无机材料学报 ›› 2015, Vol. 30 ›› Issue (1): 9-16.DOI: 10.15541/jim20140175 CSTR: 32189.14.10.15541/jim20140175
陈爱兵, 于奕峰, 臧文伟, 齐国禄, 于运红, 李月彤
收稿日期:2014-04-08
修回日期:2014-06-16
出版日期:2015-01-20
网络出版日期:2014-12-29
作者简介:陈爱兵(1978–), 男, 博士, 副教授. E-mail:chen_ab@163.com
基金资助:CHEN Ai-Bing, YU Yi-Feng, ZANG Wen-Wei, QI Guo-Lu, YU Yun-Hong, LI Yue-Tong
Received:2014-04-08
Revised:2014-06-16
Published:2015-01-20
Online:2014-12-29
About author:CHEN Ai-Bing. E-mail:chen_ab@163.com
Supported by:摘要:
CO2作为温室气体, 其捕集和存储有着重要的现实意义。多孔碳材料掺杂N原子后可以极大地改变材料的表面化学性质, 增强表面碱性, 在CO2吸附领域具有广泛的应用。基于N掺杂最新研究进展, 本文系统地介绍了原位、后处理等掺N方法和不同孔道结构对CO2吸附分离或扩散传质的影响, 总结归纳了材料的物理结构参数、表面化学性质与CO2吸附分离性能的关系, 指出了各种制备方法存在的问题及解决的方法, 为高性能的CO2吸附剂的定向设计、制备以及工业化提供了理论参考。
中图分类号:
陈爱兵, 于奕峰, 臧文伟, 齐国禄, 于运红, 李月彤. 掺氮多孔碳在二氧化碳吸附分离中的应用[J]. 无机材料学报, 2015, 30(1): 9-16.
CHEN Ai-Bing, YU Yi-Feng, ZANG Wen-Wei, QI Guo-Lu, YU Yun-Hong, LI Yue-Tong. Nitrogen-doped Porous Carbon for CO2 Adsorption[J]. Journal of Inorganic Materials, 2015, 30(1): 9-16.
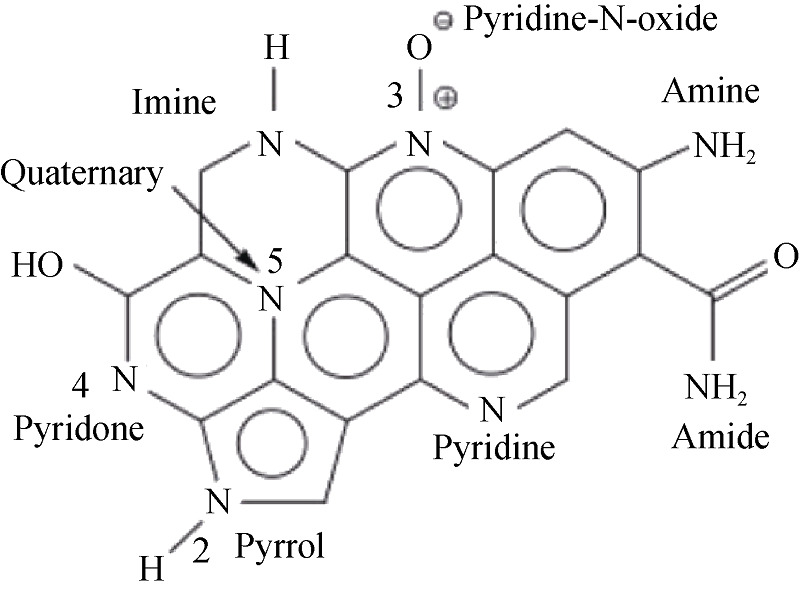
图 1 N原子在碳材料中的可能存在形式示意图[9]
Fig. 1 Possible positions of N atom in the carbon materials [9].(1-Anmio-N; 2-Pyrrolic; 3-Nitrosyl-N; 4-Pyridone; 5-Quaternary)
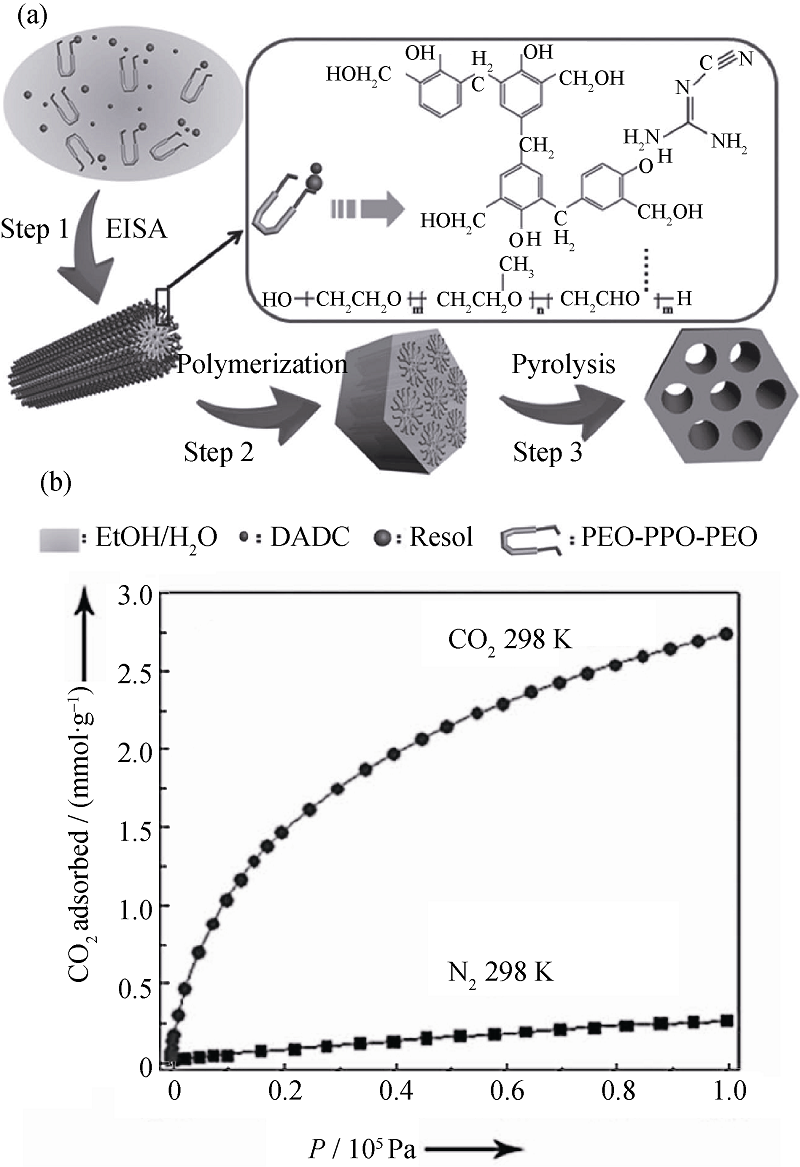
图2 EISA法添加双腈胺制备碳材料的合成机理图(a)及CO2/N2选择性吸附图(b)[16]
Fig. 2 Formation process of N-doped mesoporous carbon using dicyandiamide as an additive (a); CO2 and N2 adsorption isotherms (b)[16]
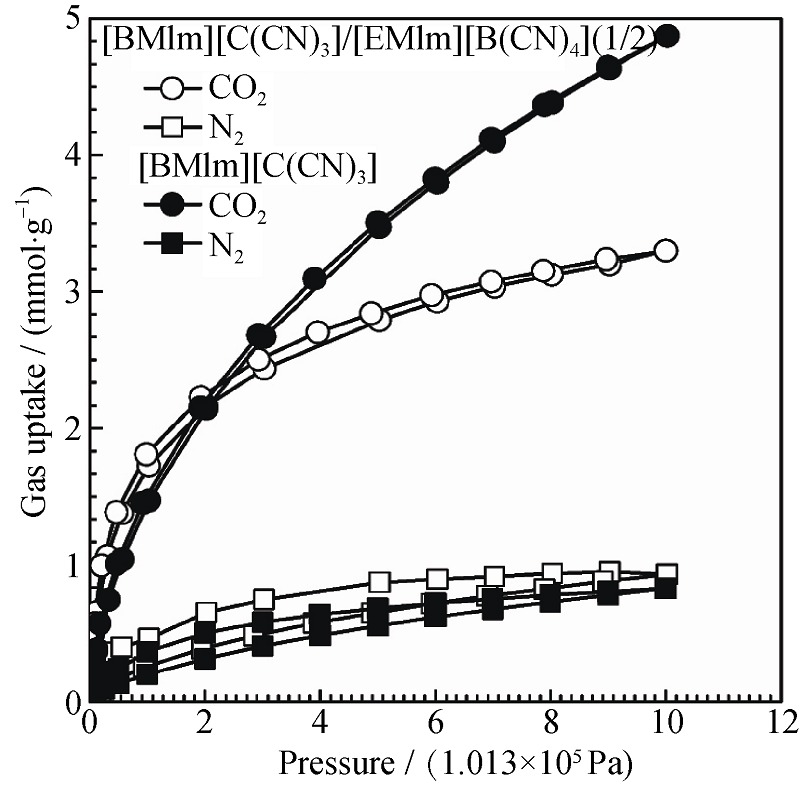
图 3 高压下[BMIm][C(CN)3]/[EMIm][B(CN)4]=1:2碳混合样品的CO2、N2吸附-脱附等温曲线[17]
Fig. 3 CO2 and N2 adsorption-desorption isotherms as a function of pressure of the carbons from [BMIm] [C(CN)3]/ [EMIm] [B(CN)4]=1:2[17]
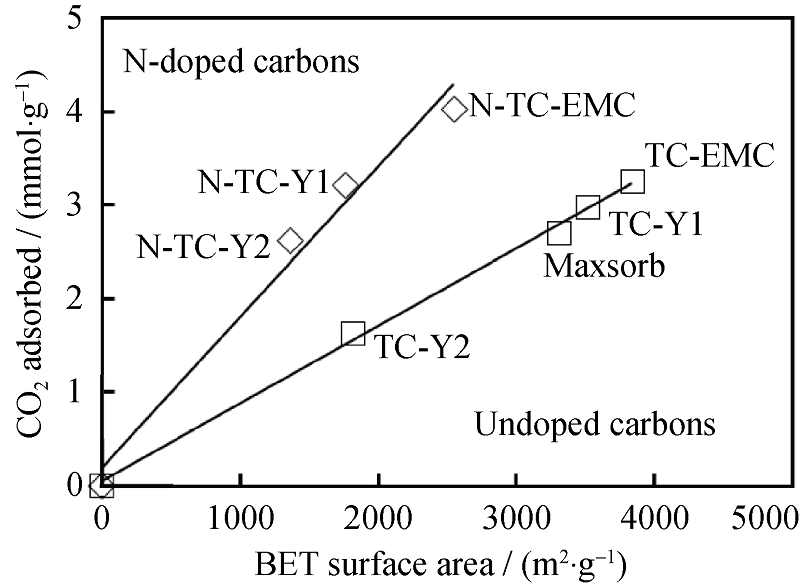
图 4 CO2吸附量与氮掺杂和未氮掺杂的碳材料比表面积关系(1.013×105 Pa, 298 K), 氮含量在6wt%~7wt%[26]
Fig. 4 Relationships between the CO2 adsorption capacities (at 298 K and 1 .013×105 Pa) and the BET surface area of nitrogen- doped and undoped carbons. The N-doped carbons contain 6wt%-7wt% N[26]
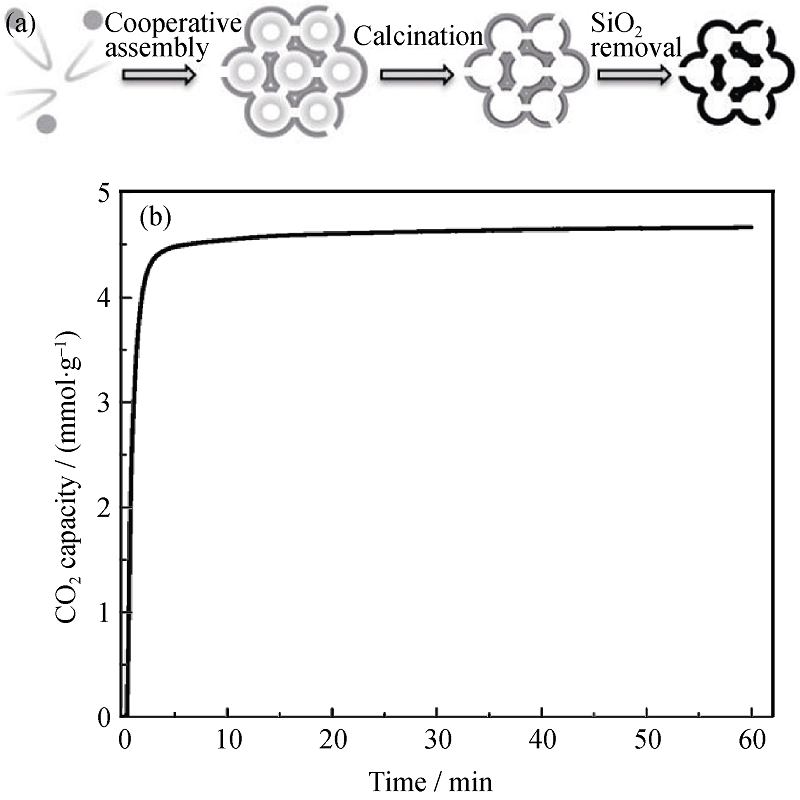
