无机材料学报 ›› 2023, Vol. 38 ›› Issue (1): 43-54.DOI: 10.15541/jim20220578 CSTR: 32189.14.10.15541/jim20220578
所属专题: 【生物材料】抗菌与肿瘤治疗(202506)
• 专栏:抗疫生物材料(特邀编辑: 杨勇) • 上一篇 下一篇
收稿日期:2022-09-29
修回日期:2022-11-09
出版日期:2023-01-20
网络出版日期:2022-12-09
通讯作者:
范克龙, 教授. E-mail: fankelong@ibp.ac.cn作者简介:吴雪彤(2000-), 女,博士研究生. E-mail: wuxuetong21@mails.ucas.ac.cn
WU Xuetong1,2( ), ZHANG Ruofei2, YAN Xiyun2, FAN Kelong2(
), ZHANG Ruofei2, YAN Xiyun2, FAN Kelong2( )
)
Received:2022-09-29
Revised:2022-11-09
Published:2023-01-20
Online:2022-12-09
Contact:
FAN Kelong, professor. E-mail: fankelong@ibp.ac.cnAbout author:WU Xuetong (2000-), female, PhD candidate. E-mail: wuxuetong21@mails.ucas.ac.cn
Supported by:摘要:
细菌和病毒一直对人类健康构成威胁。SARS-CoV-2已经在世界各地肆虐了近三年, 给人类健康带来了巨大危险。面对细菌的抗药性和抗生素治疗效果不佳等种种挑战, 人们迫切需要新的方法来对抗致病微生物。最近, 具有内在酶活性的纳米酶作为一种有前途的新型“抗生素”, 通过催化生成大量活性氧, 在生理条件下表现出卓越的抗菌和抗病毒活性。此外, 基于纳米酶的治疗中, 纳米材料在独特的物理化学特性(如光热和光动力效应)的帮助下可以增强治疗效果。本文综述了纳米酶在抗菌、抗病毒-方向的研究进展, 从机制角度系统总结分析了纳米酶消除细菌、病毒等微生物的原理, 对未来的新型纳米抗菌抗病毒材料的研发方向及其所面临的挑战进行了展望, 为开发下一代抗微生物感染纳米酶提供了思路。
中图分类号:
吴雪彤, 张若飞, 阎锡蕴, 范克龙. 纳米酶: 一种抗微生物感染新方法[J]. 无机材料学报, 2023, 38(1): 43-54.
WU Xuetong, ZHANG Ruofei, YAN Xiyun, FAN Kelong. Nanozyme: a New Approach for Anti-microbial Infections[J]. Journal of Inorganic Materials, 2023, 38(1): 43-54.
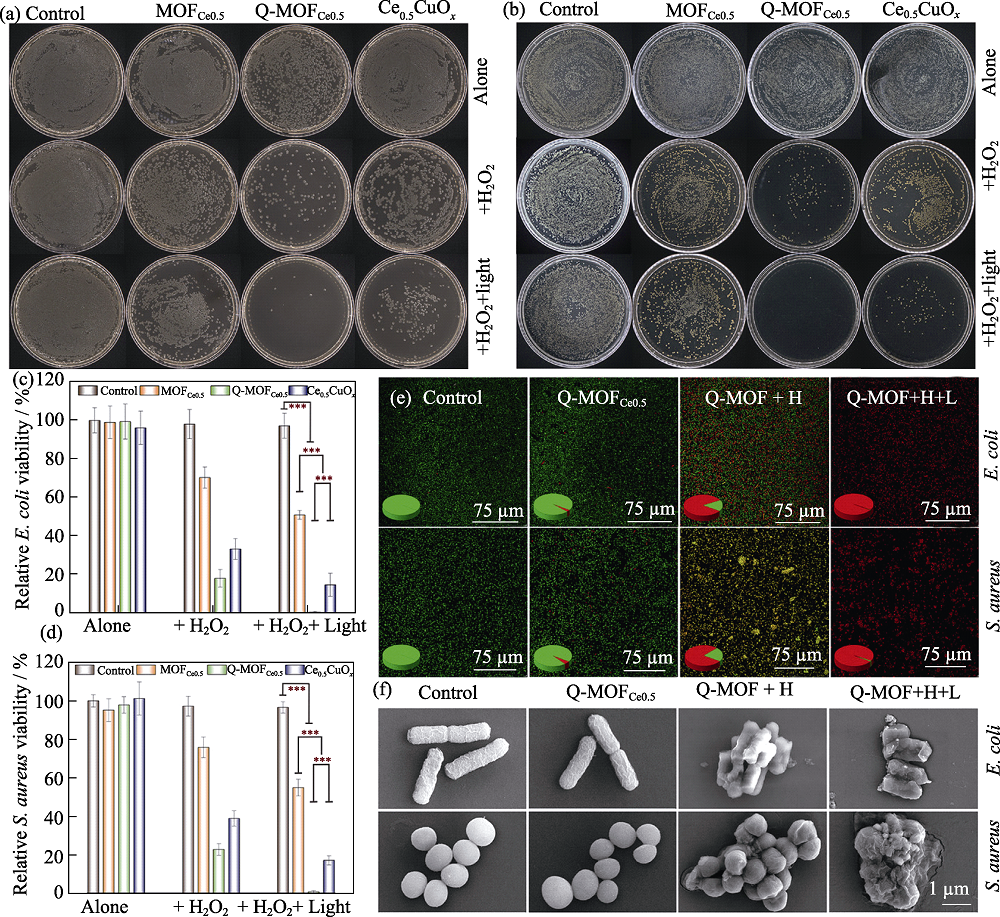
Fig. 2 Antibacterial killing effect of a nanozyme, Q-MOFCe0.5[32] (a, b) Plate count results of the antibacterial effects of Q-MOFCe0.5 on E. coli (a) and S. aureus (b); (c, d) Statistical results of the corresponding bacterial viability rates of E. coli (c) and S. aureus (d); (e) The live (SYTO-9, green)/dead (PI, red) staining results of E. coli and S. aureus; (f) Scanning electron microscope (SEM) results of bacterial samples treated by nanozymes Colorful figures are available on website
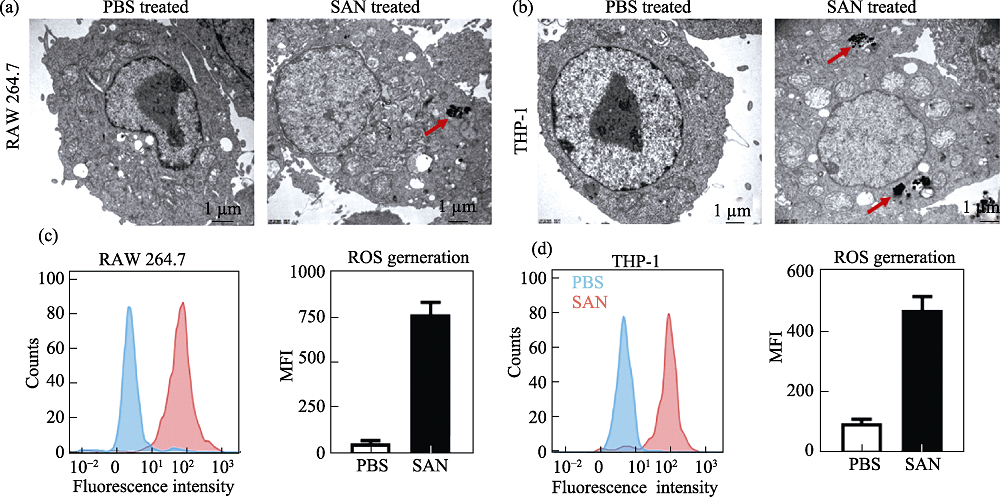
Fig. 3 Characterization and ROS generation ability of a nanozyme, Ag-TiO2 SAN[34] (a, b) Transmission electron microscope (TEM) images of Ag-TiO2 SAN co-localized with lysosomes of mouse (RAM 264.7) and human (THP-1) macrophages; (c, d) Ag-TiO2 SAN enhanced ROS generation. THP-1: a kind of human monocytic leukemia cell Colorful figures are available on website
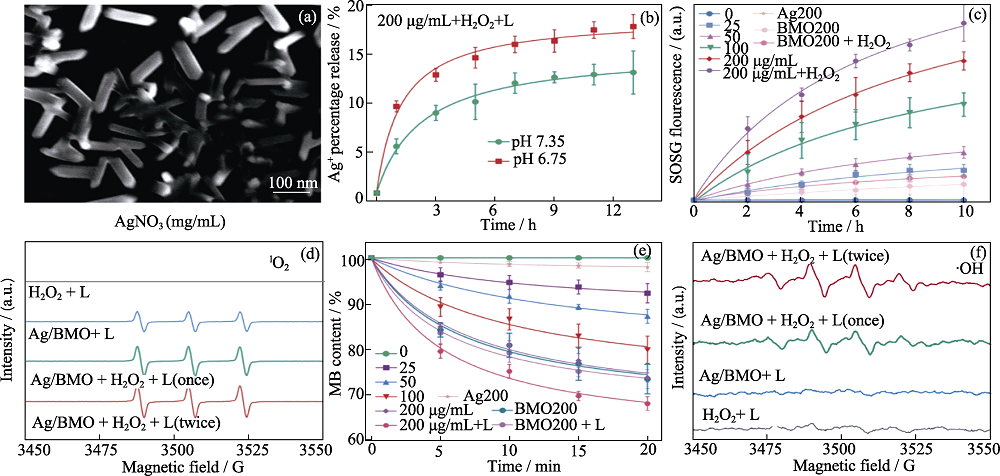
Fig. 4 Characterization, Ag+ release, and ROS generation ability of Ag/BMO NPs through photodynamic combination therapy[47] (a) SEM image of Ag/BMO NPs; (b) Percentage release of Ag+ at pH 7.35 and pH 6.75 showing photo-enhanced ROS generation ability of Ag/BMO; (c) 1O2 detection result by probe of single oxygen sensor green; (d) ESR spectra of 1O2; (e) ·OH detection result by MB indicator; (f) ESR spectra of ·OH. L: 1064 nm laser (1 W·cm-2, 10 min) Colorful figures are available on website
