无机材料学报 ›› 2015, Vol. 30 ›› Issue (8): 793-801.DOI: 10.15541/jim20140646 CSTR: 32189.14.10.15541/jim20140646
郑艳彬1, 2, 姜志刚1, 朱品文1
收稿日期:2014-12-16
修回日期:2015-03-24
出版日期:2015-08-20
网络出版日期:2015-07-21
作者简介:郑艳彬(1983–), 男, 讲师, 博士研究生. E-mail: zhengyb14@mails.jlu.edu.cn
基金资助:ZHENG Yan-Bin1, 2, JIANG Zhi-Gang1, ZHU Pin-Wen1
Received:2014-12-16
Revised:2015-03-24
Published:2015-08-20
Online:2015-07-21
About author:ZHENG Yan-Bin. E-mail: zhengyb14@mails.jlu.edu.cn
摘要:
洋葱碳独特的结构, 使其具有优异的物理化学性能。本文首先介绍了洋葱碳的分类和结构, 对几种传统的制备方法(包括电弧放电法、等离子体、电子束辐射、化学气相沉积、纳米金刚石真空退火、热解法)的优缺点进行归纳、总结。其次, 介绍了近年来发展起来的制备方法。随后,对近年来洋葱碳在锂离子二次电池负极、染料敏化太阳能电池对电极、电化学储氢电极、超级电容器电极、摩擦和磨损、催化领域的应用做一概述。最后, 指出了目前洋葱碳在制备和应用方面的不足, 对今后的研究做了展望。
中图分类号:
郑艳彬, 姜志刚, 朱品文. 洋葱碳的制备与应用研究进展[J]. 无机材料学报, 2015, 30(8): 793-801.
ZHENG Yan-Bin, JIANG Zhi-Gang, ZHU Pin-Wen. Development on the Preparation and Application of Onion-like Carbon[J]. Journal of Inorganic Materials, 2015, 30(8): 793-801.
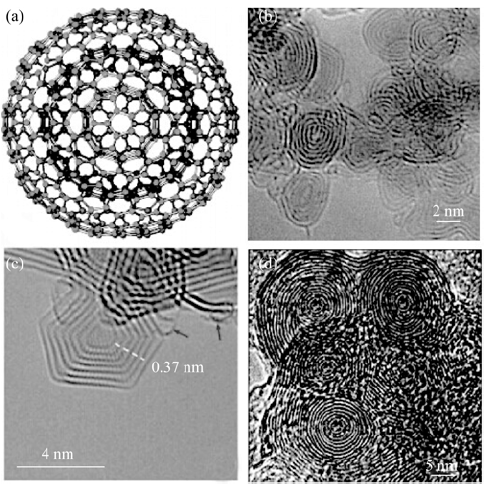
图1 (a)理想的中空OLC的结构模型[7], (b)准球形结构的OLC[8], (c)多面体结构的OLC [9]和(d)多核结构的OLC[10]
Fig. 1 (a) Structure model of ideal hollow OLC[7]with (b) quasi-spherical structure[8], (c) polyhedral structure[9] and (d) multi-core structure[10]
| Preparation methods | Advantages | Disadvantages |
|---|---|---|
| Arc discharge | High crystallization,low defect density carbon onions prepared in bulk quantities using an arc in water | Containing a large amount of carbonaceous impurities such as amorphous carbon, CNTs, CNT-like structures, graphitic debris, and metallic impurities |
| Plasma | Low cost and prepared in bulk quantities | Containing amorphous carbon and metallic impurities |
| Electron-beam radiation | In-situ observations achieved helpful to study the growth mechanism of OLC | High cost and low yield |
| Chemical vapor deposition | Simple, cheap, easy to implement and to realize mass production | Purification to remove amorphous carbon, CNTs, graphite, catalyst support, etc |
| Nanodiamond annealing in vacuum | Narrow-sized distribution and produced in massive amount | Requiring nanodiamond precursor and high-temperature vacuum oven |
| Thermolysis | Massive amount of carbon onions produced at low cost by using simple device | Larger size CNOs and/or purification requirement |
表1 OLC的主要制备方法优缺点比较
Table 1 Advantages and disadvantages of OLC's main preparation methods
| Preparation methods | Advantages | Disadvantages |
|---|---|---|
| Arc discharge | High crystallization,low defect density carbon onions prepared in bulk quantities using an arc in water | Containing a large amount of carbonaceous impurities such as amorphous carbon, CNTs, CNT-like structures, graphitic debris, and metallic impurities |
| Plasma | Low cost and prepared in bulk quantities | Containing amorphous carbon and metallic impurities |
| Electron-beam radiation | In-situ observations achieved helpful to study the growth mechanism of OLC | High cost and low yield |