图 5 介孔碳泡沫的合成示意图(a)和75℃下PEI浸渍介孔碳泡沫的CO2吸附动力学曲线(b)[35]
Fig. 5 Schematic illustration of the synthesis method of mesoporous carbon foams(a) and CO2 adsorption kinetics of the PEI-impregnated mesoporous carbon sorbent at 75℃(b)[35]
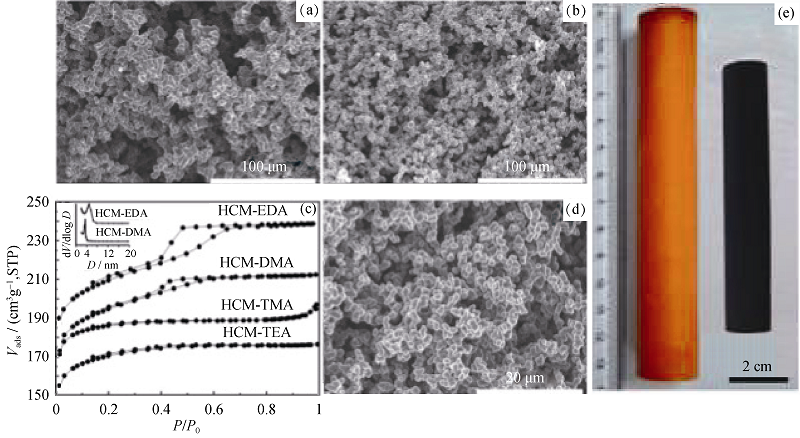
图 6 分级整块碳材料的SEM照片、N2吸附-脱附曲线和孔径分布图(a~d)和(e)光学照片[46]
Fig. 6 SEM images of hierarchical porous carbon and N2 adsorption-desorption and PSD (a-d) and optical images (e)[46]
| Samples | Nitrogen source | Nitrogen content/wt% | Functionalities | Surface area /(m2·g-1) | CO2 capacity /(mmol·g-1) |
|---|---|---|---|---|---|
| N-doped microporous carbon [ | Imine-linked polymer | 5.58-8.74 | Pyridinic, quaternary | 263-366 | 1.95 |
| N-doped mesoporous carbon[ | Melamine resin | -8.00 | Pyridinic, pyrrolic, quaternary | -750 | 1.80 |
| KOH activated porous carbon[ | Polypyrrole | -10.14 | Pyridinic,quaternary | 1700 | 3.10 |
| Organic amine grafting carbon[ | Glucose | NA | Amide, tertiary amines, primary amines | <10 | 4.10 |
| Macro-micro hollow carbon spheres [ | Melamine | 14.80 | NA | 767 | 2.67 |
| Sustainable biomass [ | Glgae | 1.1-4.7 | Pyridinic, pyrrolic, quaternary | 1300-2400 | 7.40 |
| Template carbon [ | HNO3 | 6.73 | Pyridinic, pyrrolic, quaternary | 1979 | 4.30 |
| N-doped activated carbon monoliths [ | Polyacrylonitrile | 1.80 | NA | 2501 | 5.14 |
表 1 不同掺氮碳材料对CO2的吸附能力比较
Table 1 Different nitrogen doped carbon materials for CO2 adsorption capacity
| Samples | Nitrogen source | Nitrogen content/wt% | Functionalities | Surface area /(m2·g-1) | CO2 capacity /(mmol·g-1) |
|---|---|---|---|---|---|
| N-doped microporous carbon [ | Imine-linked polymer | 5.58-8.74 | Pyridinic, quaternary | 263-366 | 1.95 |
| N-doped mesoporous carbon[ | Melamine resin | -8.00 | Pyridinic, pyrrolic, quaternary | -750 | 1.80 |
| KOH activated porous carbon[ | Polypyrrole | -10.14 | Pyridinic,quaternary | 1700 | 3.10 |
| Organic amine grafting carbon[ | Glucose | NA | Amide, tertiary amines, primary amines | <10 | 4.10 |
