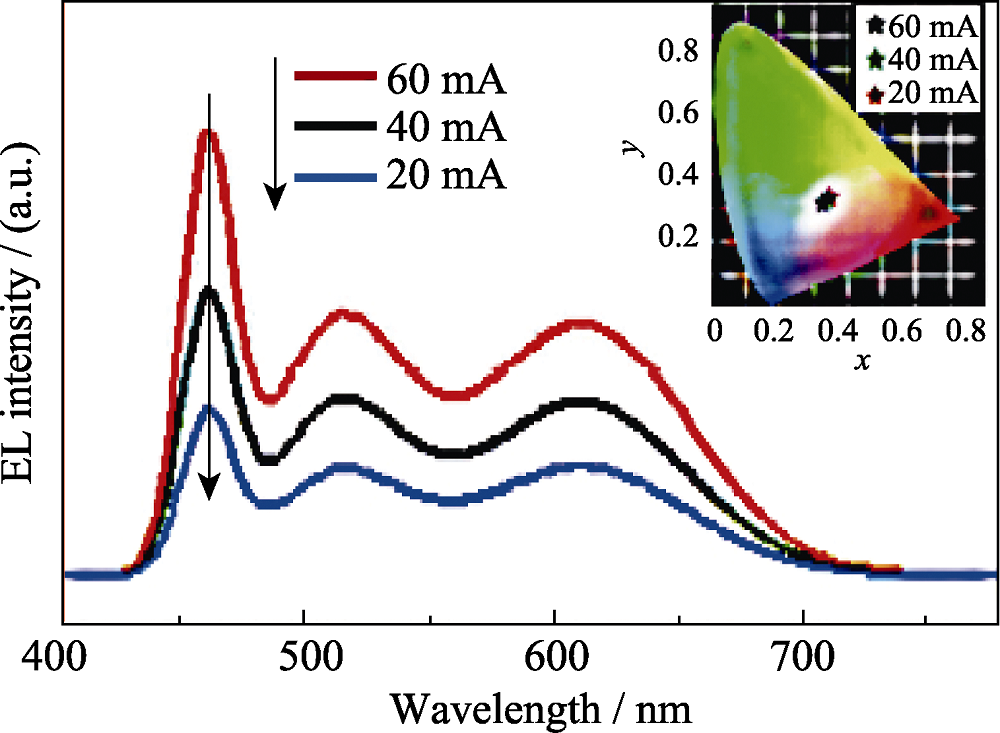
图9 不同电流下单相双激发的Mn2+掺杂Zn-Cu-In-S纳米晶材料的发光光谱图[57]
Fig. 9 Electroluminescence spectra of the WLED operated under various currents of 20 to 60 mA. Inset: the variation in CIE chromaticity coordinates of the WLED under various currents[57]
| Sample | KVO3 | RbVO3 | CsVO3 | Mg3V2O8 | Zn3V2O8 |
|---|---|---|---|---|---|
| η/% | 4 | 79 | 87 | 6 | 52 |
| CIE(x, y) | 0.362, 0.453 | 0.316, 0.424 | 0.306, 0.418 | 0.449, 0.475 | 0.432, 0.478 |
| CCT/K | 4859 | 5993 | 6334 | 3318 | 3583 |
表3 AVO3(A=K, Ru, Cs)和M3V2O8 (M=Mg, Zn)的光学性质[60]
Table 3 Luminescence property of AVO3 (A=K, Ru, Cs) and M3V2O8 (M=Mg, Zn)[60]
| Sample | KVO3 | RbVO3 | CsVO3 | Mg3V2O8 | Zn3V2O8 |
|---|---|---|---|---|---|
| η/% | 4 | 79 | 87 | 6 | 52 |
| CIE(x, y) | 0.362, 0.453 | 0.316, 0.424 | 0.306, 0.418 | 0.449, 0.475 | 0.432, 0.478 |
| CCT/K | 4859 | 5993 | 6334 | 3318 | 3583 |
| AVO3 phase | PLE peak/nm | PL peak/nm | FWHM/nm | CIE(x, y) | CCT/K |
|---|---|---|---|---|---|
| CsVO3 (W) | 356 | 487 | 151 | (0.2421, 0.3283) | 12050 |
| CsVO3 (Y) | 342 | 503 | 138 | (0.2671, 0.3855) | 8444 |
| RbVO3 (W) | 357 | 491 | 149 | (0.2462, 0.3379) | 11152 |
| RbVO3 (R) | 342 | 510 | 149 | (0.2797, 0.3960) | 7700 |
表4 不同AVO3荧光粉的光学性能[63]
Table 4 Optical property of heterogeneous AVO3[63]
| AVO3 phase | PLE peak/nm | PL peak/nm | FWHM/nm | CIE(x, y) | CCT/K |
|---|---|---|---|---|---|
| CsVO3 (W) | 356 | 487 | 151 | (0.2421, 0.3283) | 12050 |
| CsVO3 (Y) | 342 | 503 | 138 | (0.2671, 0.3855) | 8444 |
| RbVO3 (W) | 357 | 491 | 149 | (0.2462, 0.3379) | 11152 |
| RbVO3 (R) | 342 | 510 | 149 | (0.2797, 0.3960) | 7700 |
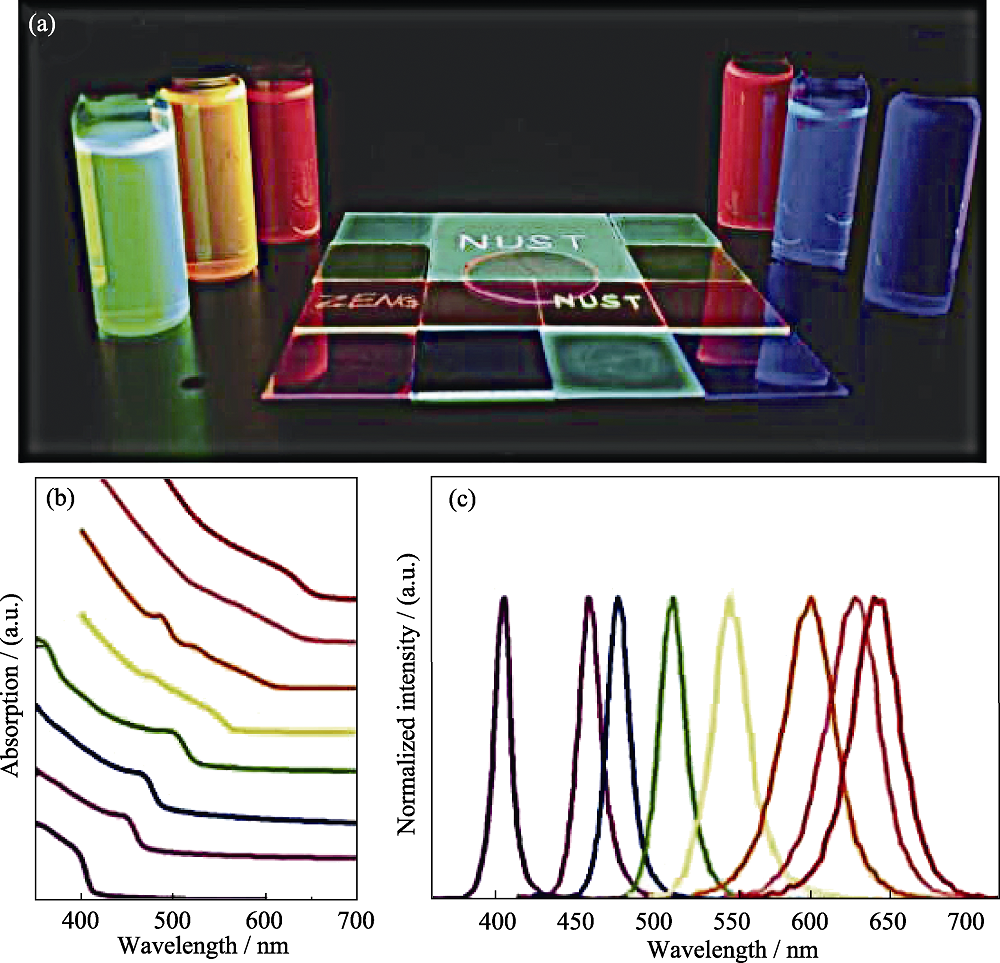
图11 (a)365 nm紫外光激发下的CsPbX3(X = Cl, Br, I)无机钙钛矿量子点溶液及薄膜样品, (b)吸收曲线, (c)不同组分的发射光谱[65]
Fig. 11 Controllable photoluminescence (a) Optical images of solution and film samples with different bandgaps under a 365 nm UV lamp; (b) Optical absorption; (c) Photoluminescence spectra of IPQDs with different composition[65]
| Phosphors | Advantages | Disadvantages | |
|---|---|---|---|
| Rare earth ion doped system | Single activator ion doped systems | High quantum conversion efficiency; wide emission spectrum range | Low color rendering index; high price; harmful to the environment |
| Multi-ions co-doping systems | |||
| Rare earth ion free systems | Semiconductor nanocrystal | Large absorption coefficient; wide excitation and emission band; high quantum yield; easy to be combined with packaging materials | Expensive raw materials; complex synthesis process; poor stability |
| Vanadate | High luminous efficiency; low preparation temperature | High color temperature; low intensity in red region | |
