无机材料学报 ›› 2017, Vol. 32 ›› Issue (7): 681-690.DOI: 10.15541/jim20160484 CSTR: 32189.14.10.15541/jim20160484
蒋久信1,2, 吴 月1, 何 瑶1, 高 松1, 张 晨1, 沈 彤3, 刘嘉宁3,4
收稿日期:2016-08-29
修回日期:2016-10-29
出版日期:2017-07-20
网络出版日期:2017-06-23
作者简介:蒋久信(1974—), 男, 博士, 副教授. E-mail: jiuxinjiang@hotmail.com
基金资助:JIANG Jiu-Xin1,2, WU Yue1, HE Yao1, GAO Song1, ZHANG Chen1, SHEN Tong3, LIU Jia-Ning3,4
Received:2016-08-29
Revised:2016-10-29
Published:2017-07-20
Online:2017-06-23
About author:JIANG Jiu-Xin. E-mail: jiuxinjiang@hotmail.com
Supported by:摘要:
球霰石以其独特的机械、物理和化学性能, 在日用品与生物医学等领域表现出广阔的应用前景。然而在三种无水碳酸钙晶体中, 球霰石的热力学性能最不稳定, 在后续的反应和处理过程中常常转变为更稳定的文石或方解石, 因此如何抑制球霰石向稳定晶型转变一直都是碳酸钙领域研究的热点。本文在概述了球霰石晶体结构、性质、应用及其转化途径的基础上, 以碳酸钙的三种基本制备体系为线索, 综述了碳化法、复分解法、微乳液法和溶剂热法等传统方法以及自组装单分子膜法、仿生合成法和热分解法等一些新型调控制备球霰石相方法的研究进展, 还就利用添加剂促进球霰石形成与稳定的相关机制加以剖析。文章旨在为球霰石相碳酸钙的有效制备提供理论和实践的参考。
中图分类号:
蒋久信, 吴 月, 何 瑶, 高 松, 张 晨, 沈 彤, 刘嘉宁. 亚稳态球霰石相碳酸钙的调控制备进展[J]. 无机材料学报, 2017, 32(7): 681-690.
JIANG Jiu-Xin, WU Yue, HE Yao, GAO Song, ZHANG Chen, SHEN Tong, LIU Jia-Ning. Progress in Tuning of Metastable Vaterite Calcium Carbonate[J]. Journal of Inorganic Materials, 2017, 32(7): 681-690.
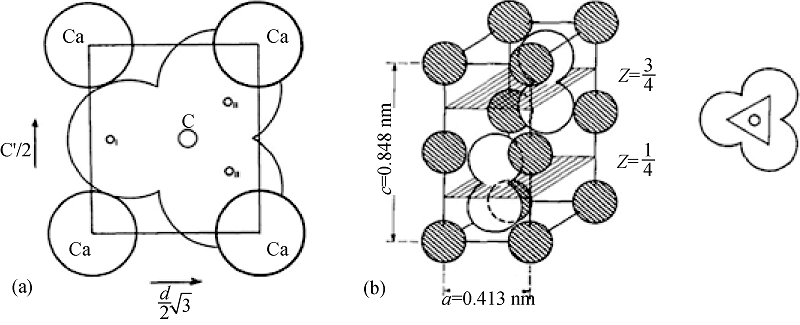
图1 (a)球霰石中碳酸根和钙原子位置的垂直投影[10]; (b)球霰石的晶胞结构[11]
Fig. 1 (a) Vertical projection of vaterite showing orientation of carbonate group relative to calcium atoms[10]; (b) Structure of the vaterite unit cell[11]
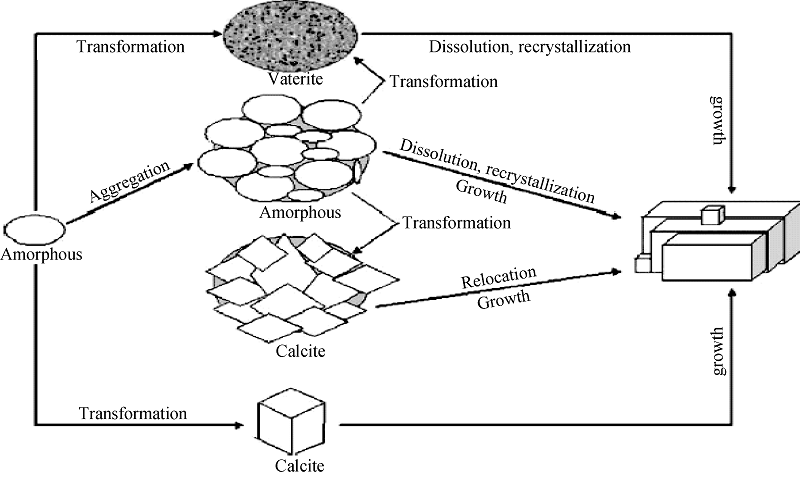
图2 CaCO3从非晶相转变为球霰石球晶和典型方解石的形成示意图[25]
Fig. 2 Schematic depiction of the CaCO3 transformation from amorphous phase to vaterite spherulites and typical calcite[25]
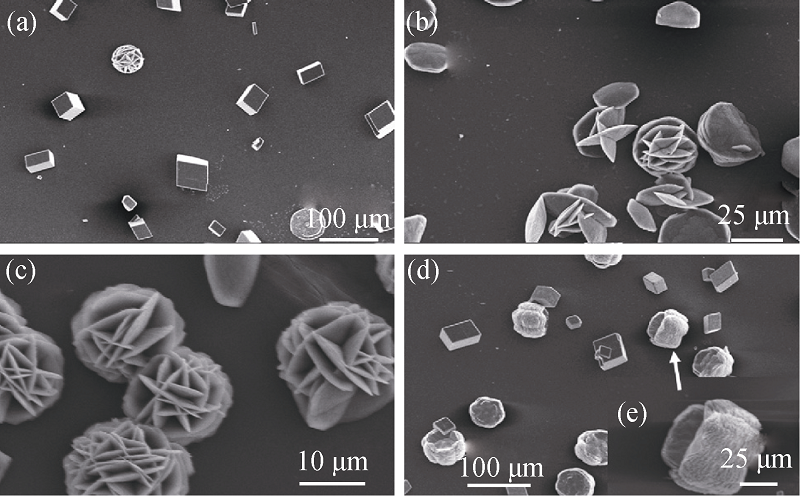
图3 无水对氨基苯磺酸与L-赖氨酸不同混合比CaCO3晶体的SEM照片[29]
Fig. 3 SEM images of CaCO3 particles in different concentration ratios between p-aminobenzene sulfonic acid anhydrous and l-Lys solutions [29] (a) 0.1 g/L:0.1 g/L; (b) 0.1 g/L:0.3 g/L; (c) 0.1 g/L:0.5 g/L; (d) 0.5 g/L:0.1 g/L; (e) High magnification of selective area of (d)
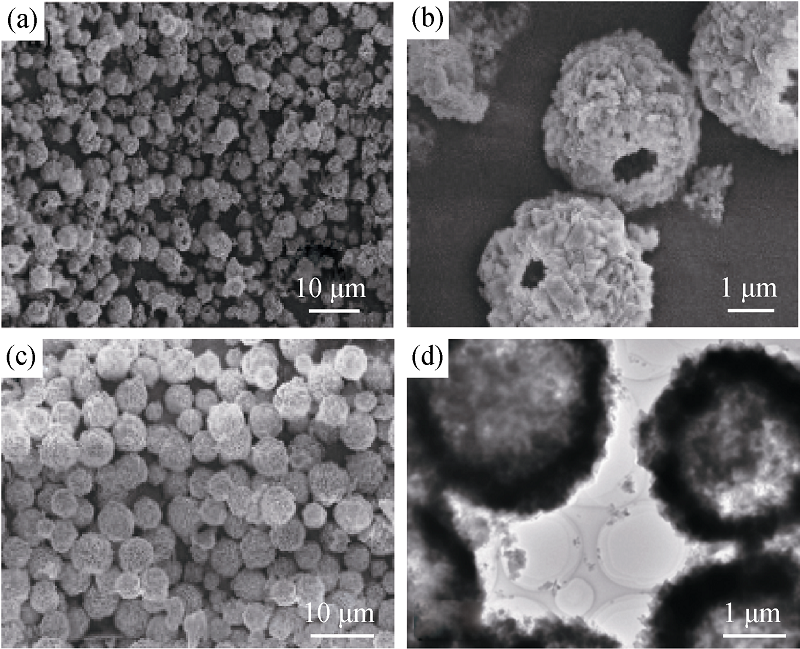
图4 添加PEG10000-SDS的SEM和TEM照片[35]