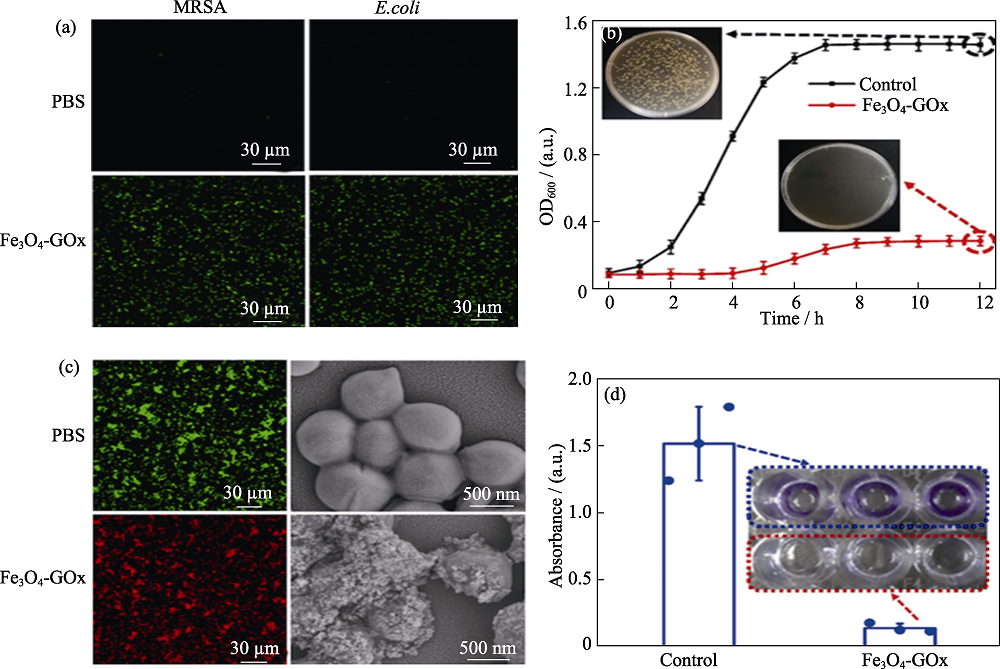
Fig. 5 In vitro antimicrobial performance of Fe3O4-GOx based on cascaded reaction[50] (a) 2',7'-Dichlorofluorescein staining images of E. coli and MRSA; (b) Growth curves of MRSA; (c) Representative SEM and live/dead staining images of MRSA; (d) Crystal violet staining image and its absorbance for integrated MRSA biofilm Colorful figures are available on website
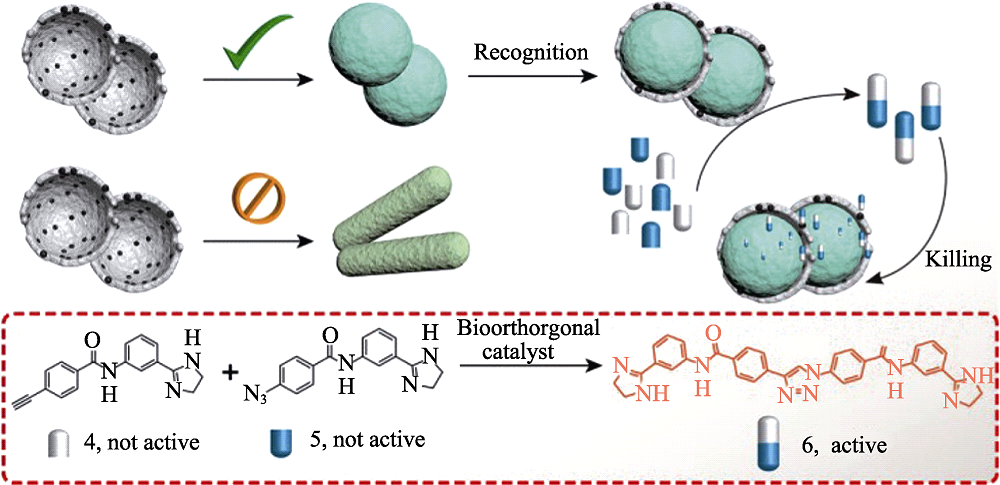
Fig. 6 S-Ab can be used as bio-orthogonal catalyst to complete shape-based selective recognition of bacteria and catalyze precursors into active antibacterial molecule[56] Colorful figure is available on website
| Mechanism | Nanozyme | Catalytic activity | Mechanism | Pathogens | Ref. |
|---|---|---|---|---|---|
| Generation ROS | Cu-MOF | OXD | ROS | E. coli; S. aureus | [ |
| Cu-CD | POD | ROS | E. coli; S. aureus | [ | |
| Chitosan grafted Fe-doped-carbon dots (CS@Fe/CDs) | POD | ROS | P. aeruginosa; S. aureus. | [ | |
| Ag-TiO2 SAN | POD | ROS | SARS-CoV-2 | [ | |
| Fmoc-diphenylalanine hydrogel-eEncapsulated Pt | OXD; POD | ROS | E. coli; S. aureus | [ | |
| CFO@BFO nanozyme-eel | CAT | ROS | E. coli; MRSA | [ | |
| Cu2O@CuO; Cu@Cu2S nanodot | OXD; POD | ROS | E. coli; S. aureus | [ | |
| FeCo@PDA NPs | POD | ROS | E. coli; S. aureus | [ | |
| Fe3O4@SiO2@dendritic mesoporous silica@small-Fe3O4 nanoparticles | POD | ROS | E. coli | [ | |
| Mesoporous vanadium oxide nanospheres | POD | ROS | E. coli; S. aureus | [ | |
| Au3+-UiO-67 NMOFs | OXD; POD | ROS | E. coli; S. aureus | [ | |
| CS@Fe3O4 | POD; SOD; CAT | ROS | A. baumannii | [ | |
| N/Cl-CDs + Ag NPs | OXD | ROS | E. coli; S. aureus; MRSA | [ | |
| Cu-N-C | OXD; POD | ROS | E. coli; S. aureus; B. cereus; C. albicans; MRSA | [ | |
| PEGMA-co-GMA-co-AAm-HBPL-MnO2 hydrogel | CAT; POD | ROS | P. aeruginosa; S. aureus; E. coli | [ | |
| GOx-MOF hydrogel | GOx; POD | ROS | E. coli; S. aureus | [ | |
| Taurine-Cu-3(PO4)(2) hybrid nanoflower | POD | ROS | E. coli; S. aureus; B. cereus; C.albicans | [ | |
| FePO4-HG | POD; SOD; CAT | ROS | E. coli; S. aureus | [ | |
| Combination therapy | Au/MoO3-x | POD | Photothermal; ROS(O2•-) | MRSA | [ |
| Cu-MOFN nanosheet | POD | Hot electron transferred ROS | S. aureus | [ | |
| CuSeNPs@MPBA | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Hollow mesoporous Prussian blue nanoparticles (HMPBNPs) | POD | ROS; Photothermal | E. coli; S. aureus | [ | |
| Bacitracin-functionalized dextran-MoSe2(AMP/dex-MoSe2 NSs) | POD | Photothermal; ROS | E. coli | [ | |
