无机材料学报 ›› 2015, Vol. 30 ›› Issue (8): 825-832.DOI: 10.15541/jim20140675 CSTR: 32189.14.10.15541/jim20140675
曲 婷, 黄 强, 赵振波
收稿日期:2014-12-25
修回日期:2015-04-07
出版日期:2015-08-20
网络出版日期:2015-07-21
作者简介:曲 婷(1990–), 女, 硕士研究生. E-mail:chemicalqt@126.com
QU Ting, HUANG Qiang, ZHAO Zhen-Bo
Received:2014-12-25
Revised:2015-04-07
Published:2015-08-20
Online:2015-07-21
About author:QU Ting. E-mail:chemicalqt@126.com
摘要:
采用水热法和共沉淀法结合制备Bi2MoO6/Ni-Fe LDH复合材料, 通过XRD、FT-IR、SEM、TEM、XPS和N2物理吸附等对样品的结构和形貌进行表征。以甲基橙、亚甲基蓝、罗丹明B和苯酚为目标降解物, 在可见光下进行复合材料的光催化性能测试, 以降解甲基橙溶液为例研究复合材料的光催化反应机理。结果表明, 复合材料的BET比表面积随着Ni-Fe LDH含量的增加而增大, 光催化活性明显提高。Bi2MoO6/Ni-Fe LDH复合材料中Ni-Fe LDH的含量为4.5%时具有最好的光催化效果, 可见光照射60 min, 甲基橙的降解率达91%, 较Bi2MoO6和Ni-Fe LDH分别提高52%和16%。Bi2MoO6/Ni-Fe LDH复合材料具有良好的稳定性, 循环使用5次, 甲基橙(MO)的降解率为88%。复合材料光催化降解甲基橙反应遵循一级反应动力学。
中图分类号:
曲 婷, 黄 强, 赵振波. Bi2MoO6/Ni-Fe LDH复合材料的制备及可见光催化性能[J]. 无机材料学报, 2015, 30(8): 825-832.
QU Ting, HUANG Qiang, ZHAO Zhen-Bo. Preparation and Visible Light Responsive Photocatalytic Activity of Bi2MoO6/Ni-Fe LDH Composites[J]. Journal of Inorganic Materials, 2015, 30(8): 825-832.
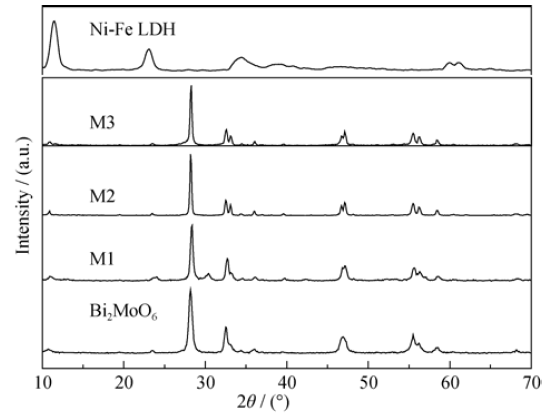
图1 Bi2MoO6, Ni-Fe LDH和他们的复合物M1、M2、M3的XRD图谱
Fig. 1 XRD patterns of Bi2MoO6, Ni-Fe LDH and their composites M1, M2 and M3 at Ni-Fe LDH contents of 2%, 4.5% and 15%, respectively

图2 Bi2MoO6、Ni-Fe LDH和他们的复合物M1、M1、M3的FT-IR图谱
Fig. 2 FT-IR spectra of Bi2MoO6 and Ni-Fe LDH and their composites M1, M2 and M3 at Ni-Fe LDH contents of 2%, 4.5% and 15%, respectively

图3 Bi2MoO6(a,b)和Bi2MoO6/Ni-Fe LDH(c,d)复合材料在低倍(a,c)和高倍(b,d)下的SEM照片
Fig. 3 SEM images of Bi2MoO6 (a,b) and Bi2MoO6/Ni-Fe LDH(c,d)) at low (a,c) and high (b,d) magnifications
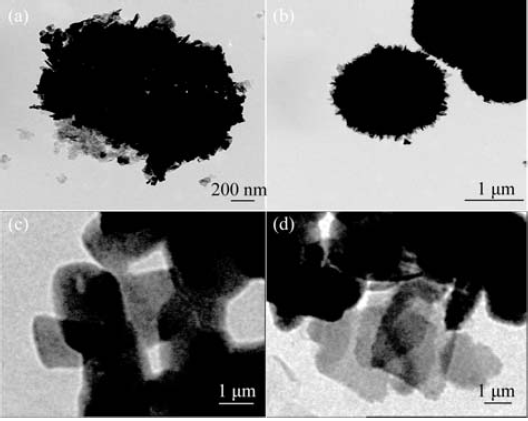
图4 Bi2MoO6 (a,b)和Bi2MoO6/Ni-Fe LDH复合材料(c,d)在低倍(a,c)和高倍(b,d)下的TEM照片
Fig. 4 TEM images of Bi2MoO6 (a,b) and Bi2MoO6/Ni-Fe LDH(c,d) at low (a,c) and high (b,d) magnifications
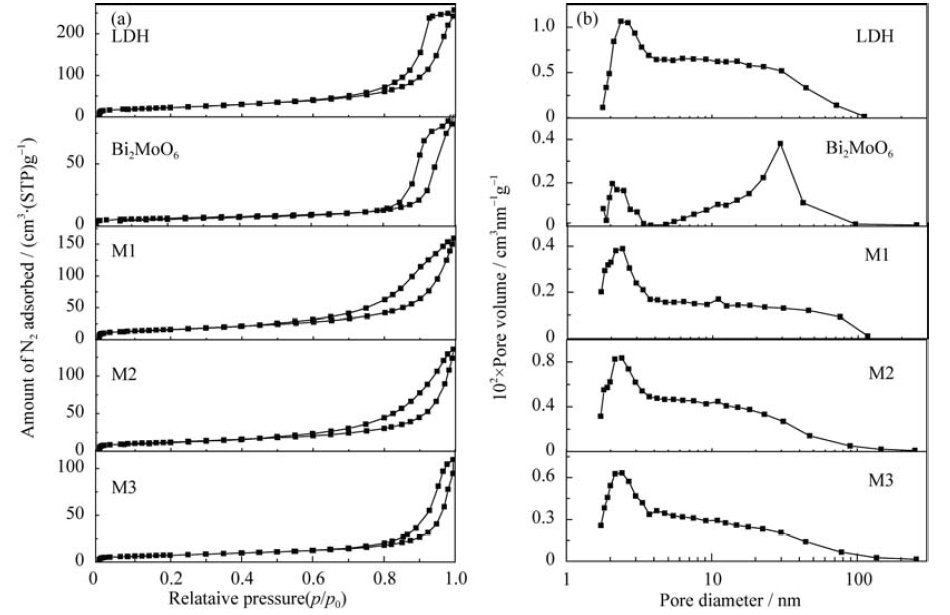