Fig. 4 SEM images of CaCO3 hollow spheres obtained in the presence of PEG10000-SDS[35](a) Low magnification image, (b) high and (c) middle magnification SEM images; and (d) TEM image of CaCO3 hollow spheres obtained in the presence of PEG2000-SDS

图5 不同浓度表面活性剂下制备的CaCO3晶体的SEM照片[38]
Fig. 5 SEM images of CaCO3 prepared at different surfactant concentrations [38](a) 0.1 mol/L; (b) Magnification of (a); (c) 0.07 mol/L; (d) 0.04 mol/L

图6 蒸发水包油饱和微乳液制备海绵状球霰石的扫描电镜照片[41]
Fig. 6 SEM images showing sponge-like vaterite spheroids prepared by evaporation[41] from water-in-oil supersaturated microemulsions with compositions of (a) octane︰SDS︰CaHCO3 =71︰4︰25(wt%); (b) octane︰dodecanol︰SDS︰CaHCO3 = 70.8︰0.7︰3.5︰25(wt%); (c) schematic diagram showing the mechanism for the formation of vaterite microsponges in water-in-oil microemulsions
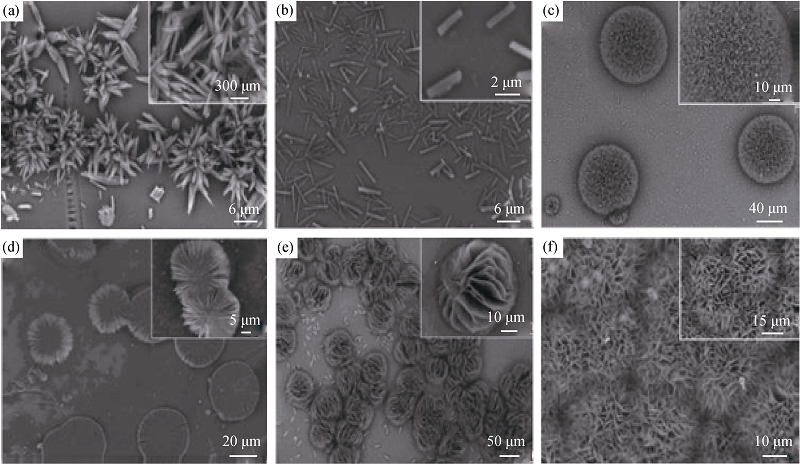
图7 CaCO3的SEM照片, 其中(a~c)和(d~f)分别为加入10 mL、20 mL的丝素蛋白, 且所加镁离子浓度(mmol/L)分别为(a) 10; (b) 20; (c) 50; (d) 10; (e) 20; (f) 50[42]
Fig. 7 SEM images of CaCO3 where (a-c) and (d-f) with 10 mL and 20 mL of silk fibroin, respectively, affter being added Mg2+ at the concentration of (a) 10; (b) 20; (c) 50; (d) 10; (e) 20; (f) 50 mmol/L[42]
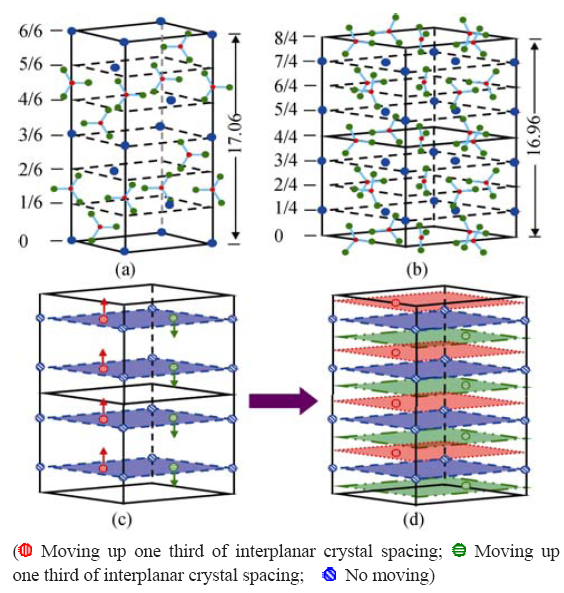
图11 (a)和(b)方解石和球霰石的原子结构, (c)球霰石结构中(0001)晶面Ca2+的排列以及在搅拌影响下的移动方向, (d)移动后Ca2+的排列及移动过程[ 59]
Fig. 11 Atomic structure of (a) calcite and (b) vaterite, (c) Ca2+ ions stacking in vaterite and the motion direction in the (0001) faces under agitation, and (d) Ca2+ ions stacking in the (0001) faces after motion[59]
| [1] | CUI Z, CUI C, ZHU Y, et al.Multiple phase inversion of emulsions stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of fatty acids.Langmuir, 2011, 28(1): 314-320. |
| [2] | LIU S. Stabilized Vaterite.U.S. Patent Application, US20030717 310. 2003.11.19. |
| [3] | DEVENNEY M, FERNANDEZ M, MORGAN S.Non-cementitious Compositions Comprising Vaterite and Methods Thereof. U.S. Patent Application, US201313804558. 2013.3.14. |