| Mechanism | Nanozyme | Catalytic activity | Mechanism | Pathogens | Ref. |
| Combination therapy | Ag/Bi2MoO6 | POD | Photodynamic; ROS | S. aureus | [ |
| CuxO-PDA | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Histidine-containing carbon nanodots | OXD | Photodynamic; ROS | E. coli | [ | |
| VOx-artificial enzyme | OXD, POD | Electron enhanced ROS | S. aureus | [ | |
| EM@MoS2 | POD | Photothermal; ROS | S. aureus | [ | |
| LS-CuS@PVA | POD | Photothermal; Photodynamic; ROS | E. coli; S. aureus | [ | |
| D-A-conjugated COF | POD | Photothermal; Photodynamic; ROS | E. coli; S. aureus | [ | |
| Cu3SnS4 NSs | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Mn3O4HNSs@ICG | OXD | Photothermal; Photodynamic; ROS | E. faecalis; E. coli; P. aeruginosa | [ | |
| Au NCs@PCN MOF | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Ag (8.5%)@NiS2-x | POD | Photothermal; ROS | E. coli | [ | |
| Cascaded reaction | Fe3O4-GOx | GOx; CAT; POD | ROS | E. coli; S. aureus | [ |
| Fe2(MoO4)3@GOx | GOx; POD | ROS | E. coli; S. aureus | [ | |
| ODex/gC/MoS2@Au@BSA Hydrogel | POD | ROS | E. coli; S. aureus | [ | |
| CuO nanospheres | POD; CAT | ROS | E. coli; S. aureus | [ | |
| MnFe2O4@MIL/Au&GOx(MMAG) | GOx; POD | ROS | E. coli; S. aureus | [ | |
| Bio-orthogonal catalysis | Man-NZs(Au, FeTPP) | Bio-orthogonal catalysis | Salmonella; Lactobacillus sp. | [ | |
| Man-NZ | Bio-orthogonal catalysis | E. coli; MSRA | [ | ||
| E-Ab and S-Ab | Bio-orthogonal catalysis | E. coli; S. aureus | [ | ||
| Others | CeO2@ZrO2 | Haloperoxidase | HBr- | E. coli; S. aureus | [ |
Table 1 Nanozymes for anti-microbial infections
| Mechanism | Nanozyme | Catalytic activity | Mechanism | Pathogens | Ref. |
|---|---|---|---|---|---|
| Generation ROS | Cu-MOF | OXD | ROS | E. coli; S. aureus | [ |
| Cu-CD | POD | ROS | E. coli; S. aureus | [ | |
| Chitosan grafted Fe-doped-carbon dots (CS@Fe/CDs) | POD | ROS | P. aeruginosa; S. aureus. | [ | |
| Ag-TiO2 SAN | POD | ROS | SARS-CoV-2 | [ | |
| Fmoc-diphenylalanine hydrogel-eEncapsulated Pt | OXD; POD | ROS | E. coli; S. aureus | [ | |
| CFO@BFO nanozyme-eel | CAT | ROS | E. coli; MRSA | [ | |
| Cu2O@CuO; Cu@Cu2S nanodot | OXD; POD | ROS | E. coli; S. aureus | [ | |
| FeCo@PDA NPs | POD | ROS | E. coli; S. aureus | [ | |
| Fe3O4@SiO2@dendritic mesoporous silica@small-Fe3O4 nanoparticles | POD | ROS | E. coli | [ | |
| Mesoporous vanadium oxide nanospheres | POD | ROS | E. coli; S. aureus | [ | |
| Au3+-UiO-67 NMOFs | OXD; POD | ROS | E. coli; S. aureus | [ | |
| CS@Fe3O4 | POD; SOD; CAT | ROS | A. baumannii | [ | |