| Macro-micro hollow carbon spheres [ | Melamine | 14.80 | NA | 767 | 2.67 |
| Sustainable biomass [ | Glgae | 1.1-4.7 | Pyridinic, pyrrolic, quaternary | 1300-2400 | 7.40 |
| Template carbon [ | HNO3 | 6.73 | Pyridinic, pyrrolic, quaternary | 1979 | 4.30 |
| N-doped activated carbon monoliths [ | Polyacrylonitrile | 1.80 | NA | 2501 | 5.14 |
| [1] | ROCHELLE G T.Amine scrubbing for CO2 capture. Science, 2009, 325(5948):1652-1654. |
| [2] | CHOMAA J, JEDYNAKB K, JARONIECC M, et al.Microporosity development in phenolic resin-based mesoporouscarbons for enhancing CO2 adsorption at ambient conditions.Applied Surface Science, 2014, 289(2): 592-600. |
| [3] | LUO J, PENG F, ZHENG W X, et al.Aerobic liquid-phase oxidation of ethyl benzene to acetophenone catalyzed by carbon nanotubes.ChemCatChem, 2013, 5(6):1578-1586. |
| [4] | ALAM N, MOKAYA R, Evolution of optimal porosity for improved hydrogen storage in template zeolite-like carbons.Energy Environ. Sci., 2010, 3(11): 1773-1781. |
| [5] | WANG J X, XUE C F, LV Y Y, et al.Kilogram-scale synthesis of ordered mesoporous carbons and their electrochemical performance.Carbon, 2011; 49(13): 4580-4588. |
| [6] | CHANG H, JOO S H, PAK C.Synthesis and characterization of mesoporouscarbonfor fuel cell applications. J. Mater. Chem., 2007, 17(30): 3078-3088. |
| [7] | WU Z X, PAUL A, ZHAO D Y, et.al. Post-enrichment of nitrogen in soft-templated ordered mesoporouscarbonmaterials for highly efficient phenol removal and CO2 capture.J. Mater. Chem., 2012, 22(22):11379-11389. |
| [8] | LIU L, DENG Q F, YUAN Z Y, et.al. Ordered mesoporous carbons: citric acid-catalyzed synthesis, nitrogen doping and CO2 capture.J. Mater. Chem., 2011, 21(40): 16001-16009. |
| [9] | WANG X Q, LIU C G, DAI S, et al.Nitrogen-enriched ordered mesoporous carbons through direct pyrolysis in ammonia with enhanced capacitive performance. J. Mater. Chem. A, 2013, 1(27):7920-7926. |