| Perovskite | Optical band gap adjustable; high quantum conversion efficiency | Pollution from soluble heavy metal Pb |
表5 各类单基质白光荧光粉的优缺点
Table 5 Advantages and disadvantages of single-phase WLEDs phosphors
| Phosphors | Advantages | Disadvantages | |
|---|---|---|---|
| Rare earth ion doped system | Single activator ion doped systems | High quantum conversion efficiency; wide emission spectrum range | Low color rendering index; high price; harmful to the environment |
| Multi-ions co-doping systems | |||
| Rare earth ion free systems | Semiconductor nanocrystal | Large absorption coefficient; wide excitation and emission band; high quantum yield; easy to be combined with packaging materials | Expensive raw materials; complex synthesis process; poor stability |
| Vanadate | High luminous efficiency; low preparation temperature | High color temperature; low intensity in red region | |
| Perovskite | Optical band gap adjustable; high quantum conversion efficiency | Pollution from soluble heavy metal Pb |
| [1] | LIU X Y . Development perspective of LED and backlight. Lamps & Lighting, 2008,12(4):14-17. |
| [2] | KIM J S, JEON P E, CHOI J C , et al. Emission color variation of M2SiO4:Eu 2+(M=Ba, Sr, Ca) phosphor for light-emitting diode. Solid State Commun. 2005,133(3):187-190. |
| [3] | KUO C H, SHEU J K, CHANG S J , et al. n-UV + blue/green/red white light emitting diode lamps. Jpn. J. Appl. Phys., 2003,42(4B):2284-2287. |
| [4] | CHO J, PARK J H, KIM J K , et al. White light-emitting diodes history, progress, and future. Laser Photonics. Rev., 2017,11(2):1600147. |
| [5] | ZHONG J, CGEN D, CHEN X , et al. Efficient rare-earth free red-emitting Ga2YSbO6: Mn 4+, M (M=Li +, Na +, K +, Mg 2+) phosphors for white light emitting diodes. Dalton Transactions, 2018,47:6528-6537. |
| [6] | PAVITRA E, SEETA RAMA RAJU G, KRISHNA B , et al. Evolution of highly efficient rare-earth free Cs(1-x)RbxVO3 phosphors as a single emitting component for NUV-based white LEDs. J. Mater. Chem. C, 2018,6(46):12746-12757. |
| [7] | LIN C C, TANG Y S, HU S F , et al. KBaPO4:Ln (Ln = Eu, Tb, Sm) phosphors for UV excitable white light-emitting diodes. J. Lumin., 2009,129(12):1682-1684. |
| [8] | LIN C C, LIU R S . Advances in phosphors for light-emitting diodes. J. Phys. Chem. Lett., 2011,2(11):1268-1277. |
| [9] | YE S, XIAO F, PAN X Y , et al. Phosphors in phosphor-converted white light-emitting diodes: recent advances in materials, techniques and properties. Mater. Sci. Eng., R, 2010,71(1):1-34. |
| [10] | MUTHU S, SCHUUMANS F J P, PASHLEY M D S . Red, green, and blue leds for white light illumination. IEEE J. Sel. Top. Quantum Electronics, 2002,8(2):333-338. |