图5 Bi2MoO6,Ni-Fe LDH和他们的复合物M1~M3的N2吸附-脱附等温线(a)及孔径分布(b)
Fig. 5 N2 adsorption-desorption isotherms (a) and pore size distributions (b) of Bi2MoO6 and Ni-Fe LDH and their composites M1-M3 samples with different Ni-Fe LDH contents (2%; 4.5%; 15%)
| Catalyst | Bi2MoO6 | Ni-Fe LDH | M1 | M2 | M3 |
|---|---|---|---|---|---|
| BET surface area /(m2·g-1) | 20.07 | 80.25 | 26.64 | 43.81 | 58.41 |
| Vpore /(m2·g-1) | 0.153 | 0.376 | 0.146 | 0.210 | 0.248 |
| dpore /nm | 30.14 | 16.14 | 20.26 | 17.47 | 15.18 |
表1 Bi2MoO6、Ni-Fe LDH和复合材料M1~M3的比表面积、孔体积和孔径
Table1 BET surface area, pore volume and pore size of Bi2MoO6, Ni-Fe LDH and their composites M1-M3
| Catalyst | Bi2MoO6 | Ni-Fe LDH | M1 | M2 | M3 |
|---|---|---|---|---|---|
| BET surface area /(m2·g-1) | 20.07 | 80.25 | 26.64 | 43.81 | 58.41 |
| Vpore /(m2·g-1) | 0.153 | 0.376 | 0.146 | 0.210 | 0.248 |
| dpore /nm | 30.14 | 16.14 | 20.26 | 17.47 | 15.18 |
| C0 /(mg·L-1) | First-order reaction kinetics equation | R |
|---|---|---|
| 10 | ln(c0/c)=0.03765t-0.0223 | 0.9965 |
| 12 | ln(c0/c)= 0.02122t-0.04275 | 0.9971 |
| 14 | ln(c0/c)= 0.0166t-0.03749 | 0.9958 |
| 16 | ln(c0/c)= 0.01578t-0.01939 | 0.9962 |
表2 Bi2MoO6/Ni-Fe LDH复合材料光催化降解甲基橙溶液动力学参数
Table 2 Kinetic parameter of Bi2MoO6/Ni-Fe LDH composites for photocatalytic degradation of MO
| C0 /(mg·L-1) | First-order reaction kinetics equation | R |
|---|---|---|
| 10 | ln(c0/c)=0.03765t-0.0223 | 0.9965 |
| 12 | ln(c0/c)= 0.02122t-0.04275 | 0.9971 |
| 14 | ln(c0/c)= 0.0166t-0.03749 | 0.9958 |
| 16 | ln(c0/c)= 0.01578t-0.01939 | 0.9962 |
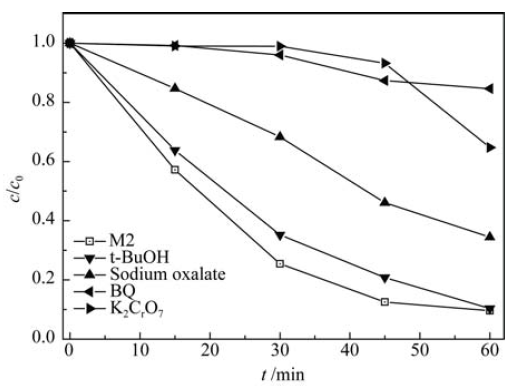
图10 外加不同清除剂(20 mmol/L的 t-BuOH、0.2 mmol/L的草酸钠、10 mmol/L的K2Cr2O7和0.1 mmol/L 的BQ)时, Bi2MoO6/Ni-Fe LDH复合材料光催化降解MO曲线
Fig. 10 Photocatalytic degradation efficiency for MO by the catalyst Bi2MoO6/Ni-Fe LDH composite after addition of different scavengers (20 mmol/L t-BuOH, 0.2 mmol/L sodium oxalate, 10 mmol/L K2Cr2O7 and 0.1 mmol/L BQ)
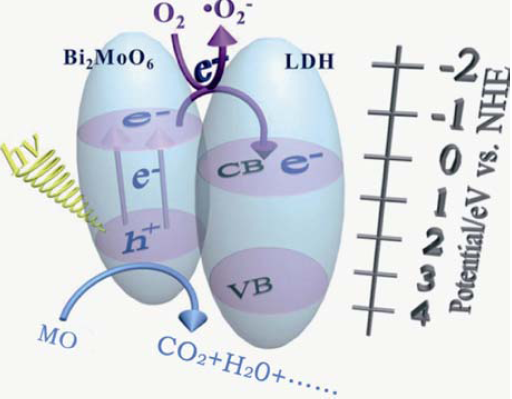