| Chemical vapor deposition | Simple, cheap, easy to implement and to realize mass production | Purification to remove amorphous carbon, CNTs, graphite, catalyst support, etc |
| Nanodiamond annealing in vacuum | Narrow-sized distribution and produced in massive amount | Requiring nanodiamond precursor and high-temperature vacuum oven |
| Thermolysis | Massive amount of carbon onions produced at low cost by using simple device | Larger size CNOs and/or purification requirement |
| Metal oxides | Theoretical capacity/(mAh·g-1) | Ref |
|---|---|---|
| SnO2 | 782 | [54] |
| CuO | 674 | [4] |
| CdO | 1046 | [55] |
| Cr2O3 | 1058 | [56] |
| MoO2 | 838 | [57] |
| NiO | 718 | [3] |
| MnO | 755 | [58] |
| MnO2 | 1233 | [59] |
| Mn2O3 | 1019 | [60] |
| Fe2O3 | 1007 | [60] |
| Fe3O4 | 926 | [61] |
| CoO | 715 | [62] |
| Co3O4 | 890 | [5] |
表2 不同金属氧化物的理论比容量
Table 2 Theoretical capacities of various metal oxides
| Metal oxides | Theoretical capacity/(mAh·g-1) | Ref |
|---|---|---|
| SnO2 | 782 | [54] |
| CuO | 674 | [4] |
| CdO | 1046 | [55] |
| Cr2O3 | 1058 | [56] |
| MoO2 | 838 | [57] |
| NiO | 718 | [3] |
| MnO | 755 | [58] |
| MnO2 | 1233 | [59] |
| Mn2O3 | 1019 | [60] |
| Fe2O3 | 1007 | [60] |
| Fe3O4 | 926 | [61] |
| CoO | 715 | [62] |
| Co3O4 | 890 | [5] |
| Anode materials | OLC encapsulation | Initial discharge ability of nanoparticles electrode at different rates | Cycle performance | Ref |
|---|---|---|---|---|
| NiO | No | NiO, 2C, 558.8 mAh/g | 0.5C, 1150 mAh/g (initial discharge) and 383.5 mAh/g (after 50 cycles) | [3] |
| Yes | NiO/C, 2C, 1105.6 mAh/g | 0.5C, 1689.4 mAh/g (initial discharge) and 1157.7 mAh/g (after 50 cycles) | ||
| CuO | No | CuO, 1.2C, 178.9 mAh/g | 100 mA/g , 270.2 mAh/g (after 50 cycles) | [4] |
| Yes | CuO/C, 1.2C, 535.6 mAh/g | 100 mA/g, 628.7 mAh/g (after 50 cycles) with a high Coulombic efficiency of 98.6% | ||
| Co3O4 | No | Co3O4, 2C, 459 mAh/g | 1248.8 mAh/g (initial discharge) and 471.5 mAh/g (after 50 cycles) | [5] |
| Yes | Co3O4/C, 2C, 925 mAh/g | 0.5C, 1467.6 mAh/g (initial discharge) and 1026.9 mAh/g (after 50 cycles) | ||
| SnO2 | No | / | 0.2 mA/cm2, 849 mAh/g (initial discharge) to 123 mAh/g (after 50 cycles) | [63] |
| Yes | / | 0.2 mA/cm2, 755 mAh/g(initial discharge) and 446 mAh/g (after 50 cycles) |
表3 金属氧化物负极材料性能比较
Table 3 Performance comparison of metal oxides used as anode materials
| Anode materials | OLC encapsulation | Initial discharge ability of nanoparticles electrode at different rates | Cycle performance | Ref |
|---|---|---|---|---|
| NiO | No | NiO, 2C, 558.8 mAh/g | 0.5C, 1150 mAh/g (initial discharge) and 383.5 mAh/g (after 50 cycles) | [3] |
| Yes | NiO/C, 2C, 1105.6 mAh/g | 0.5C, 1689.4 mAh/g (initial discharge) and 1157.7 mAh/g (after 50 cycles) | ||
| CuO | No | CuO, 1.2C, 178.9 mAh/g | 100 mA/g , 270.2 mAh/g (after 50 cycles) | [4] |