| N/Cl-CDs + Ag NPs | OXD | ROS | E. coli; S. aureus; MRSA | [ | |
| Cu-N-C | OXD; POD | ROS | E. coli; S. aureus; B. cereus; C. albicans; MRSA | [ | |
| PEGMA-co-GMA-co-AAm-HBPL-MnO2 hydrogel | CAT; POD | ROS | P. aeruginosa; S. aureus; E. coli | [ | |
| GOx-MOF hydrogel | GOx; POD | ROS | E. coli; S. aureus | [ | |
| Taurine-Cu-3(PO4)(2) hybrid nanoflower | POD | ROS | E. coli; S. aureus; B. cereus; C.albicans | [ | |
| FePO4-HG | POD; SOD; CAT | ROS | E. coli; S. aureus | [ | |
| Combination therapy | Au/MoO3-x | POD | Photothermal; ROS(O2•-) | MRSA | [ |
| Cu-MOFN nanosheet | POD | Hot electron transferred ROS | S. aureus | [ | |
| CuSeNPs@MPBA | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Hollow mesoporous Prussian blue nanoparticles (HMPBNPs) | POD | ROS; Photothermal | E. coli; S. aureus | [ | |
| Bacitracin-functionalized dextran-MoSe2(AMP/dex-MoSe2 NSs) | POD | Photothermal; ROS | E. coli | [ | |
| Mechanism | Nanozyme | Catalytic activity | Mechanism | Pathogens | Ref. |
| Combination therapy | Ag/Bi2MoO6 | POD | Photodynamic; ROS | S. aureus | [ |
| CuxO-PDA | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Histidine-containing carbon nanodots | OXD | Photodynamic; ROS | E. coli | [ | |
| VOx-artificial enzyme | OXD, POD | Electron enhanced ROS | S. aureus | [ | |
| EM@MoS2 | POD | Photothermal; ROS | S. aureus | [ | |
| LS-CuS@PVA | POD | Photothermal; Photodynamic; ROS | E. coli; S. aureus | [ | |
| D-A-conjugated COF | POD | Photothermal; Photodynamic; ROS | E. coli; S. aureus | [ | |
| Cu3SnS4 NSs | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Mn3O4HNSs@ICG | OXD | Photothermal; Photodynamic; ROS | E. faecalis; E. coli; P. aeruginosa | [ | |
| Au NCs@PCN MOF | POD | Photothermal; ROS | E. coli; S. aureus | [ | |
| Ag (8.5%)@NiS2-x | POD | Photothermal; ROS | E. coli | [ | |
| Cascaded reaction | Fe3O4-GOx | GOx; CAT; POD | ROS | E. coli; S. aureus | [ |
| Fe2(MoO4)3@GOx | GOx; POD | ROS | E. coli; S. aureus | [ | |
| ODex/gC/MoS2@Au@BSA Hydrogel | POD | ROS | E. coli; S. aureus | [ | |
| CuO nanospheres | POD; CAT | ROS | E. coli; S. aureus | [ | |
| MnFe2O4@MIL/Au&GOx(MMAG) | GOx; POD | ROS | E. coli; S. aureus | [ | |
| Bio-orthogonal catalysis | Man-NZs(Au, FeTPP) | Bio-orthogonal catalysis | Salmonella; Lactobacillus sp. | [ | |
| Man-NZ | Bio-orthogonal catalysis | E. coli; MSRA | [ | ||
| E-Ab and S-Ab | Bio-orthogonal catalysis | E. coli; S. aureus | [ | ||
| Others | CeO2@ZrO2 | Haloperoxidase | HBr- | E. coli; S. aureus | [ |
| [1] |