| [11] | CRAWFORD M H . LEDs for solid state lighting: performance challenges and recent advances. IEEE J. Sel. Top. Quantum Electronics, 2009,15(4):1028-1040. |
| [12] | SHANG M M, LI C, LIN J . How to produce white light in a single- phase host? Chem. Soc. Rev. 2014,43(5):1372-1386. |
| [13] | LI G G, TIAN Y, ZHAO Y , et al. Recent progress in luminescence tuning of Ce 3+ and Eu 2+-activated phosphors for pc-WLEDs. Chem. Soc. Rev, 2015,44(23):8688-8713. |
| [14] | XIA Z G, MEJJERINK A . Ce 3+-doped garnet phosphors: composition modification, luminescence properties and applications . Chem. Soc. Rev., 2017,46(1):275-299. |
| [15] | KO M J, YOON H C, YOO H Y , et al. Highly efficient green Zn-Ag-In-S/Zn-In-S/Zns qds by a strong exothermic reaction for down-converted green and tripackage white LEDs. Adv. Funct. Mater., 2017,27(4):1602638. |
| [16] | CUI Y J, YUE Y F, QIAN G D , et al. Luminescent functional metal-organic frameworks. Chem. Rev., 2012,112(2):1126-1162. |
| [17] | HAIDER G, USMAN M, CHEN T P , et al. Electrically driven white light emission from intrinsic metal-organic framework. ACS Nano, 2016,10(9):8366-8375. |
| [18] | PATHAK S, SAKAI N, RIVAROLA F W R , et al. Perovskite crystals for tunable white light emission. Chem. Mater., 2015,27(23):8066-8075. |
| [19] | HE H, SONG X F, FU R L , et al. Crystal structure and luminescence of Li2Ca0.7Sr0.3SiO4:Eu 2+ and its application in multi-phosphor converted white LEDs. J. Alloys Compd, 2010,493(1/2):401-405. |
| [20] | WANG Z J, YANG Z P, GUO Q L , et al. Luminescence characteristics of Eu 2+ activated Ca2SiO4, Sr2SiO4 and Ba2SiO4 phosphors for white LEDs. Chin. Phys. B, 2009,18(5):2068-2071. |
| [21] | KIM J S, PARK Y H, KIM S M , et al. Temperature-dependent emission spectra of M2SiO4:Eu 2+(M=Ca, Sr, Ba) phosphors for green and greenish white LEDs. Solid State Commun., 2005,133(7):445-448. |
| [22] | KWON B J, GANDHI S, WOO H J , et al. Optical properties of CaSrSiO4:Eu2+ phosphors prepared by using a solid-state reaction method for white light-emitting diodes. Journal of the Korean Physical Society, 2015,67(3):556-562. |
| [23] | ANNADURAI G, LI B, DERAKUMAR B , et al. Synthesis, structural and photoluminescence properties of novel orange-red emitting Ba3Y2B6O15:Eu 3+ phosphors. Journal of Luminescence, 2019,208:75-81. |
| [24] | ZHANG W, SHEN H, HU X L , et al. Solid-state synthesis, structure and spectroscopic analysis of Dy:CaYAl3O7 phosphors. Journal of Alloys and Compounds, 2019,781:255-260. |