| Yes | CuO/C, 1.2C, 535.6 mAh/g | 100 mA/g, 628.7 mAh/g (after 50 cycles) with a high Coulombic efficiency of 98.6% | ||
| Co3O4 | No | Co3O4, 2C, 459 mAh/g | 1248.8 mAh/g (initial discharge) and 471.5 mAh/g (after 50 cycles) | [5] |
| Yes | Co3O4/C, 2C, 925 mAh/g | 0.5C, 1467.6 mAh/g (initial discharge) and 1026.9 mAh/g (after 50 cycles) | ||
| SnO2 | No | / | 0.2 mA/cm2, 849 mAh/g (initial discharge) to 123 mAh/g (after 50 cycles) | [63] |
| Yes | / | 0.2 mA/cm2, 755 mAh/g(initial discharge) and 446 mAh/g (after 50 cycles) |
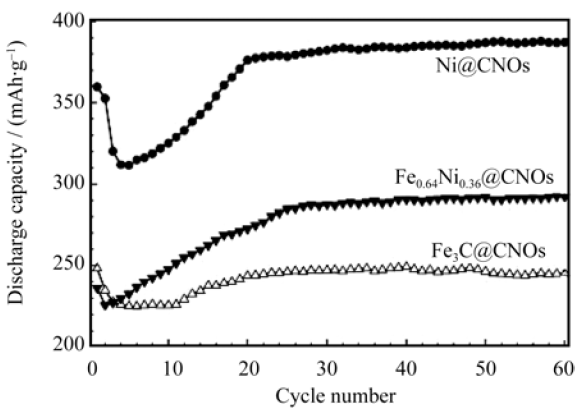
图3 室温下, 充放电电流密度为500 mA/g时三种电极的放电容量随循环次数的变化[21]
Fig. 3 Discharge capcity of the three types of CNOs versus cycle number at a charge-discharge current density of 500 mA/g at room temperature[21]
| Types of materials | Specific capacitances/(F·g-1) | Ref |
|---|---|---|
| CNOs | 30 | [67] |
| CNOs/KOH activation | <122 | [66] |
| CNOs/H2SO4 activation | 20-40 | [68] |
| CNOs/RuO2·xH2O | 96-334 | [69] |
| CNOs/NiO | 218.2-290.6 | [70] |
| CNOs/Ni(OH)2 | 727.4-1225.2 | [70] |
| CNOs/MnO2 | 177.5 | [71] |
| CNOs/PDDA | 20-30 | [72] |
| CNOs/chit | 20-30 | [72] |
| CNOs/PANI | <525 | [73] |
| CNOs/PQ | 267 | [67] |
| CNOs/NQ | 91 | [67] |
| CNOs/PY | 130 | [67] |
表3 CNOs电极和CNOs基复合材料电极的比电容
Table 3 Specific capacitances of various CNOs and CNOs-based composite electrode
| Types of materials | Specific capacitances/(F·g-1) | Ref |
|---|---|---|
| CNOs | 30 | [67] |
| CNOs/KOH activation | <122 | [66] |
| CNOs/H2SO4 activation | 20-40 | [68] |
| CNOs/RuO2·xH2O | 96-334 | [69] |
| CNOs/NiO | 218.2-290.6 | [70] |
| CNOs/Ni(OH)2 | 727.4-1225.2 | [70] |
| CNOs/MnO2 | 177.5 | [71] |
| CNOs/PDDA | 20-30 | [72] |
| CNOs/chit | 20-30 | [72] |
| CNOs/PANI | <525 | [73] |
| CNOs/PQ | 267 | [67] |
| CNOs/NQ | 91 | [67] |
| CNOs/PY | 130 | [67] |
| [1] | UGARTE D.Curling and closure of graphitic networks under electron-beam irradiation.Nature, 1992, 359: 707-709. |
| [2] | ABDULLAEVA Z, OMURZAK E, IWAMOTO C, et al.Onion- like carbon-encapsulated Co, Ni, and Fe magnetic nanoparticles with low cytotoxicity synthesized by a pulsed plasma in a liquid.Carbon, 2012, 50: 1776-1785. |
| [3] | LIU X G, OR S W, JIN C G, et al.NiO/C nanocapsules with onion- like carbon shell as anode material for lithium ion batteries.Carbon, 2013, 60: 215-220. |
| [4] | LIU X G, BI N N, FENG C, et al.Onion-like carbon coated CuO nanocapsules: a highly reversible anode material for lithium ion batteries. Journal of Alloys and Compounds, 2014, 587: 1-5. |
| [5] | LIU X G, OR S W, JIN C G, et al.Co3O4/C nanocapsules with onion- like carbon shells as anode material for lithium ion batteries.Electrochimica Acta, 2013, 100: 140-146. |
| [6] | QIAO Z J, LI J J, ZHAO N Q, et al.Graphitization and microstructure transformation of nanodiamond to onion-like carbon. Scripta Materialia, 2006, 54: 225-229. |