WIERSINGA W J, RHODES A, CHENG A C, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA-Journal of the American Medical Association, 2020, 324(8): 782.
DOI URL |
| [2] |
GRIEF S N, LOZA J K. Guidelines for the evaluation and treatment of pneumonia. Primary Care, 2018, 45(3): 485.
DOI PMID |
| [3] |
BLACK R E, MORRIS S S, BRYCE J. Where and why are 10 million children dying every year? Lancet, 2003. 361(9376): 2226,
DOI PMID |
| [4] |
DENYS G A, RELICH R F. Antibiotic resistance in nosocomial respiratory infections. Clinics in Laboratory Medicine, 2014, 34(2): 257.
DOI PMID |
| [5] |
YAP V, DATTA D, METERSKY M L. Is the present definition of health care-associated pneumonia the best way to define risk of infection with antibiotic-resistant pathogens? Infectious Disease Clinics of North America, 2013, 27(1): 1.
DOI PMID |
| [6] |
BOUCHER H W, TALBOT G H, BRADLEY J S, et al. Bad bugs, no drugs: no eskape! An update from the infectious diseases society of America. Clinical Infectious Diseases, 2009, 48(1): 1.
DOI PMID |
| [7] |
PITOUT J D D, LAUPLAND K B. Extended-spectrum beta-lactamase-producing enterobacteriaceae: an emerging public-health concern. Lancet Infectious Diseases, 2008, 8(3): 159.
DOI PMID |
| [8] |
TONG S Y C, DAVIS J S, EICHENBERGER E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical Microbiology Reviews, 2015, 28(3): 603.
DOI PMID |
| [9] |
YANG R, XU J, XU L, et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano, 2018, 12(6): 5121.
DOI PMID |
| [10] |
ZHU G Z, LYNN G M, JACOBSON O, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nature Communications, 2017, 8: 1954.
DOI URL |
| [11] |
LU J, LIONG M, LI Z, et al. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small, 2010, 6(16): 1794.
DOI PMID |
| [12] |
TIAN Y, LI S, SONG J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 2014, 35(7): 2383.
DOI PMID |
| [13] |
YANG Y, LI Z Y, YAMAGUCHI K, et al. Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale, 2012, 4(8): 2663.
DOI PMID |
| [14] |
GAO L, ZHUANG J, NIE L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology, 2007, 2(9): 577.
DOI PMID |
| [15] |
XU B, WANG H, WANG W, et al. A single-atom nanozyme for wound disinfection applications. Angewandte Chemie International Edition, 2019, 58(15): 4911.
DOI URL |
| [16] |
ZHAO H Q, ZHANG R F, YAN X Y, et al. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. Journal of Materials Chemistry B, 2021, 9(35): 6939.
DOI PMID |
| [17] |
HU X, LI F Y, XIA F, et al. Biodegradation-mediated enzymatic activity-tunable molybdenum oxide nanourchins for tumor-specific cascade catalytic therapy. Journal of the American Chemical Society, 2020, 142(3): 1636.