| [4] | TAS A.Use of vaterite and calcite in forming calcium phosphate cement scaffolds.Ceram. Eng. Sci. Proc., 2009, 28(9): 135-150. |
| [5] | OHGUSHI H, OKUMURA M, YOSHIKAWA T, et al.Bone formation processin porous calcium carbonate and hydroxyapatite.J. Biomed. Mater. Res., 1992, 26(7): 885-895. |
| [6] | BUKREEVA T, MARCHENKO I, BORODINA T, et al.Calcium carbonate and titanium dioxide particles as a basis for container fabrication for brain delivery of compounds.Dokl. Phys. Chem., 2011, 440(1): 165-167. |
| [7] | LI Q, DING Y, LI F, et al.Solvothermal growth of vaterite in the presence of ethylene glycol, 1, 2-propanediol and glycerin. J. Cryst. Growth, 2002, 236(1): 357-362. |
| [8] | FLATEN E, SEIERSTEN M, ANDREASSEN J.Polymorphism and morphology of calcium carbonate precipitated in mixed solvents of ethylene glycol and water.J. Cryst. Growth, 2009, 311(13): 3533-3538. |
| [9] | DUPONT L, PORTEMER F.Synthesis and study of a well crystallized CaCO3 vaterite showing a new habitus.J. Mater. Chem., 1997, 7(5): 797-800. |
| [10] | KAMHI S.On the structure of vaterite CaCO3.Acta Crystallogr., 1963, 16(8): 770-772. |
| [11] | MEYER H.Struktur und fehlordnung des vaterits.Z. Kristallogr., 1969, 128(1-6): 183-212. |
| [12] | DEMICHELIS R, RAITERI P, GALE J, et al.A new structural model for disorder in vaterite from first-principles calculations.CrystEngComm, 2012, 14(1): 44-47. |
| [13] | DEMICHELIS R, RAITERI P, GALE J, et al.The multiple structures of vaterite.Cryst. Growth Des., 2013, 13(6): 2247-2251. |
| [14] | NAKA K, TANAKA Y, CHUJO Y.Effect of anionic starburst dendrimers on the crystallization of CaCO3 in aqueous solution: size control of spherical vaterite particles.Langmuir, 2002, 18(9): 3655-3658. |
| [15] | IMAI H, TOCHIMOTO N, NISHINO Y, et al.Oriented nanocrystal mosaic in monodispersed CaCO3 microspheres with functional organic molecules.Cryst. Growth Des., 2012, 12(2): 876-882. |
| [16] | YU S, CÖLFEN H, ANTONIETTI M. Polymer-controlled morphosynthesis and mineralization of metal carbonate superstructures.J. Phys. Chem. B, 2003, 107(30): 7396-7405. |