| [7] | XU B S.Prospects and research progress in nano onion-like fullerenes.New carbon materials, 2008, 23(4): 289-301. |
| [8] | LIN Y M, PAN X L, QI W, et al.Nitrogen-doped onion-like carbon: a novel and efficient metal-free catalyst for epoxidation reaction. J. Mater. Chem. A, 2014, 2: 12475-12483. |
| [9] | BOGDANOV K, FEDOROV A, OSIPOV V, et al.Annealing- induced structural changes of carbon onions: high-resolution transmission electron microscopy and Raman studies.Carbon, 2014, 73: 78-86. |
| [10] | XU B S, TANAKA S I.Multiple-nuclei Onion Like Fullerenes Cultivated by Electron Beam Irradiation. Proc. Int. Conf. ICSE,Cambridge, 1997: 355-360. |
| [11] | WANG Q, SUN X L, HE D Y, et al.Preparation and study of carbon nano-onion for lithium storage. Materials Chemistry and Physics, 2013, 139: 333-337. |
| [12] | WANG Y, HAN Z J, YU S F, et al.Core-leaf onion-like carbon/ MnO2 hybrid nano-urchins for rechargeable lithium-ion batteries.Carbon, 2013, 64: 230-236. |
| [13] | WU G, NELSON M, MA S G, et al.Synthesis of nitrogen-doped onion-like carbon and its use in carbon-based CoFe binary non-precious-metal catalysts for oxygen-reduction.Carbon, 2011, 49: 3972-3982. |
| [14] | MATSUMOTO N, JOLY-POTTUZ L, KINOSHITA H, et al.Application of onion-like carbon to micro and nanotribology.Diamond & Related Materials, 2007, 16: 1227-1230. |
| [15] | JOLY-POTTUZ L, MATSUMOTO N, KINOSHITA H, et al.Diamond-derived carbon onions as lubricant additives.Tribology International, 2008, 41: 69-78. |
| [16] | MATSUMOTO N, MISTRY K K, KIM J H, et al.Friction reducing properties of onion-like carbon based lubricant under high contact pressure.Tribology, 2012, 6(3): 116-120. |
| [17] | WANG Y, XING G Z, HAN Z J, et al.Pre-lithiation of onion-like carbon/MoS2 nano-urchin anodes for high-performance rechargeable lithium ion batteries.Nanoscale, 2014, 6: 8884-8890. |
| [18] | WANG Y, YAN F, LIU S W, et al.Onion-like carbon matrix supported Co3O4 nanocomposites: a highly reversible anode material for lithium ion batteries with excellent cycling stability.J. Mater. Chem. A, 2013, 1(17): 5212-5216. |
| [19] | BU I Y Y. Synthesis of graphitic carbon nano-onions for dye sensitized solar cells.Solar Energy, 2014, 105: 236-242. |
| [20] | ZHANG C G, LI J J, LIU E Z, et al.Synthesis of hollow carbon nano-onions and their use for electrochemical hydrogen storage.Carbon, 2012, 50: 3513-3521. |
| [21] | ZHANG C G, LI J J, SHI C S, et al.Effect of Ni, Fe and Fe-Ni alloy catalysts on the synthesis of metal contained carbon nano-onions and studies of their electrochemical hydrogen storage properties.Journal of Energy Chemistry, 2014, 23: 324-330. |
| [22] | MCDONOUGH J K, FROLOV A I, PRESSER V, et al.Influence of the structure of carbon onions on their electrochemical performance in supercapacitor electrodes.Carbon, 2012, 50: 3298-3309. |