DOI PMID |
| [18] |
MENG X, LI D, CHEN L, et al. High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano, 2021, 15(3): 5735.
DOI PMID |
| [19] | JIANG B, YAN L, ZHANG J, et al. Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Applied Materials & Interfaces, 2019, 11(10): 9747. |
| [20] |
JIANG D W, NI D L, ROSENKRANS Z T, et al. Nanozyme: new horizons for responsive biomedical applications. Chemical Society Reviews, 2019, 48(14): 3683.
DOI PMID |
| [21] |
WANG Z, LI G, GAO Y, et al. Trienzyme-like iron phosphates-based (FePOs) nanozyme for enhanced anti-tumor efficiency with minimal side effects. Chemical Engineering Journal, 2021, 404: 125574.
DOI URL |
| [22] | ZHANG Y A, LI Y N, ZHANG J L, et al. Nanocage-based capture-detection system for the clinical diagnosis of autoimmune disease. Small, 2021, 17(25): 2101655. |
| [23] |
DUAN D, FAN K, ZHANG D, et al. Nanozyme-strip for rapid local diagnosis of ebola. Biosensors & Bioelectronics, 2015, 74: 134.
DOI URL |
| [24] |
MENG X, ZOU S, LI D, et al. Nanozyme-strip for rapid and ultrasensitive nucleic acid detection of SARS-CoV-2. Biosensors & Bioelectronics, 2022, 217: 114739.
DOI URL |
| [25] |
MAO Z, CHEN J, WANG Y, et al. Copper metal organic framework as natural oxidase mimic for effective killing of gram- negative and gram-positive bacteria. Nanoscale, 2022, 14(26): 9474.
DOI URL |
| [26] | CAO M, CHANG Z, TAN J, et al. Superoxide radical-mediated self-synthesized Au/MoO(3-x) hybrids with enhanced peroxidase-like activity and photothermal effect for anti-MRSA therapy. ACS Applied Materials & Interfaces, 2022, 14(11): 13025. |
| [27] | WANG Y, YAO J, CAO Z, et al. Peroxidase-mimetic copper-doped carbon-dots for oxidative stress-mediated broad-spectrum and efficient antibacterial activity. Chemistry-a European Journal, 2022, 28: e202104174. |
| [28] |
FUENTES K M, ONNA D, RIOUAL T, et al. Copper upcycling by hierarchical porous silica spheres functionalized with branched polyethylenimine: antimicrobial and catalytic applications. Microporous and Mesoporous Materials, 2021, 327: 111391.
DOI URL |
| [29] |
SINGH N, SAVANUR M A, SRIVASTAVA S, et al. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson's disease model. Angewandte Chemie International Edition, 2017, 56(45): 14267.
DOI URL |
| [30] |
MU X, WANG J, LI Y, et al. Redox trimetallic nanozyme with neutral environment preference for brain injury. ACS Nano, 2019, 13(2): 1870.
DOI PMID |
| [31] |
AI Y, YOU J, GAO J, et al. Multi-shell nanocomposites based multienzyme mimetics for efficient intracellular antioxidation. Nano Research, 2021, 14(8): 2644.
DOI URL |
| [32] |
HUANG L, SUN D W, PU H. Photosensitized peroxidase mimicry at the hierarchical 0D/2D heterojunction-like quasi metal-organic framework interface for boosting biocatalytic disinfection. Small, 2022, 18(20): 2200178.
DOI URL |
| [33] |
PAN T, CHEN H, GAO X, et al. Engineering efficient artificial nanozyme based on chitosan grafted Fe-doped-carbon dots for bacteria biofilm eradication. Journal of Hazardous Materials, 2022, 435: 128996.
DOI URL |
| [34] |
WANG D, ZHANG B, DING H, et al. TiO2 supported single Ag atoms nanozyme for elimination of SARS-COV2. Nano Today, 2021, 40: 101243.
DOI URL |
| [35] |
NI Y, WANG J, WANG M, et al. COVID-19-inspired "artificial virus" to combat drug-resistant bacteria by membrane-intercalation- photothermal-photodynamic multistage effects. Chemical Engineering Journal, 2022, 446: 137322.
DOI URL |
| [36] |
SARINA S, WACLAWIK E R, ZHU H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chemistry, 2013, 15(7): 1814.
DOI URL |
| [37] |
MU M, WEN S, HU S, et al. Putting surface-enhanced Raman spectroscopy to work for nanozyme research: methods, materials and applications. Trac-Trends in Analytical Chemistry, 2022, 152: 116603.
DOI URL |
| [38] |
LIAO X, XU Q, SUN H, et al. Plasmonic nanozymes: localized surface plasmonic resonance regulates reaction kinetics and antibacterial performance. Journal of Physical Chemistry Letters, 2022, 13(1): 312.
DOI PMID |
| [39] | LI L, YANG J, WEI J, et al. SERS monitoring of photoinduced- enhanced oxidative stress amplifier on Au@carbon dots for tumor catalytic therapy. Light-Science & Applications, 2022, 11(1): 286. |
| [40] |
WANG D, FANG Y, YU W, et al. Significant solar energy absorption of mxene Ti3C2Tx nanofluids via localized surface plasmon resonance. Solar Energy Materials and Solar Cells, 2021, 220: 110850.
DOI URL |
| [41] |
ZANDI O, AGRAWAL A, SHEARER A B, et al. Impacts of surface depletion on the plasmonic properties of doped semiconductor nanocrystals. Nature Materials, 2018, 17(8): 710.