图11 可见光下Bi2MoO6/Ni-Fe LDH复合材料降解MO的示意图
Fig. 11 Schematic illustrati on showing MO degradation over Bi2MoO6/Ni-Fe LDH composite under visible light irradiation
| [1] | CHEN X B, SHEN S H, GUO L J, et al.Semiconductor-based photocatalytic hydrogen generation.Chem. Rev., 2010, 110(11): 6503-6570. |
| [2] | MARYE ANNE FOX, MARIA T, DULAY. Heterogeneous photocatalysis.Chem. Rev., 1993, 93(1): 341-357. |
| [3] | MICHAEL R HOFFMANN, SCOT T MARTIN, DETLEF W BAHNEMANN, et al.Environmental applications of semiconductor photocatalysis.Chem. Rev., 1995, 95(1): 69-96. |
| [4] | SURENDAR TONDA, SANTOSH KUMAR, VISHNU SHANKER, et al.Synthesis of Cr and La-codoped SrTiO3 nanoparticles for enhanced photocatalytic performance under sunlight irradiation.Chem. Phys., 2014, 16: 23819-23828. |
| [5] | ZHOU X, JI H B, HUANG X J.Photocatalytic degradation of methyl orange over metallopopphyrins supported on TiO2 degussa P25.Molecules, 2012, 17(2): 1149-1158. |
| [6] | JIMMY C YU, YU J G, ZHANG L Z, et al.Effects of F- doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders.Chemistry of Materials, 2002, 14(9): 3808-3816. |
| [7] | GE L, HAN C C, LIU J. Novel visible linght-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B, 2011, 108-109: 100-107. |
| [8] | YANG J X, ZHOU X, LI C, et al.A theoretical study on the mechanism of photocatalytic oxygen evolution on BiVO4 in aqueous solution.Chem. Eur., 2013, 19(4): 1320-1326. |
| [9] | TIAN G H, WANG C J, FU H G, et al.In situ growth of Bi2MoO6 on reduced graphene oxide nanosheets for improved visible-light photocatalytic activity.Cryst. Eng. Comm., 2014, 16: 842-849. |
| [10] | XU Y S, ZHANG W D.Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance.Dalton Trans., 2013, 42(4): 1094-1101. |
| [11] | XU Y S, ZHANG W D. Anion exchange strategy for construction of sesame-biscuit-like Bi2O2CO3/Bi2MoO6 nanocomposites with enhanced photocatalytic activity. Applied Catalysis B: Environmental, 2013, 140-141: 306-316. |
