无机材料学报 ›› 2017, Vol. 32 ›› Issue (9): 923-930.DOI: 10.15541/jim20160662 CSTR: 32189.14.10.15541/jim20160662
赵 晗1, 2, 周晓霞1, 潘琳钰1, 2, 陈航榕1
收稿日期:2016-11-28
修回日期:2017-01-16
出版日期:2017-09-30
网络出版日期:2017-08-29
作者简介:赵 晗(1991–), 男, 博士研究生. E-mail: zhaohan@student.sic.ac.cn
基金资助:ZHAO Han1, 2, ZHOU Xiao-Xia1, PAN Lin-Yu1, 2, CHEN Hang-Rong1
Received:2016-11-28
Revised:2017-01-16
Published:2017-09-30
Online:2017-08-29
About author:ZHAO Han. E-mail: zhaohan@student.sic.ac.cn
Supported by:摘要:
采用尿素均相沉淀法合成了CuCeZrOx(CCZ)三元复合氧化物催化剂。采用X射线衍射分析(XRD)、N2-吸脱附测试、X射线光电子能谱(XPS)、扫描透射电镜(STEM)、程序升温还原(脱附、氧化)等技术, 考察煅烧温度对催化剂的物化性质和催化碳烟燃烧活性的影响。结果表明: 350℃煅烧产物CCZ-350表现出最优的催化活性: 在空速为12000 mL/(gcatalyst·h), O2浓度为10vol%, NO浓度为500×10-6, 催化剂和碳烟以10︰1质量比松散接触条件下, 碳烟颗粒最大燃烧速率温度T50 = 407℃, 同时表现出极佳的抗水汽中毒和抗SO2中毒性能, 这与活性组分的高度分散以及催化剂表面大量高活性吸附氧物种的存在有关。此外, 催化剂材料具有疏松多孔的鸟巢状结构, 有利于催化剂和碳烟颗粒的充分接触。该催化剂在柴油车尾气排放温度范围内(150~400℃)还能完全催化氧化柴油车尾气中CO、C3H6和C3H8等其他污染物, 显示出优异的催化净化柴油车尾气的综合性能。
中图分类号:
赵 晗, 周晓霞, 潘琳钰, 陈航榕. 鸟巢状CuCeZrOx三元复合氧化物的合成及其对碳烟颗粒的催化氧化性能研[J]. 无机材料学报, 2017, 32(9): 923-930.
ZHAO Han, ZHOU Xiao-Xia, PAN Lin-Yu, CHEN Hang-Rong. Birdnest-like CuCeZr Mixed Oxides: Synthesis and Excellent Catalysts for Diesel Exhaust Oxidatio[J]. Journal of Inorganic Materials, 2017, 32(9): 923-930.
| Sample | Lattice parameter /nm a | SBET/ (m2·g-1) b | VBJH/ (cm3·g-1) c | Dp/nm c |
|---|---|---|---|---|
| CCZ-300 | 5.416 | 198.0 | 0.960 | 33.8 |
| CCZ-350 | 5.415 | 186.0 | 0.900 | 32.6 |
| CCZ-400 | 5.420 | 160.0 | 0.850 | 33.6 |
| CCZ-500 | 5.418 | 128.0 | 0.800 | 32.5 |
| CCZ-600 | 5.420 | 110.0 | 0.700 | 32.9 |
| CCZ-800 | 5.415 | 12.0 | 0.150 | 49.0 |
| CCZ-1000 | 5.389 | 0.1 | 0.001 | No data |
表1 不同温度煅烧产物CCZ-x的晶胞参数、比表面积、孔容和孔径(x = 300~1000)
Table 1 Structural properties of different CCZ-x catalysts (x = 300-1000)
| Sample | Lattice parameter /nm a | SBET/ (m2·g-1) b | VBJH/ (cm3·g-1) c | Dp/nm c |
|---|---|---|---|---|
| CCZ-300 | 5.416 | 198.0 | 0.960 | 33.8 |
| CCZ-350 | 5.415 | 186.0 | 0.900 | 32.6 |
| CCZ-400 | 5.420 | 160.0 | 0.850 | 33.6 |
| CCZ-500 | 5.418 | 128.0 | 0.800 | 32.5 |
| CCZ-600 | 5.420 | 110.0 | 0.700 | 32.9 |
| CCZ-800 | 5.415 | 12.0 | 0.150 | 49.0 |
| CCZ-1000 | 5.389 | 0.1 | 0.001 | No data |
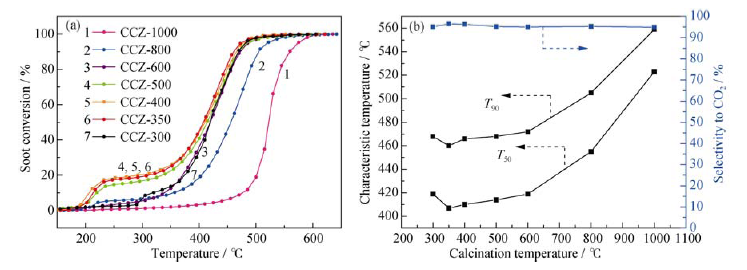
图2 CCZ-x(x=300-1000)催化碳烟氧化过程的(a)碳烟转化率曲线, (b)特征温度T90、T50及CO2选择性随煅烧温度的变化
Fig. 2 Temperature-programmed oxidation of soot catalyzed by different CCZ-x catalysts (x = 300-1000): soot conversion curves (a), and the change in characteristic temperatures (T90 and T50) and selectivity to CO2 () with increasing calcination temperature (b)