| [17] | NEHRKE G, VAN CAPPELLEN P.Framboidal vaterite aggregates and their transformation into calcite: a morphological study.J. Cryst. Growth, 2006, 287(2): 528-530. |
| [18] | CÖLFEN H, QI L. A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer.Chem.-Eur. J., 2001, 7(1): 106-116. |
| [19] | HU Q, ZHANG J, TENG H, et al.Growth process and crystallographic properties of ammonia-induced vaterite.Am. Mineral., 2012, 97(8/9): 1437-1445. |
| [20] | FRICKE M, VOLKMER D, KRILL C, et al.Vaterite polymorph switching controlled by surface charge density of an amphiphilic dendron-calix [4] arene.Cryst. Growth Des., 2006, 6(5): 1120-1123. |
| [21] | GEHRKE N, CÖLFEN H, PINNA N, et al. Superstructures of calcium carbonate crystals by oriented attachment.Cryst. Growth Des., 2005, 5(4): 1317-1319. |
| [22] | QI L, LI J, MA J.Biomimetic morphogenesis of calcium carbonate in mixed solutions of surfactants and double-hydrophilic block copolymers.Adv. Mater., 2002, 14(4): 300-303. |
| [23] | MUGNAIOLI E, ANDRUSENKO I, SCHÜLER T, et al. Ab initio structure determination of vaterite by automated electron diffraction.Angew. Chem. Int. Ed., 2012, 51(28): 7041-7045. |
| [24] | WEI H, MA N, SONG B, et al.Formation of multilayered vaterite via phase separation, crystalline transformation, and self-assembly of nanoparticles at the air/water interface.J. Phys. Chem. C, 2007, 111(15): 5628-5632. |
| [25] | WEI H, SHEN Q, ZHAO Y, et al.Influence of polyvinylpyrrolidone on the precipitation of calcium carbonate and on the transformation of vaterite to calcite.J. Cryst. Growth, 2003, 250(3): 516-524. |
| [26] | POLITI Y, ARAD T, KLEIN E, et al.Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase.Science, 2004, 306(5699): 1161-1164. |
| [27] | 章峻, 包富荣, 戴冬萍, 等. 马来酸酐 (MAH) 表面改性纳米碳酸钙粉体的制备及表面性能. 无机化学学报, 2007, 23(5): 822-826. |