| [25] | WU Z C, LIU J, HOU W G , et al. A new single-host white-light- emitting BaSrMg(PO4)2: Eu 2+ phosphor for white-light-emitting diodes. J. Alloys Compd, 2010,498(2):139-142. |
| [26] | KIM J S, JEON P E, PARK Y H , et al. White-light generation through ultraviolet-emitting diode and white-emitting phosphor. Appl. Phys. Lett., 2004,85(17):3696. |
| [27] | SHANG M M, LI G G, KANG X J , et al. LaOF : Eu 3+ nanocrystals: hydrothermal synthesis, white and color-tuning emission properties. Dalton Trans., 2012,41(18):5571-5580. |
| [28] | LI C X, ZHANG C M, HOU Z Y , et al. β-NaYF4 and β-NaYF4:Eu 3+ microstructures: morphology control and tunable luminescence properties. J. Phys. Chem. C, 2009,113(6):2332-2339. |
| [29] | LIU X M, LI C X, QUAN Z W , et al. Tunable luminescence properties of CaIn2O4:Eu3+ phosphors. J. Phys. Chem. C, 2007 , 111(44):16601-16607. |
| [30] | LIANG C H, TEOH L G, LIU K T , et al. Near white light emission of BaY2ZnO5 doped with Dy3+ ions. J. Alloys Compd., 2012,517:9-13. |
| [31] | AUZEL F, PELLE F . Concentration and excitation efffects in multiphonon non-radiative transitions of rare-earth ions. Journal of Luminescence, 1996,69(5/6):249-255. |
| [32] | BLASSE G . Energy transfer in oxiidic phosphors. Physics Letters A, 1968,28(6):444-445. |
| [33] | LU M, ZHU C F, CHEN Z T , et al. Ca10Na(PO4)7: Eu 2+, Mn 2+ phosphors for ultraviolet light emitting diodes. Polyhedron., 2018,153:139-144. |
| [34] | HUANG C H, LIU W R, CHEN T M . Single-phased white-light phosphors Ca9Gd(PO4)7:Eu 2+,Mn 2+ under near-ultraviolet excitation . J. Phys. Chem. C, 2010,114(43):18698-18701. |
| [35] | YANG W J, LUO L Y, CHEN T M , et al. Luminescence and energy transfer of Eu- and Mn-coactivated CaAl2Si2O8 as a potential phosphor for white-Light UVLED. Chem. Mater., 2005,17(15):3883-3888. |
| [36] | HSU C H, DAS S, LU C H . Color-tunable, single phased MgY4Si3O13: Ce 3+, Mn 2+ phosphors with efficient energy transfer for white-light-emitting diodes . J. Electrochem. Soc., 2012,159(5):J193-J199. |
| [37] | LIU Y F, ZHANG X, HAO Z D , et al. Tunable full-color-emitting Ca3Sc2Si3O12:Ce 3+, Mn2+ phosphorvia charge compensation and energy transfer. Chem. Commun., 2011,47(38):10677-10679. |
| [38] | LAKSHMINARASIMHAN N, VARADARAJU U V . White-light generation in Sr2SiO4:Eu2+, Ce3+ under near-UV excitation: a novel phosphor for solid-state lighting. J. Electrochem. Soc., 2005,152(9):H152-H156. |
| [39] | CHANG C K, CHEN T M . Sr3B2O6:Ce 3+, Eu 2+: a potential single- phased white-emitting borate phosphor for ultraviolet light-emitting diodes . Appl. Phys. Lett., 2007,91(8):081902. |