| [23] | PECH D, BRUNET M, DUROU H, et al.Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon.Nature Nanotechnology, 2010, 5: 651-654. |
| [24] | BUSHUEVA E G, GALKIN P S, BULUSHEVA L G, et al.Double layer supercapacitor properties of onion-like carbon materials.Phys. Stat. Sol.(b), 2008, 245(10): 2296-2299. |
| [25] | SHENDEROVA O, GRISHKO V, CUNNINGHAM G, et al.Onion- like carbon for terahertz electromagnetic shielding.Diamond & Related Materials, 2008, 17: 462-466. |
| [26] | SANO N, WANG H, ALEXANDROU I, et al.Properties of carbon onions produced by an arc discharge in water.Journal of Applied Physics, 2002, 92(5): 2783-2788. |
| [27] | BORGOHAIN R, YANG J, SELEGUE J P, et al.Controlled synthesis, efficient purification, and electrochemical characterization of arc-discharge carbon nano-onions.Carbon, 2014, 66: 272-284. |
| [28] | XING G, JIA X L, SHI Z Q.The production of carbon nano-materials by arc discharge under water or liquid nitrogen .New Carbon Materials, 2007, 22(4): 337-341. |
| [29] | SANO N, WANG H, CHHOWALLA M, et al.Nanotechnology: synthesis of carbon 'onions' in water.Nature, 2001, 414: 506-507. |
| [30] | ZHOU J F, SHEN Z Y, HOU S M, et al.Adsorption and manipulation of carbon onions on highly oriented pyrolytic graphite studied with atomic force microscopy.Applied Surface Science, 2007, 253: 3237-3241. |
| [31] | LIU W, MIAO Y, MENG Q S.Synthesis of Onion-Like Fullerenes by Arc Discharge in Non-Toxic Organic Liquid. Integrated Ferroelectrics, 2012, 138: 77-82. |
| [32] | CHEN X H, DENG F M, WANG J X, et al.New method of carbon onion growth by radio-frequency plasma-enhanced chemical vapor deposition.Chemical Physics Letters, 2001, 336: 201-204. |
| [33] | SZERENCSI M, RADNOCZI G.The mechanism of growth and decay of carbon nano-onions formed by ordering of amorphous particles.Vacuum, 2010, 84: 197-201. |
| [34] | HE C N, TIAN F, LIU S J, et al.Characterization and magnetic property of carbon coated metal nanoparticles and hollow carbon onions fabricated by CVD of methane. Materials Letters, 2008, 62: 3697-3699. |
| [35] | HE C N, ZHAO N Q, DU X W, et al.Low-temperature synthesis of carbon onions by chemical vapor deposition using a nickel catalyst supported on aluminum.Scripta Materialia, 2006, 54: 689-693. |
| [36] | WANG X M, XU B S, LIU X G, et al.Synthesis of Fe-included onion-like fullerenes by chemical vapor deposition.Diamond & Related Materials, 2006, 15: 147-150. |
| [37] | ZHANG C G, LI J J, SHI C S, et al.The efficient synthesis of carbon nano-onions using chemical vapor deposition on an unsupported Ni-Fe alloy catalyst.Carbon, 2011, 49: 1151-1158. |
| [38] | MYKHAYLYK O O, SOLONIN Y M, BATCHELDER D N, et al. Transformation of nanodiamond into carbon onions: a comparative study by high-resolution transmission electron microscopy, electron energy-lossspectroscopy, X-ray diffraction, small-angle X-ray scattering,ultraviolet Raman spectroscopy. Journal of applied Physics, 2005, 97: 074302-1-6. |