| [10] | DARGE T C, ARENILLA S A, SNAPE C E.Preparation of carbon dioxide adsorbents from the chemical activation of urea- formaldehyde and melamine-formaldehyde resins.Fuel, 2007, 86(1/2): 22-31. |
| [11] | NANDI M, OKADA K, UYAMA D H.Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation.Chem. Commun., 2012, 48(83): 10283-10285. |
| [12] | ZHAO Y F, ZHAO L, HAN Y, et al.Novel porous carbon materials with ultrahigh nitrogen contents for selective CO2 capture.J. Mater. Chem., 2012, 22(37): 19726-19731. |
| [13] | SEVILLA M, VIGÓN P V, FUERTES A B. N-doped polypyrrole-based porous carbons for CO2capture.Adv. Funct. Mater., 2011, 21(14): 2781-2787. |
| [14] | YU J Y, GUO M Y, ZHU G S, et al.One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol-urea-formaldehyde resin for CO2 capture.Carbon, 2014, 69(4): 502-514. |
| [15] | FENG C M, LI H X, WAN Y.Fabrication of N-doped highly ordered mesoporous polymers and carbons.J. Nanosci. Nanotechnol, 2009, 9(2):1558-1563. |
| [16] | WEI J, ZHOU D D, ZHAO D Y, et al.A Controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors. Adv. Funct. Mater., 2013, 23(18): 2322-2328. |
| [17] | FULVIO P F, LEE J S, DAI S, et al.Boron and nitrogen-rich carbons from ionic liquid precursors with tailorable surface properties.Phys. Chem. Chem. Phys., 2011, 13(30): 13486-13491. |
| [18] | CHEN A B, YU Y F, LV H J, et al.Thin-walled, mesoporous and nitrogen-doped hollow carbon spheres using ionic liquids as precursors.J. Mater. Chem., 2013, 1(4): 1045-1047. |
| [19] | CHEN A B, LIU C, YU Y F, et al.A co-confined carbonization approach to aligned nitrogen-doped mesoporous carbon nanofibers and its application as an adsorbent.J. Hazard. Mater., 2014, 276(13): 192-199. |
| [20] | PLAZA M G, PEVIDA C, ARENILLAS A, et al.CO2 capture by adsorption with nitrogen enriched carbons.Fuel, 2007, 86(14): 2204-2212. |
| [21] | SONG J, SHEN W Z, WANG J G, et al.Superior carbon-based CO2 adsorbents prepared from poplar anthers.Carbon, 2014, 69(4): 255-263. |