| [40] | WEN Y, WANG Y H, ZHANG F , et al. Near-ultraviolet excitable Ca4Y6(SiO4)6O: Ce3+, Tb3+ white phosphors for light-emitting diodes. Mater. Chem. Phys., 2011,129(3):1171-1175. |
| [41] | JIAO H Y, WANG Y H . Ca2Al2SiO7: Ce 3+, Tb 3+: a white-light phosphor suitable for white-light-emitting diodes . J. Electrochem. Soc., 2009,156(5):J117-J120. |
| [42] | GONG Y, WANG Y H, LI Y Q . Ce 3+, Dy 3+ Co-doped white-light long-lasting phosphor: Sr2Al2SiO7 through energy transfer . J. Electrochem. Soc., 2010,157(6):J208-J211. |
| [43] | LIU X L, LIU Y X, YAN D T , et al. Single-phased white-emitting 12CaO·7Al2O3:Ce 3+, Dy 3+ phosphors with suitable electrical conductivity for field emission displays. J. Mater. Chem., 2012,22(3):16839-16843. |
| [44] | XUAN T T, LIU J Q, XIE R J , et al. Microwave-assisted synthesis of CdS/ZnS:Cu quantum dots for white light-emitting diodes with high color rendition. Chem. Mater., 2015,27(4):1187-1193. |
| [45] | KRAUSE M M, MOONEY J, KAMBHAMPATI P . Chemical and thermodynamic control of the surface of semiconductor nanocrystals for designer white light emitters. ACS Nano, 2013,7(7):5922-5929. |
| [46] | SHEN C C, TSENG W L . One-step synthesis of white-light emitting quantum dots at low temperature. Inorg. Chem., 2009,48(18):8689-8694. |
| [47] | ZIEGLER J, XU S, KUCUR E , et al. Silica-coated InP/ZnS nanocrystals as converter material in white LEDs. Adv. Mater., 2008,20(21):4068-4073. |
| [48] | LIM J H, PARK M J, BAE W K , et al. Highly efficient cadmium- free quantum dot light emitting diodes enabled by the direct formation of excitons within InP@ZnSeS quantum dots. ACS Nano, 2013,7(10):9019-9026. |
| [49] | BOL A A, MEJJERINK A . Luminescence of nanocrystalline ZnS: Pb 2+ . Phys. Chem., 2001,3(11):2105-2112. |
| [50] | CHEN H S, WANG S J J, LO C J , et al. White-light emission from organics-capped ZnSe quantum dots and application in white- light-emitting diodes. Appl. Phys. Lett., 2005,86(13):131905. |
| [51] | NIZAMOGLU S, MUTLUGUN E, AKYUZO , et al. White emitting CdS quantum dot nanoluminophores hybridized on near-ultraviolet LEDs for high-quality white light generation and tuning. New J. Phys., 2008,10:023026. |
| [52] | BOWERS M J, MCBRIDE J R, ROSENTHAL S J . White-light emission from magic-sized cadmium selenide nanocrystals. J. Am. Chem. Soc., 2005,127(44):15378-15379. |
| [53] | ROSSON T E, CLAIBOME S M, MCBRIDE J R , et al. Bright white light emission from ultrasmall cadmium selenide nanocrystals. J. Am. Chem. Soc., 2012,134(19):8006-8009. |
| [54] | LIU Q H, DENG R P, JI X L , et al. Alloyed Mn-Cu-In-S nanocrystals: a new type of diluted magnetic semiconductor quantum dots. Nanotechnology, 2012,23(25):255706. |