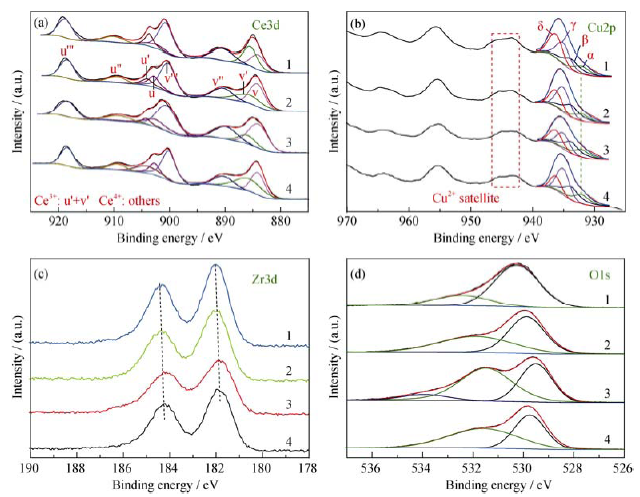
图3 CCZ-x (4: x = 300, 3: x = 350, 2: x = 500, 1: x = 800) 的XPS谱图: Ce 3d (a), Cu 2p (b), Zr 3d (c), O 1s (d)
Fig. 3 Ce 3d (a) , Cu 2p (b), Zr 3d (c), and O 1s (d) XPS patterns of CCZ-x (4: x = 300, 3: x = 350, 2: x = 500, 1: x = 800)
| Sample | Cu: Ce: Zr a | Oads/(Oads+Olatt) |
|---|---|---|
| CCZ-800 | 0.17: 0.60: 0.23 | 0.23 |
| CCZ-500 | 0.15: 0.65: 0.20 | 0.46 |
| CCZ-350 | 0.12: 0.67: 0.21 | 0.64 |
| CCZ-300 | 0.12: 0.69: 0.19 | 0.57 |
表2 不同煅烧温度产物CCZ-x(x = 800, 500, 350, 300)表面化学组分的XPS分析
Table 2 XPS surface analyses of different CCZ-x (x = 800, 500, 350, 300)
| Sample | Cu: Ce: Zr a | Oads/(Oads+Olatt) |
|---|---|---|
| CCZ-800 | 0.17: 0.60: 0.23 | 0.23 |
| CCZ-500 | 0.15: 0.65: 0.20 | 0.46 |
| CCZ-350 | 0.12: 0.67: 0.21 | 0.64 |
| CCZ-300 | 0.12: 0.69: 0.19 | 0.57 |
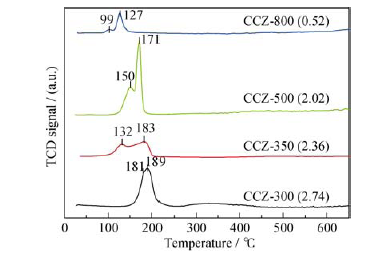
图4 不同煅烧温度产物CCZ-x (x = 300, 350, 500, 800)的H2-TPR 曲线 (括号中的数据为H2-TPR曲线的积分面积)
Fig. 4 H2-TPR profiles of the selected CCZ-x catalysts (x= 300, 350, 500, and 800) Data in the brackets indicate the integrated area of the H2-TPR curves

图5 不同煅烧温度产物CCZ-x (x = 300, 350, 500, 800)的O2-TPD曲线 (括号中的数据为O2-TPD曲线的积分面积)
Fig. 5 O2-TPD profiles of the selected CCZ-x catalysts (x= 300, 350, 500, and 800) Data in the brackets indicate the integrated area of the O2-TPD curves
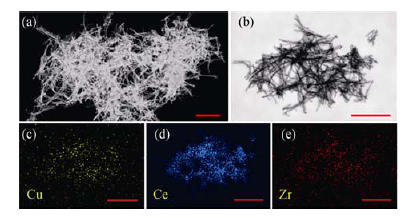
图6 CCZ-350的STEM (a, b) 图像和元素分布图(c-e)
Fig. 6 Dark-field (a) and bright-field (b) STEM images and elements mapping (c-e) (corresponding to TEM image in b) of CCZ-350 Scale bars: 100 nm
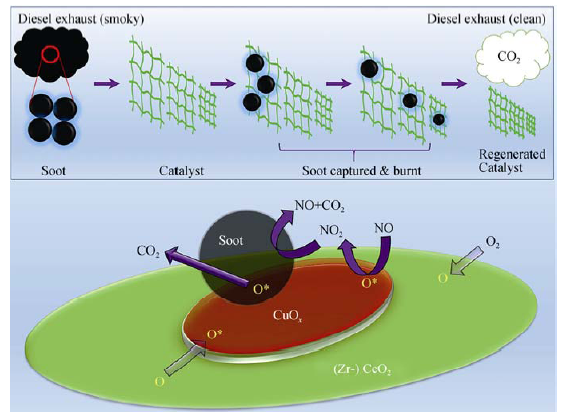
图7 CCZ-350催化碳烟燃烧过程中鸟巢结构“过滤+催化”作用示意图(a), 活性氧物种对碳烟氧化的催化作用机制(b)
Fig. 7 Schematic representation of (a) the role of the “nest structure” of CCZ-350 in soot combustion process, and (b) the soot combustion in NOx/O2 over CCZ-350 catalyst
| [1] | HORVATH H.Atmospheric light absorption—a revie.Atmospheric Environment, 1993, 27(3): 293-317. |
| [2] | VAN SETTEN B A A L, MAKKEE M, MOULIJN J A. Science and technology of catalytic diesel particulate filter.Catalysis Reviews-Science and Engineering, 2001, 43(4): 489-564. |
| [3] | YU X H, ZHAO Z, WEI Y C,et al.Synthesis of K-doped three-dimensionally ordered macroporous Mn0.5Ce0.5Oδ catalysts and their catalytic performance for soot oxidatio. Chinese Journal of Catalysis, 2015, 36(11): 1957-1967. |