DOI PMID |
| [42] |
GENG X, ZHANG D, ZHENG Z, et al. Integrated multifunctional device based on Bi2S3/Pd: localized heat channeling for efficient photothermic vaporization and real-time health monitoring. Nano Energy, 2021, 82: 105700.
DOI URL |
| [43] |
CHEN X, WANG X, FANG Y, et al. Long-lasting chemiluminescence- based poct for portable and visual pathogenic detection and in situ inactivation. Analytical Chemistry, 2022, 94: 8382.
DOI URL |
| [44] |
LI Y, ZHU Y, WANG C, et al. Mild hyperthermia induced by hollow mesoporous prussian blue nanoparticles in alliance with a low concentration of hydrogen peroxide shows powerful antibacterial effect. Molecular Pharmaceutics, 2022, 19(3): 819.
DOI URL |
| [45] | LIN T, JIANG G, LIN D, et al. Bacitracin-functionalized dextran- mose 2 with peroxidase-like and near-infrared photothermal activities for low-temperature and synergetic antibacterial applications. ACS Applied Biomaterials, 2022, 5(5): 2347. |
| [46] |
SONGCA S P, ADJEI Y. Applications of antimicrobial photodynamic therapy against bacterial biofilms. International Journal of Molecular Sciences, 2022, 23(6): 3209.
DOI URL |
| [47] |
GAO C, ZHANG T, YANG N, et al. POD nanozyme optimized by charge separation engineering for light/pH activated bacteria catalytic/photodynamic therapy. Signal Transduction and Targeted Therapy, 2022, 7(1): 86.
DOI PMID |
| [48] | HE S, FENG Y, SUN Q, et al. Charge-switchable CuxO nanozyme with peroxidase and near-infrared light enhanced photothermal activity for wound antibacterial application. ACS Applied Materials & Interfaces, 2022, 14(22): 25042. |
| [49] |
LOUKANOV A, KURIBARA A, NIKOLOVA S, et al. Light-activated oxidize-mimicking nanozyme for inhibition of pathogenic escherichia coli. Microscopy Research and Technique, 2022, 85(5): 1949.
DOI PMID |
| [50] |
DU X, JIA B, WANG W, et al. pH-switchable nanozyme cascade catalysis: a strategy for spatial-temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer. Journal of Nanobiotechnology, 2022, 20(1): 12.
DOI PMID |
| [51] | ZHANG Y, LI D, XU Y, et al. Application of a cascaded nanozyme in infected wound recovery of diabetic mice. ACS Biomaterials Science & Engineering, 2022, 8(4): 1522. |
| [52] |
FEDELI S, IM J, GOPALAKRISHNAN S, et al. Nanomaterial-based bioorthogonal nanozymes for biological applications. Chemical Society Reviews, 2021, 50(24): 13467.
DOI PMID |
| [53] |
NGUYEN S S, PRESCHER J A. Developing bioorthogonal probes to span a spectrum of reactivities. Nature Reviews Chemistry, 2020, 4(9): 476.
DOI PMID |
| [54] |
HARDIE J, MAKABENTA J M, GUPTA A, et al. Selective treatment of intracellular bacterial infections using host cell-targeted bioorthogonal nanozymes. Materials Horizons, 2022, 9(5): 1489.
DOI URL |
| [55] |
GUPTA A, DAS R, MAKABENTA J M, et al. Erythrocyte-mediated delivery of bioorthogonal nanozymes for selective targeting of bacterial infections. Materials Horizons, 2021, 8(12): 3424.
DOI PMID |
| [56] |
NIU J, WANG L, CUI T, et al. Antibody mimics as bio-orthogonal catalysts for highly selective bacterial recognition and antimicrobial therapy. ACS Nano, 2021, 15(10): 15841.
DOI PMID |
| [57] | CHEN J, ZHANG S, CHEN X, et al. A self-assembled fmoc-diphenylalanine hydrogel-encapsulated Pt nanozyme as oxidase-and peroxidase-like breaking ph limitation for potential antimicrobial application. Chemistry-a European Journal, 2022, 28(26): e202104247. |
| [58] |
DENG Q, ZHANG L, LIU X, et al. Magnetoelectrically ignited nanozyme-eel for combating bacterial biofilms. Chemical Communications, 2022, 58(55): 7634.
DOI URL |
| [59] |
HE Y, YIN M, SUN J, et al. Excellent catalytic properties of luminescent Cu@Cu2S nanozymes and their antibacterial applications. Chemical Communications, 2022, 58(18): 2995.
DOI URL |
| [60] |
KUANG F, CHEN Y, SHAN W, et al. Biomimetic FeCo@Pd a nanozyme platform with fenton catalytic activity as efficient antibacterial agent. Journal of Materials Chemistry B, 2022, 10(29): 5582.
DOI URL |
| [61] |
HUANG Y, LIU D, GUO R, et al. Magnetic-controlled dandelion-like nanocatalytic swarm for targeted biofilm elimination. Nanoscale, 2022, 14(17): 6497.
DOI URL |
| [62] |
LI P, FENG Y, CHENG D, et al. Self-template synthesis of mesoporous vanadium oxide nanospheres with intrinsic peroxidase-like activity and high antibacterial performance. Journal of Colloid and Interface Science, 2022, 625: 435.
DOI PMID |
| [63] |
PAN M M, OUYANG Y, SONG Y L, et al. Au3+-functionalized UIO-67 metal-organic framework nanoparticles: O2-(center dot-) and center dot OH generating nanozymes and their antibacterial functions. Small, 2022, 18(23): 2200548.
DOI URL |
| [64] |
WANG W, WU Z, SHI P, et al. Antibacterial effect of chitosan-modified Fe3O4 nanozymes on Acinetobacter baumannii. Journal of Microbiology and Biotechnology, 2022, 32(2): 263.