| [28] | KONTREC J, KRALJ D, BREČEVIĆ L, et al. Influence of some polysaccharides on the production of calcium carbonate filler particles.J. Cryst. Growth, 2008, 310(21): 4554-4560. |
| [29] | ZHANG Q, REN L, SHENG Y, et al.Control of morphologies and polymorphs of CaCO3 via multi-additives system.Mater. Chem. Phys., 2010, 122(1): 156-163. |
| [30] | 汪小红, 张群, 董晓庆, 等. 超声辅助的荔枝状球霰石的制备与表征. 人工晶体学报, 2013, 42(10): 2164-2169. |
| [31] | YANG YA-NAN, ZHU XIAO-LI, KONG XIANG-ZHENG.Controls of crystal morphology, size and structure in spontaneous preparation of calcium carbonate.Journal of Inorganic Materials, 2013, 28(12): 1313-1320. |
| [32] | RAMESH T, INCHARA S A, PALLAVI K.Para-amino benzoic acid-mediated synthesis of vaterite phase of calcium carbonate.J. Chem. Sci., 2015, 127(5): 843-848. |
| [33] | 夏宏宇, 张群, 王刚, 等. 球形和橄榄形球霰石的简易制备研究. 人工晶体学报, 2015, 44(6): 1701-1706. |
| [34] | ZOU JIAN-PENG, YANG HONG-ZHI, XIAO-PING, et al.Controllable fabrication of calcium carbonate hollow microspheres with micro-nano hierarchical structure.Journal of Inorganic Materials, 2016, 31(7): 711-718. |
| [35] | JI X, LI G, HUANG X.The synthesis of hollow CaCO3 microspheres in mixed solutions of surfactant and polymer. Mater. Lett., 2008, 62(4): 751-754. |
| [36] | 徐国峰, 王洁欣, 沈志刚, 等. 单分散纳米碳酸钙的制备和表征. 北京化工大学学报(自然科学版), 2009, 36(5): 27-30. |
| [37] | KANG S, HIRASAWA I, KIM W, et al.Morphological control of calcium carbonate crystallized in reverse micelle system with anionic surfactants SDS and AOT.J. Colloid. Interf. Sci. 2005, 288(2): 496-502. |
| [38] | JIANG J, MA Y, ZHANG T, et al.Morphology and size control of calcium carbonate crystallized in a reverse micelle system with switchable surfactants.RSC Adv., 2015, 5: 80216-80219. |
| [39] | 陈银霞, 纪献兵, 赵改青, 等. 低温溶剂热法合成圆饼状球霰石碳酸钙. 材料导报, 2010, 24(12): 99-102. |
| [40] | 陈先勇, 唐琴, 刘代俊. 独特形貌碳酸钙的微波水热合成与表征. 功能材料, 2012, 43(9): 1109-1112. |
| [41] | WALSH D, LEBEAU B, MANN S.Morphosynthesis of calcium carbonate (vaterite) microsponges.Adv. Mater., 1999, 11(4): 324-328. |