| [39] | BUTENKO Y V, KRISHNAMURTHY S, CHAKRABORTY A K, et al. Photoemission study of onionlike carbons produced by annealing nanodiamonds. Physical Review B, 2005, 71: 075420-1-10. |
| [40] | CEBIK J, MCDONOUGH J K, PEERALLY F, et al. Raman spectroscopy study of the nanodiamond-to-carbon onion transformation. Nanotechnology, 2013, 24: 205703-1-10. |
| [41] | BYSTRZEJEWSKI M, RUMMELI M H, GEMMING T, et al.Catalyst-free synthesis of onion-like carbon nanoparticles.New Carbon Materials, 2010, 25(1): 1-8. |
| [42] | GORELIK T, URBAN S, FALK F, et al.Carbon onions produced by laser irradiation of amorphous silicon carbide.Chemical Physics Letters, 2003, 373: 642-645. |
| [43] | HU S L, BAI P K, TIAN F, et al.Hydrophilic carbon onions synthesized by millisecond pulsed laser irradiation.Carbon, 2009, 47: 876-883. |
| [44] | DOROBANTU D, BOTA P M, BOERASU I, et al.Pulse laser ablation system for carbon nano-onions fabrication.Surface Engineering and Applied Electrochemistry, 2014, 50(5): 390-394. |
| [45] | ZHANG H M, LIANG C H, LIU J, et al.The formation of onion-like carbon-encapsulated cobalt carbide core/shell nanoparticles by the laser ablation of metallic cobalt in acetone.Carbon, 2013, 55: 108-115. |
| [46] | GAO Y, ZHOU Y S, PARK J B, et al. Resonant excitation of precursor molecules in improving the particle crystallinity, growth> rate and optical limiting performance of carbon nano- onions. Nanotechnology, 2011, 22(16): 165604-1-6. |
| [47] | XIAO J, OUYANG G, LIU P, et al.Reversible nanodiamond- carbon onion phase transformations.Nano letters, 2014, 14: 3645-3652. |
| [48] | CABIOC’H T, JAOUEN M, THUNE E, et al. Carbon onions formation by high-dose carbon ion implantation into copper and silver. Surface and Coatings Technology, 2000, 128-129: 43-50. |
| [49] | HUANG J Y, YASUDA H AND MORI H. Highly curved carbon nanostructures produced by ball-milling.Chemical Physics Letters, 1999, 303: 130-134. |
| [50] | HAN F D, YAO B AND BAI Y J. Preparation of carbon nano-onions and their application as anode materials for rechargeable lithium-ion batteries. J. Phys. Chem. C, 2011, 115: 8923-8927. |
| [51] | GHOSH M, SONKAR S K, SAXENA M, et al.Carbon nano-onions for imaging the life cycle of drosophila melanogaster.Small, 2011, 7(22): 3170-3177. |
| [52] | CHOUCAIR M, STRIDE J A.The gram-scale synthesis of carbon onions.Carbon, 2012, 50: 1109-1115. |
| [53] | HE C N, WU S, ZHAO N Q, et al.Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material.ACS Nano, 2013, 7(5): 4459-4469. |
| [54] | HAN Q Y, ZAI J T, XIAO Y L, et al.Direct growth of SnO2 nanorods on graphene as high capacity anode materials for lithium ion batteries.RSC Adv., 2013, 3: 20573-20578. |