| [4] | OBEID E, LIZARRAGA L, TSAMPAS M N,et al. Continuously regenerating diesel particulate filters based on ionically conducting ceramics. Journal of Catalysis, 2014, 309: 87-96. |
| [5] | UCHISAWA J O, OBUCHI A, ZHAO Z,et al. Carbon oxidation with platinum supported catalyst. Applied Catalysis B: Environmental, 1998, 18(3/4): L183-L187. |
| [6] | LIU J, ZHAO Z, WANG J Q,et al.The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustio. Applied Catalysis B: Environmental, 2008, 84(1/2): 185-195. |
| [7] | WU X D, LIU S, LIN F,et al.Nitrate storage behavior of Ba/MnOx-CeO2 catalyst and its activity for soot oxidation with heat transfer limitation. Journal of Hazardous Materials, 2010, 181(1/2/3): 722-728. |
| [8] | XU J F, LIU J, ZHAO Z,et al.Easy synthesis of three-dimensionally ordered macroporous La1-xKxCoO3 catalysts and their high activities for the catalytic combustion of soo. Journal of Catalysis, 2011, 282(1): 1-12. |
| [9] | CAO C M, ZHANG Y X, LIU D S,et al. Gravity-driven multiple collision-enhanced catalytic soot combustion over a space-open array catalyst consisting of ultrathin ceria nanobelt. Small, 2015, 11(30): 3659-3664. |
| [10] | AMADINE O, MATTI H, ABDELOUHADI K,et al.Ceria-supported copper nanoparticles: a highly efficient and recyclable catalyst for N-arylation of indol. Journal of Molecular Catalysis A: Chemical, 2014, 395: 409-419. |
| [11] | LIU L J, YAO Z J, LIU B,et al.Correlation of structural characteristics with catalytic performance of CuO/CexZr1-xO2 catalysts for NO reduction by C. Journal of Catalysis, 2010, 275(1): 45-60. |
| [12] | JIANG D, WANG W Z, ZHANG L,et al.A strategy for improving deactivation of catalytic combustion at low temperature via synergistic photo catalysi. Applied Catalysis B: Environmental, 2015, 165: 399-407. |
| [13] | KONSOLAKIS M.The role of copper-ceria interactions in catalysis science: recent theoretical and experimental advance.Applied Catalysis B: Environmental, 2016, 198: 49-66. |
| [14] | SAINZ-VIDAL A, BALMASEDA J, LARTUNDO-ROJAS L,et al. Preparation of Cu-mordenite by ionic exchange reaction under milling: a favorable route to form the mono-(μ-oxo) dicopper active specie. Microporous and Mesoporous Materials, 2014, 185: 113-120. |
| [15] | VERHELST J, DECROUPET D, DE VOS D.Catalytic self-cleaning coatings for thermal oxidation of organic deposits on glas.Catalysis Science & Technology, 2013, 3(6): 1579-1590. |
| [16] | DUPIN J C, GONBEAU D, VINATIER P,et al. Systematic XPS studies of metal oxides, hydroxides and peroxide. Physical Chemistry Chemical Physics, 2000, 2(6): 1319-1324. |