| [22] | SAYARI A, BELMABKHOUT Y.Stabilization of amine- containing CO2 adsorbents: dramatic effect of water vapor. J. Am. Chem. Soc., 2010, 132(18): 6312-6314. |
| [23] | WANG J C, LIU Q.An efficient one-step condensation and activationstrategy to synthesize porous carbons with optimal micropore sizes for highly selective CO2 adsorption.Nanoscale, 2014, 6(8): 4148-4156. |
| [24] | ZHANG Z.S, ZHOU J, QIAO S Z, et al. Critical role of small micropores in high CO2 uptake.Phys. Chem. Chem. Phys., 2013, 15(7): 2523-2529. |
| [25] | ZHOU J, LI W, ZHUO S P, et al.Carbon dioxide adsorption performance of N-doped zeolite Y template carbons.RSC Adv., 2012, 2(1): 161-167. |
| [26] | WANG L, YANG R T.Significantly increased CO2 adsorption performance of nanostructured templated carbon by tuning surface area and nitrogen doping.J. Phys. Chem. C, 2012, 116(1): 1099-1106. |
| [27] | MANGUN C L, BENAK K R, ECONOMY J, et al.Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon, 2001, 39(12): 1809-1820. |
| [28] | SHEN W Z, ZHANG S C, FAN W B, et al.Hierarchical porous polyacrylonitrile-based activated carbon fibers for CO2 capture.J. Mater. Chem., 2011, 21(36): 14036-14040. |
| [29] | HAO G P, JIN Z Y, LU A H.Porous carbon nanosheets with precisely tunablethickness and selective CO2 adsorption properties.Energy Environ. Sci., 2013, 6(12): 3740-3747. |
| [30] | CHANDRA V, YU S U, KIM K S, et al.Highly selective CO2 capture on N-doped carbon produced by chemicalactivation of polypyrrole functionalized graphene sheets.Chem. Commun., 2012, 48(5): 735-737. |
| [31] | SHEN Y M, BAI J F.A new kind CO2/CH4 separation material: open ended nitrogen doped carbon nanotubes formed by direct pyrolysis of metal organicframeworks.Chem. Commun., 2010, 46(8): 1308-1310. |
| [32] | ZHAO Y X, SEREDYCH M, BANDOSZ T J.Superior performance of copper based MOF and aminatedgraphite oxide composites as CO2 adsorbents at room temperature.ACS Appl. Mater Interfaces, 2013, 5(11): 4951-4959. |