| [55] | FENG J K, XIONG S L, QIAN Y T, et al.Synthesis of nanosized cadmium oxide (CdO) as a novel high capacity anode material for Lithium-ion batteries: influence of carbon nanotubes decoration and binder choice.Electrochimica Acta, 2014, 129: 107-112. |
| [56] | GUO B K, CHI M F, SUN X G, et al.Mesoporous carbon-Cr2O3 composite as an anode material for lithium ion batteries.Journal of Power Sources, 2012, 205: 495-499. |
| [57] | CHEN A, LI C X, TANG R, et al.MoO2-ordered mesoporous carbon hybrids as anode materials with highly improved rate capability and reversible capacity for lithium-ion battery.Phys. Chem. Chem. Phys., 2013, 15: 13601-13610. |
| [58] | LIU B, HU X L, XU H H, et al.Encapsulation of MnO nanocrystals in electrospun carbon nanofibers as high-performance anode materials for lithium-ion batteries.Scientific Reports, 2014, 4: 4229. |
| [59] | ZANG J, CHEN J J, ZHANG C L, et al.The synthesis of a core-shell MnO2/3D-ordered hollow carbon sphere composite and its superior electrochemical capability for lithium ion batteries. J. Mater. Chem. A, 2014, 2(18): 6343-6347. |
| [60] | LEPPLE M, ADAM R, CUPID D M, et al.Thermodynamic investigations of copper oxides used as conversion type electrodes in lithium ion batteries.J. Mater. Sci., 2013, 48: 5818-5826. |
| [61] | WANG J, ZHAO H L, ZENG Z P, et al.Nano-sized Fe3O4 /carbon as anode material for lithium ion battery.Materials Chemistry and Physics, 2014, 148: 699-704. |
| [62] | QIN D, YAN P, LI G Z, et al. Synthesis of hierarchical CoO nano/microstructures as anode materials for lithium-ion batteries. Journal of Nanomaterials, 2014, 2014: 489862-1-5. |
| [63] | ZHANG H J, SONG H H, ZHOU J S, et al.Preparation and electrochemical properties of SnO2/onion-like hollow carbon nanoparticle composites as anode materials for lithium-ion batteries.Acta Phys.-Chim. Sin., 2010, 26(5): 1259-1263. |
| [64] | STROBEL R, GARCHE J, MOSELEY P T, et al.Hydrogen storage by carbon materials.Journal of Power Sources, 2006, 159: 781-801. |
| [65] | PORTET C, YUSHIN G, GOGOTSI Y.Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors.Carbon, 2007, 45: 2511-2518. |
| [66] | GAO Y, ZHOU Y S, QIAN M, et al.Chemical activation of carbon nano-onions for high-rate supercapacitor electrodes.Carbon, 2013, 51: 52-58. |
| [67] | ANJOS D M, MCDONOUGH J K, PERRE E, et al.Pseudocapacitance and performance stability of quinone-coated carbon onions.Nano energy, 2013, 2: 702-712. |
| [68] | PLONSKA-BRZEZINSKA M E, ECHEGOYEN L. Carbon nano-onions for supercapacitor electrodes: recent developments and applications.Journal of Materials Chemistry A, 2013, 1: 13703-13714. |
| [69] | BORGOHAIN R, LI J C, SELEGUE J P, et al.Electrochemical study of functionalized carbon nano-onions for high-performance supercapacitor electrodes.J. Phys. Chem. C, 2012, 116: 15068-15075. |