| [17] | HUO C L, OUYANG J, YANG H M.CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous material.via direct synthetic route. Scientific Reports, 2014, 4: 3682. |
| [18] | FEI Z Y, LU P, FENG X Z,et al. Geometrical effect of CuO nanostructures on catalytic benzene combustio. Catalysis Science & Technology, 2012, 2(8): 1705-1710. |
| [19] | BUENO-LOPEZ A.Diesel soot combustion ceria catalyst.Applied Catalysis B: Environmental, 2014, 146: 1-11. |
| [20] | ANEGGI E, DE LEITENBURG C, DOLCETTI G, et al. Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2-ZrO2.Catalysis Today, 114(1): 40-47. |
| [21] | KASPAR J, FORNASIERO P, GRAZIANI M.Use of CeO2-based oxides in the three-way catalysi.Catalysis Today, 1999, 50(2): 285-298. |
| [22] | SETIABUDI A, MAKKEE M, MOULIJN J A.The role of NO2 and O2 in the accelerated combustion of soot in diesel exhaust gase.Applied Catalysis B: Environmental, 2004, 50(3): 185-194. |
| [1] | 王晓波, 朱于良, 薛稳超, 史汝川, 骆柏锋, 罗骋韬. PT含量变化对PMN-PT单晶的大功率性能影响[J]. 无机材料学报, 2025, 40(7): 840-846. |
| [2] | 汤新丽, 丁自友, 陈俊锐, 赵刚, 韩颖超. 基于稀土铕离子荧光标记的磷酸钙纳米材料体内分布与代谢研究[J]. 无机材料学报, 2025, 40(7): 754-764. |
| [3] | 余乐洋阳, 赵芳霞, 张舒心, 徐以祥, 牛亚然, 张振忠, 郑学斌. 感应等离子球化技术制备喷涂用高熵硼化物粉体[J]. 无机材料学报, 2025, 40(7): 808-816. |
| [4] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [5] | 孙晶, 李翔, 毛小建, 章健, 王士维. 月桂酸改性剂对氮化铝粉体抗水解性能的影响[J]. 无机材料学报, 2025, 40(7): 826-832. |
| [6] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [7] | 王鲁杰, 张玉新, 李彤阳, 于源, 任鹏伟, 王建章, 汤华国, 姚秀敏, 黄毅华, 刘学建, 乔竹辉. 深海服役环境下碳化硅陶瓷材料的腐蚀及磨损行为[J]. 无机材料学报, 2025, 40(7): 799-807. |
| [8] | 李文元, 徐佳楠, 邓瀚澳, 常爱民, 张博. 钒取代对LaTaO4陶瓷微观结构和微波介电性能的影响[J]. 无机材料学报, 2025, 40(6): 697-703. |
| [9] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [10] | 董晨雨, 郑维杰, 马一帆, 郑春艳, 温峥. 压电力显微镜表征Pb(Mg,Nb)O3-PbTiO3超薄膜弛豫特性[J]. 无机材料学报, 2025, 40(6): 675-682. |
| [11] | 何国强, 张恺恒, 王震涛, 包健, 席兆琛, 方振, 王昌昊, 王威, 王鑫, 姜佳沛, 李祥坤, 周迪. Ba(Nd1/2Nb1/2)O3: 一种被低估的K40微波介质陶瓷[J]. 无机材料学报, 2025, 40(6): 639-646. |
| [12] | 张家维, 陈宁, 程原, 王博, 朱建国, 金城. Bi4Ti3O12铋层状压电陶瓷的A/B位掺杂及其电学性能[J]. 无机材料学报, 2025, 40(6): 690-696. |
| [13] | 崔宁, 张玉新, 王鲁杰, 李彤阳, 于源, 汤华国, 乔竹辉. (TiVNbMoW)Cx高熵陶瓷的单相形成过程与碳空位调控[J]. 无机材料学报, 2025, 40(5): 511-520. |
| [14] | 熊思宇, 莫尘, 朱肖伟, 朱国斌, 陈德钦, 刘来君, 施晓东, 李纯纯. 超低介电常数LiBxAl1-xSi2O6微波介质陶瓷的低温烧结[J]. 无机材料学报, 2025, 40(5): 536-544. |
| [15] | 安然, 林锶, 郭世刚, 张冲, 祝顺, 韩颖超. 铁掺杂纳米羟基磷灰石的制备及紫外吸收性能研究[J]. 无机材料学报, 2025, 40(5): 457-465. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||