| [33] | BABARAO R, DAI S, JIANG D E.Nitrogen-doped mesoporouscarbon for carbon capture-a molecular simulation study.J. Phys. Chem. C, 2012, 116(12):7106-7110. |
| [34] | YU J Y, GUO M Y, ZHU G S, et al.Simple fabrication of an ordered nitrogen-doped mesoporous carbon with resorcinol- melamine-formaldehyde resin.Microporous Mesoporous Mater., 2014, 190(8): 117-127. |
| [35] | FU L L, QI G G, GIANNELIS E P, et al.Facile synthesis and application of a carbon foam with large mesopores.Phys. Chem. Chem. Phys., 2013, 15(44): 19134-19137. |
| [36] | WHITE R J, ANTONIETTI M, TITIRICI M M, et al.Naturally inspired nitrogen doped porous carbon. J. Mater. Chem., 2009, 19(45): 8645-8650. |
| [37] | OLEJNICZAK A, LEZANSKA M, LUKASZEWICZA J P, et al.Novel nitrogen-containing mesoporous carbons prepared from chitosan.J. Mater. Chem. A, 2013, 1(31): 8961-8967. |
| [38] | SEVILLA M, FALCO C, FUERTES A B, et al.High-performance CO2 adsorbents from algae.RSC Adv., 2012, 2(33): 12792-12797. |
| [39] | WOOD K N, HAYREA R O, PYLYPENKO S.Recent progress on nitrogen·carbon structures designed for use in energy and sustainability applications.Energy Environ. Sci., 2014, 7: 1212-1249. |
| [40] | WANG J C, LIU Q.An ordered mesoporous aluminosilicateoxynitridetemplate to prepare N-incorporated ordered mesoporous carbon.J. Phys. Chem. C, 2007, 111(20): 7266-7272. |
| [41] | YANG H W, YUAN Y Z, EDMAN TSANG S C. Nitrogen- enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chemical Engineering Journal, 2012, 185-186(5): 374-379. |
| [42] | LI Q, YANG J P, ZHAO D Y, et al.Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2capture.Nano Res., 2010, 3(9): 632-642. |
| [43] | WILKE A, WEBER J.Hierarchical nanoporous melamine resin sponges with tunable porosity-porosity analysis and CO2 adsorption properties.J. Mater. Chem., 2011, 21(14): 5226-5229. |