| [70] | PLONSKA-BRZEZINSKA M E, BRUS D M, MOLINA- ONTORIA A, et al. Synthesis of carbon nano-onion and nickel hydroxide/oxide composites as supercapacitor electrodes. RSC Adv., 2013, 3: 25891-25901. |
| [71] | WANG Y, YU S F, SUN C Y, et al.MnO2/onion-like carbon nanocomposites for pseudocapacitors. J. Mater. Chem., 2012, 22: 17584-17588. |
| [72] | BRECZKO J, WINKLER K, PLONSKA-BRZEZINSKA M E, et al. Electrochemical properties of composites containing small carbon nano-onions and solid polyelectrolytes.J. Mater. Chem., 2010, 20: 7761-7768. |
| [73] | PLONSKA-BRZEZINSKA D M E, BRECZKO J, PALYS D B, et al. The electrochemical properties of nanocomposite films obtained by chemical in situ polymerization of aniline and carbon nanostructures.ChemPhysChem, 2013, 14(1): 116-124. |
| [74] | HIRATA A, IGARASHI M, KAITO T.Study on solid lubricant properties of carbon onions produced by heat treatment of diamond clusters or particles.Tribology International, 2004, 37: 899-905. |
| [75] | BUCHOLZ E W, PHILLPOT S R, SINNOTT S B.Molecular dynamics investigation of the lubrication mechanism of carbon nano-onions. Computational Materials Science, 2012, 54: 91-96. |
| [76] | HAN C, BO X J, ZHANG Y F, et al.One-pot synthesis of nitrogen and sulfur co-doped onion-like mesoporous carbon vesicle as an efficient metal-free catalyst for oxygen reduction reaction in alkaline solution.Journal of Power Sources, 2014, 272: 267-276. |
| [77] | HUANG Q, YU D L, XU B, et al.Nanotwinned diamond with unprecedented hardness and stability.Nature, 2014, 510: 250-253. |
| [78] | 龚文. 碳纳米葱无添加剂高温高压烧结聚晶金刚石的研究. 秦皇岛: 燕山大学硕士学位论文, 2012. |
| [79] | 余强华. 微米金刚石对碳纳米葱烧结PCD组织及性能的影响. 秦皇岛: 燕山大学硕士学位论文, 2013. |
| [1] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [2] | 魏相霞, 张晓飞, 徐凯龙, 陈张伟. 增材制造柔性压电材料的现状与展望[J]. 无机材料学报, 2024, 39(9): 965-978. |
| [3] | 李雷, 程群峰. 高性能MXenes纳米复合材料研究进展[J]. 无机材料学报, 2024, 39(2): 153-161. |
| [4] | 冒爱琴, 陆文宇, 贾洋刚, 王冉冉, 孙静. 柔性压电器件及其可穿戴应用[J]. 无机材料学报, 2023, 38(7): 717-730. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 王鲁凯, 冯军宗, 姜勇刚, 李良军, 冯坚. 直写3D打印陶瓷基多孔结构的研究进展[J]. 无机材料学报, 2023, 38(10): 1133-1148. |
| [7] | 白志强, 赵璐, 白云峰, 冯锋. MXenes的制备、性质及其在肿瘤诊疗中的研究进展[J]. 无机材料学报, 2022, 37(4): 361-375. |
| [8] | 刘凯, 孙策, 史玉升, 胡佳明, 张庆庆, 孙云飞, 章嵩, 涂溶, 闫春泽, 陈张伟, 黄尚宇, 孙华君. 增材制造压电陶瓷的现状与展望[J]. 无机材料学报, 2022, 37(3): 278-288. |
| [9] | 雷伟岩, 王岳, 武世然, 石东新, 沈毅, 李锋锋. VA族单元素二维纳米材料在生物医用领域的研究进展[J]. 无机材料学报, 2022, 37(11): 1181-1191. |
| [10] | 黄慧, 陈雨. 材料医学和医学材料[J]. 无机材料学报, 2022, 37(11): 1151-1169. |
| [11] | 苏莉, 杨建平, 兰悦, 王连军, 江莞. 纳米铁颗粒及其复合材料的界面设计及环境修复应用[J]. 无机材料学报, 2021, 36(6): 561-569. |
| [12] | 范宏伟, 李克睿, 侯成义, 张青红, 李耀刚, 王宏志. 多功能电致变色器件:从多器件到单器件集成[J]. 无机材料学报, 2021, 36(2): 115-127. |
| [13] | 徐放, 金平实, 罗宏杰, 曹逊. VO2热致变色智能窗: 现状、挑战及展望[J]. 无机材料学报, 2021, 36(10): 1013-1021. |
| [14] | 王堋人, 苟燕子, 王浩. 第三代SiC纤维及其在核能领域的应用现状[J]. 无机材料学报, 2020, 35(5): 525-531. |
| [15] | 贾汉祥, 曹逊, 金平实. 无机全固态电致变色材料与器件研究进展[J]. 无机材料学报, 2020, 35(5): 511-524. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||