| [42] | AN X, CAO C.Biomineralization of CaCO3 through the cooperative interactions between multiple additives and self-assembled monolayers.J. Phys. Chem. C, 2008, 112(16): 6526-6530. |
| [43] | YANG B, NAN Z.Abnormal polymorph conversion of calcium carbonate from calcite to vaterite.Mater. Res. Bull., 2012, 47(3): 521-526. |
| [44] | YANG D, YU K, AI Y, et al.The mineralization of electrospun chitosan/poly (vinyl alcohol) nanofibrous membranes.Carbohydr. Polym., 2011, 84(3): 990-996. |
| [45] | RAUTARAY D, AHMAD A, SASTRY M.Biosynthesis of CaCO3 crystals of complex morphology using a fungus and an actinomycete.J. Am. Chem. Soc., 2003, 125(48): 14656-14657. |
| [46] | BEUVIER T, CALVIGNAC B, DELCROIX G, et al.Synthesis of hollow vaterite CaCO3 microspheres in supercritical carbon dioxide medium. J. Mater. Chem., 2011, 21(26): 9757-9761. |
| [47] | 潘晓芳, 王海水. 乙醇/水混合溶剂体系中碳酸钙晶体晶型和取向的控制. 无机化学学报, 2014, 30(6): 1312-1316. |
| [48] | 韩金鑫, 连宾, 唐源, 等. 恶臭假单胞菌对碳酸钙的诱导矿化作用. 南京大学学报 (自然科学版), 2013, 49(6): 681-688. |
| [49] | 马晓明, 杨媛媛, 张晓婷, 等. 大豆胰岛素指导下具有分级结构碳酸钙的仿生合成. 河南师范大学学报(自然科学版), 2014, 42(2): 64-68. |
| [50] | 黄玉刚, 褚日环, 何明辉, 等. 富含羧基的多肽基双亲水杂化共聚物控制碳酸钙的形成. 中山大学学报(自然科学版), 2014, 53(3): 73-79. |
| [51] | GUO Y, WANG F, ZHANG J, et al.Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids.Res. Chem. Intermed., 2013, 39(6): 2407-2415. |
| [52] | 任丽英, 张群, 朱万华, 等. 仿生合成碳酸钙微环. 人工晶体学报, 2015, 44(1): 250-255. |
| [53] | LIU L, JIANG J, YU S.Polymorph selection and structure evolution of CaCO3 mesocrystals under control of poly (sodium 4-styrenesulfonate): synergetic effect of temperature and mixed solvent.Cryst. Growth Des., 2014, 14(11): 6048-6056. |
| [54] | 陈晓东, 辛梅华, 李明春, 等. N-琥珀酰基-O-羟丙基磺酸壳聚糖仿生合成球霰石碳酸钙. 材料研究学报, 2016, 30(1): 31-37. |
| [55] | 朱文杰, 蔡春华, 林嘉平. 碳酸钙在聚合物胶束控制下的仿生合成. 高分子学报, 2011, 4: 335-339. |
| [56] | 汪玉瑛. 生物成因碳酸钙矿化机制的仿生实验研究. 合肥: 中国科学技术大学博士学位论文, 2015. |
| [57] | 张群, 张清. 不同晶型碳酸钙的仿生矿化研究. 硅酸盐通报, 2014, 33(5): 1236-1240. |
| [58] | JIANG J, YE J, ZHANG G, et al.Polymorph and morphology control of CaCO3 via temperature and PEG during the decomposition of Ca(HCO3)2.J. Am. Ceram. Soc., 2012, 95(12): 3735-3738. |