| [12] | ZHANG M Y, ZHANG P, LIU Y, et al.One-dimensional Bi2MoO6/TiO2 hierarchical heterostructures with enhanced photocatalytic activity.Cryst. Eng. Comm., 2012, 14(2): 605-612. |
| [13] | ZHAO X, LIU H J, QU J H, et al.Photocatalytic reduction of bromate at C60 modified Bi2MoO6 under visible light irradiation.Appl. Catal. B, 2011, 106(1): 63-68. |
| [14] | HUANG Z J, ZHU N W, DANG Z, et al. Enhancement of photocatalytic degradation of dimethyl phthalate with nano-TiO2 immobilized onto hydrophobic layered double hydroxides: a mechanism study. Hazard. Mater, 2013, 246-247: 70-78. |
| [15] | LI H P, DENG Q H, LIU J Y, et al.Synthesis, characterization and enhanced visible light photocatalytic activity of Bi2MoO6/Zn-Al layered double hydroxide hierarchical heterostructures.Catalysis Science and Technology, 2014, 4: 1028-1037. |
| [16] | SEFTE E.M, MERTENS L M., Cool P. The influence of the Ti4+ location on the formation of delf-asserhbled nanocomposite sys- tems based on TiO2 and Mg/Al-LDHs with photocatalytic properties. Applied Catalysis B: Environmental, 2013, 134: 274-285. |
| [17] | WANG Q, DERMOT O’HARE. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets.Chem. Rev., 2012, 112: 4124-4155. |
| [18] | SHER BAHADUR RAWAL, DEVI PRASHAD OJHA, SANG DO SUNG, et al.Fe2WO6/TiO2, an efficient visible-light photocatalyst driven by hole-transport mechanism.Catalysis Communications, 2014, 56: 55-59. |
| [19] | XU Y, MARTIN A A SCHOONEN. The absolute energy positions of conduction and valence bands of selected semiconducting minerals.American Mineralogist, 2000, 85: 543-556. |
| [20] | ZHOU T, LI F, ZHAN K T, et al.Preparation and characterization of layered precursor nickel-iron hydrotalcites and magnetic materials.Acta Chimica Sinica, 2002, 60(6): 1078-1083. |