DOI URL |
| [65] |
ZHU J, LI Q, LI X, et al. Simulated enzyme activity and efficient antibacterial activity of copper-doped single-atom nanozymes. Langmuir, 2022, 38(22): 6860.
DOI URL |
| [66] |
ZHU J, LI X, WU X, et al. Nanocomposite of Ag nanoparticles and deep eutectic solvent-derived carbon dots with oxidase mimicking activity as synergistic bactericidal agent. Letters in Applied Microbiology, 2022, 74(5): 684.
DOI URL |
| [67] |
TU C, LU H, ZHOU T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials, 2022, 286: 121597.
DOI URL |
| [68] |
ZHANG S, DING F, LIU Y, et al. Glucose-responsive biomimetic nanoreactor in bacterial cellulose hydrogel for antibacterial and hemostatic therapies. Carbohydrate Polymers, 2022, 292: 119615.
DOI URL |
| [69] |
YILMAZ S G, DEMIRBAS A, KARAAGAC Z, et al. Synthesis of taurine-Cu3(PO4)2 hybrid nanoflower and their peroxidase-mimic and antimicrobial properties. Journal of Biotechnology, 2022, 343: 96.
DOI URL |
| [70] | LIAO Z Y, GAO W W, SHAO N N, et al. Iron phosphate nanozyme-hydrogel with multienzyme-like activity for efficient bacterial sterilization. ACS Applied Materials & Interfaces, 2022, 14(16): 18170. |
| [71] |
LI L, CAO S, WU Z, et al. Modulating electron transfer in vanadium-based artificial enzymes for enhanced ROS-catalysis and disinfection. Advanced Materials, 2022, 34(17): 2108646.
DOI URL |
| [72] |
SHI T, RUAN Z, WANG X, et al. Erythrocyte membrane- enveloped molybdenum disulfide nanodots for biofilm elimination on implants via toxin neutralization and immune modulation. Journal of Materials Chemistry B, 2022, 10(11): 1805.
DOI URL |
| [73] | XIE Y, GAN C, LI Z, et al. Fabrication of a lignin-copper sulfide-incorporated PVA hydrogel with near-infrared-activated photothermal/photodynamic/peroxidase-like performance for combating bacteria and biofilms. ACS Biomaterials Science & Engineering, 2022, 8(2): 560. |
| [74] | YANG G P, MENG X L, XIAO S J, et al. Construction of D-a-conjugated covalent organic frameworks with enhanced photodynamic, photothermal, and nanozymatic activities for efficient bacterial inhibition. ACS Applied Materials & Interfaces, 2022, 14(24): 28289. |
| [75] |
YANG Y, WANG C, WANG N, et al. Photogenerated reactive oxygen species and hyperthermia by Cu3SnS4 nanoflakes for advanced photocatalytic and photothermal antibacterial therapy. Journal of Nanobiotechnology, 2022, 20(1): 195.
DOI URL |
| [76] |
ZHANG X, MIN Y, ZHANG Q, et al. Functionalized Mn3O4 nanosheets with photothermal, photodynamic, and oxidase-like activities triggered by low-powered near-infrared light for synergetic combating multidrug-resistant bacterial infections. Advanced Healthcare Materials, 2022, 11(12): 2200121.
DOI URL |
| [77] | ZHAO X, CHANG L, HU Y, et al. Preparation of photocatalytic and antibacterial MOF nanozyme used for infected diabetic wound healing. ACS Applied Materials & Interfaces, 2022. 14(16): 18194. |
| [78] |
ZHANG Y, YANG D, GUO W, et al. A multifunctional hydrogel dressing with antibacterial properties for effective wound healing. Dalton Transactions, 2022, 51(17): 6817.
DOI URL |
| [79] |
LI Y, FU R, DUAN Z, et al. Injectable hydrogel based on defect-rich multi-nanozymes for diabetic wound healing via an oxygen self-supplying cascade reaction. Small, 2022, 18(18): 2200165.
DOI URL |
| [80] |
WANG P, PENG L, LIN J, et al. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose-activated cascade reaction to efficiently promote diabetic wound healing. Chemical Engineering Journal, 2021, 415: 128901.
DOI URL |
| [81] | ZHOU X, ZHANG S, LIU Y, et al. Antibacterial cascade catalytic glutathione-depleting MOF nanoreactors. ACS Applied Materials & Interfaces, 2022, 14(9): 11104. |
| [82] |
LUO Q, LI Y, HUO X, et al. Stabilizing ultrasmall ceria-cluster nanozyme for antibacterial and antibiofouling applications. Small, 2022, 18(16): 2107401.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [8] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [9] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [10] | 李建军, 陈芳明, 张梨梨, 王磊, 张丽亭, 陈慧雯, 薛长国, 徐良骥. CoFe2O4/MgAl-LDH催化剂活化过氧一硫酸盐促进抗生素降解[J]. 无机材料学报, 2025, 40(4): 440-448. |
| [11] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [12] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [13] | 贾相华, 张辉霞, 刘艳凤, 左桂鸿. 湿化学法制备Cu2O/Cu空心球异质结光催化剂[J]. 无机材料学报, 2025, 40(4): 397-404. |
| [14] | 信震宇, 郭瑞华, 乌仁托亚, 王艳, 安胜利, 张国芳, 关丽丽. Pt-Fe/GO纳米催化剂的制备及其电催化乙醇氧化性能研究[J]. 无机材料学报, 2025, 40(4): 379-387. |
| [15] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||