| [59] | JIANG J, ZHANG Y, XU D, et al.Can agitation determine the polymorphs of calcium carbonate during the decomposition of calcium bicarbonate?CrystEngComm, 2014, 16(24): 5221-5226. |
| [60] | ZENG H, YAN Z, JIAO M, et al.A novel method for preparing calcium carbonate particles: thermal decomposition from calcium hydrogen carbonate solution.Key Eng. Mater., 2016, 697: 113-118. |
| [61] | TRUSHINA D, BUKREEVA T, KOVALCHUK M, et al.CaCO3 vaterite microparticles for biomedical and personal care applications.Mater. Sci. Eng., C, 2014, 45: 644-658. |
| [62] | RODRIGUEZ-NAVARRO C, JIMENEZ-LOPEZ C, RODRIGUEZ-NAVARRO A, et al.Bacterially mediated mineralization of vaterite.Geochim. Cosmochim. Acta, 2007, 71(5): 1197-1213. |
| [63] | WANG X, WU C, TAO K, et al.Influence of ovalbumin on CaCO3 precipitation during in vitro biomineralization.J. Phys. Chem. B, 2010, 114(16): 5301-5308. |
| [64] | DONNERS J, HEYWOOD B, MEIJER E, et al.Control over calcium carbonate phase formation by dendrimer/surfactant templates.Chem.-Eur. J., 2002, 8(11): 2561-2567. |
| [65] | PARAKHONSKIY B, HAASE A, ANTOLINI R.Sub-micrometer vaterite containers: synthesis, substance loading, and release.Angew. Chem., Int. Edit., 2012, 51(5): 1195-1197. |
| [66] | SAND K, RODRIGUEZ-BLANCO J, MAKOVICKY E, et al.Crystallization of CaCO3 in water-alcohol mixtures: spherulitic growth, polymorph stabilization, and morphology change.Cryst. Growth Des., 2012, 12(2): 842-853. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 洪培萍, 梁龙, 吴炼, 马颖康, 庞浩. ZIF-67结构调控及其对盐酸金霉素的吸附性能研究[J]. 无机材料学报, 2025, 40(4): 388-396. |
| [10] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [11] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [12] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [13] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [14] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [15] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||