| [44] | GUTIERREZ M C, CARRIAZO D, MONTE F D, et al.Deep eutectic solvents as both precursors and structure directing agents in thesynthesis of nitrogen doped hierarchical carbons highly suitable for CO2.capture.Energy Environ. Sci., 2011, 4(11): 3535-3544. |
| [45] | HAO G P, LI W C, LU A H.Lysine-assisted rapid synthesis of crack-free hierarchical carbon monoliths with ahexagonal array of mesopores.Carbon, 2011, 49(12): 3762-3772. |
| [46] | HAO G P, LI W C, LU A H. Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc., 2011,133(29): 11378-11388. |
| [47] | ZHU B J, LI K X, GUO Z X, et al.Nitrogen-enriched and hierarchically porous carbon macro-spheres-ideal for large-scale CO2 Capture.J. Mater. Chem. A, 2014, 2(15): 5481-5489. |
| [48] | WANG J C, SENKOVSKA I, KASKEL S, et al.Imine-linked polymer-derived nitrogen-doped microporous carbons with excellent CO2 capture properties.ACS Appl. Mater. Interfaces, 2013, 5(8): 3160-3167. |
| [49] | CHEN H C, SUN F G, LONG D H, et al.Nitrogen doping effects on the physical and chemical properties of mesoporous carbon. J. Phys. Chem. C, 2013, 117(16): 8318-8328. |
| [50] | TITRIRICI M M, WHITE R J, ZHAO L.Nitrogen-doped hydrothermal carbons.Green, 2012, 2(1): 25-40. |
| [51] | FENG S S, LI W, ZHAO D Y, et al.Synthesis of nitrogen-doped hollow carbon nanospheres for CO2 capture.Chem. Commun., 2014, 50(3): 329-331. |
| [52] | MA X Y, CAO M H, HU C W, et al.Bifunctional HNO3 catalytic synthesis of N-doped porous carbons for CO2 capture. J. Mater. Chem. A, 2013, 1(3): 913-918. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [8] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [9] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [10] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [11] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [12] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [13] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [14] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [15] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||