| [55] | DING K, JING L H, LIU C Y , et al. Magnetically engineered Cd-free quantum dots as dualmodality probes for fluorescence/ magnetic resonance imaging of tumors. Biomaterials, 2014,35(5):1608-1617. |
| [56] | ZHANG Z L, LIU D, LI D Z , et al. Dual emissive Cu-InP/ZnS/InP/ZnS nanocrystals: single-source“greener” emitters with flexibly tunable emission from visibleto near-infrared and their application in white light emitting diodes. Chem. Mater., 2015,27(4):1405-1411. |
| [57] | PENG L H, LI D Z, ZHANG Z L , et al. Large-scale synthesis of single-source, thermally stable, and dual-emissive Mn-doped Zn-Cu-In-S nanocrystals for bright white light-emitting diodes. Nano Research., 2015,8(10):3316-3331. |
| [58] | LI J, ZHOU H F, LIU X , et al. Spectral analysis and band gap of RbVO3. Spectroscopy and Spectral Analysis, 2010,12(30):3320-3323. |
| [59] | NAKAJIMA T, ISOBE M, TSUCHIYA T . Direct fabrication of metavanadate phosphor films on organic substrates for white- light-emitting devices. Nature Materials, 2008,7(9):735-740. |
| [60] | NAKAJIMA T, ISOBE M, TSUCHIYA T . A revisit of photoluminescence property for vanadate oxides AVO3 (A: K, Ru and Cs) and M3V2O8 (M: Mg and Zn). Journal of Luminescence, 2009,3(029):1598-1601. |
| [61] | NAKAJIMA T, ISOBE M, UZAWA Y , et al. Rare earth-free high color rendering white light-emitting diodes using CsVO3 with highest quantum efficiency for vanadate phosphors. J. Mater. Chem. C, 2015,3:10748. |
| [62] | LI J, LI X, WANG C , et al. Preparation and properties of CsVO3/polymer composite material. Journal of Functional Materials, 2014,39(45):3106-3109. |
| [63] | SUN G Y, LI W J, JI S D , et al. Heterogeneity in optimized solid-state synthesis of metavanadate AVO3(A=Rb, Cs). Res. Chem, Interned., 2017,43(1):341-352. |
| [64] | CHEN X, XIA Z G, YI M . Rare-earth free self-activated and rare-earth activated Ca2NaZn2V3O12 vanadate phosphors and their color-tunable luminescence properties. Journal of Physics and Chemistry of Solids, 2013,5(74):1439-1443. |
| [65] | LI X M, WU Y, ZHANG S L , et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Advanced Functional Materials, 2016,4(26):2435-2445. |
| [66] | LUO J J, WANG X M, LI S R , et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature, 2018,563:541-545. |
| [67] | DONG Y H, ZENG S Y, HAN B N , et al. BN/CsPbX3 composite nanocrystals: synthesis and applications in white LED. Journal of Inorganic Materials, 2019,34(1):72-78. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 周帆, 田志林, 李斌. 热防护系统用碳化物超高温陶瓷抗烧蚀涂层研究进展[J]. 无机材料学报, 2025, 40(1): 1-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||