| [21] | KHASSIN ALEXANDER A, CHERMASHENTSEVA GALINA K, PARMON VALENTIN N, et al.Cobalt-aluminum co-precipitated catalysts and their performance in the Fischer-Tropsch synthesis .Catal. A: Chem., 2001, 168(1/2): 193-207. |
| [22] | SEUNGHO CHO, EUN SUN KIM, JAE SUNG LEE.Anion-doped mixed metal oxide nanostructures derived from layered double hydroxide as visible light photocatalysts.Adv. Funct. Mater., 2013, 23(19): 2348-2356. |
| [23] | MENG Y B, HUANG X, WU Y X, et al.Kinetic study and modeling on photocatalytic degradation of para-chlorobenzoate at different light intensities.Environmental Pollution, 2002, 117(2): 307-313. |
| [1] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [2] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [3] | 贾相华, 张辉霞, 刘艳凤, 左桂鸿. 湿化学法制备Cu2O/Cu空心球异质结光催化剂[J]. 无机材料学报, 2025, 40(4): 397-404. |
| [4] | 马彬彬, 钟婉菱, 韩涧, 陈椋煜, 孙婧婧, 雷彩霞. ZIF-8/TiO2复合介观晶体的制备及光催化活性[J]. 无机材料学报, 2024, 39(8): 937-944. |
| [5] | 曹青青, 陈翔宇, 吴健豪, 王筱卓, 王乙炫, 王禹涵, 李春颜, 茹菲, 李兰, 陈智. SiO2增强自敏性氮化碳微球可见光降解盐酸四环素的研究[J]. 无机材料学报, 2024, 39(7): 787-792. |
| [6] | 王兆阳, 秦鹏, 蒋胤, 冯小波, 杨培志, 黄富强. 三明治结构钌插层二氧化钛光催化四环素降解性能研究[J]. 无机材料学报, 2024, 39(4): 383-389. |
| [7] | 叶茂森, 王耀, 许冰, 王康康, 张胜楠, 冯建情. II/Z型Bi2MoO6/Ag2O/Bi2O3异质结可见光催化降解四环素[J]. 无机材料学报, 2024, 39(3): 321-329. |
| [8] | 李秋实, 殷广明, 吕伟超, 王怀尧, 李婧琳, 杨红光, 关芳芳. Na+/g-C3N4材料的制备及光催化降解亚甲基蓝机理[J]. 无机材料学报, 2024, 39(10): 1143-1150. |
| [9] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [10] | 伍林, 胡明蕾, 王丽萍, 黄少萌, 周湘远. TiHAP@g-C3N4异质结的制备及光催化降解甲基橙[J]. 无机材料学报, 2023, 38(5): 503-510. |
| [11] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [12] | 牛海滨, 黄佳慧, 李倩文, 马董云, 王金敏. 多孔NiMoO4纳米片薄膜的直接水热生长及其电致变色性能[J]. 无机材料学报, 2023, 38(12): 1427-1433. |
| [13] | 孙晨, 赵昆峰, 易志国. 甲烷完全催化氧化研究进展[J]. 无机材料学报, 2023, 38(11): 1245-1256. |
| [14] | 贾鑫, 李晋宇, 丁世豪, 申倩倩, 贾虎生, 薛晋波. Pd纳米颗粒协同氧空位增强TiO2光催化CO2还原性能[J]. 无机材料学报, 2023, 38(11): 1301-1308. |
| [15] | 马润东, 郭雄, 施凯旋, 安胜利, 王瑞芬, 郭瑞华. MoS2/g-C3N4 S型异质结的构建及光催化性能研究[J]. 无机材料学报, 2023, 38(10): 1176-1182. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||