
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (11): 1268-1276.DOI: 10.15541/jim20250009
• RESEARCH ARTICLE • Previous Articles Next Articles
WANG Hongqin1,2( ), DENG Hao2, LIANG Hua1, TIAN Qiang1, YAN Minhao1, HUANG Yi1(
), DENG Hao2, LIANG Hua1, TIAN Qiang1, YAN Minhao1, HUANG Yi1( )
)
Received:2025-01-07
Revised:2025-03-10
Published:2025-11-20
Online:2025-04-02
Contact:
HUANG Yi, lecturer. E-mail: huangyi516@163.comAbout author:WANG Hongqin (1999-), female, Master candidate. E-mail: 1523555457@qq.com
Supported by:CLC Number:
WANG Hongqin, DENG Hao, LIANG Hua, TIAN Qiang, YAN Minhao, HUANG Yi. Properties and Mechanism of U(VI) Removal by Calcium Orthovanadate[J]. Journal of Inorganic Materials, 2025, 40(11): 1268-1276.
| Metal ion | ρ0 /(mg·L-1) | Metal ion | ρ0 /(mg·L-1) |
|---|---|---|---|
| Zn2+ | 648.63 | Co2+ | 67.12 |
| Cr3+ | 101.22 | Ba2+ | 64.22 |
| Cu2+ | 72.55 | U(VI) | 121.49 |
| Ni2+ | 133.04 |
Table 1 Initial mass concentrations of several metal ions in coexisting ionic solution
| Metal ion | ρ0 /(mg·L-1) | Metal ion | ρ0 /(mg·L-1) |
|---|---|---|---|
| Zn2+ | 648.63 | Co2+ | 67.12 |
| Cr3+ | 101.22 | Ba2+ | 64.22 |
| Cu2+ | 72.55 | U(VI) | 121.49 |
| Ni2+ | 133.04 |
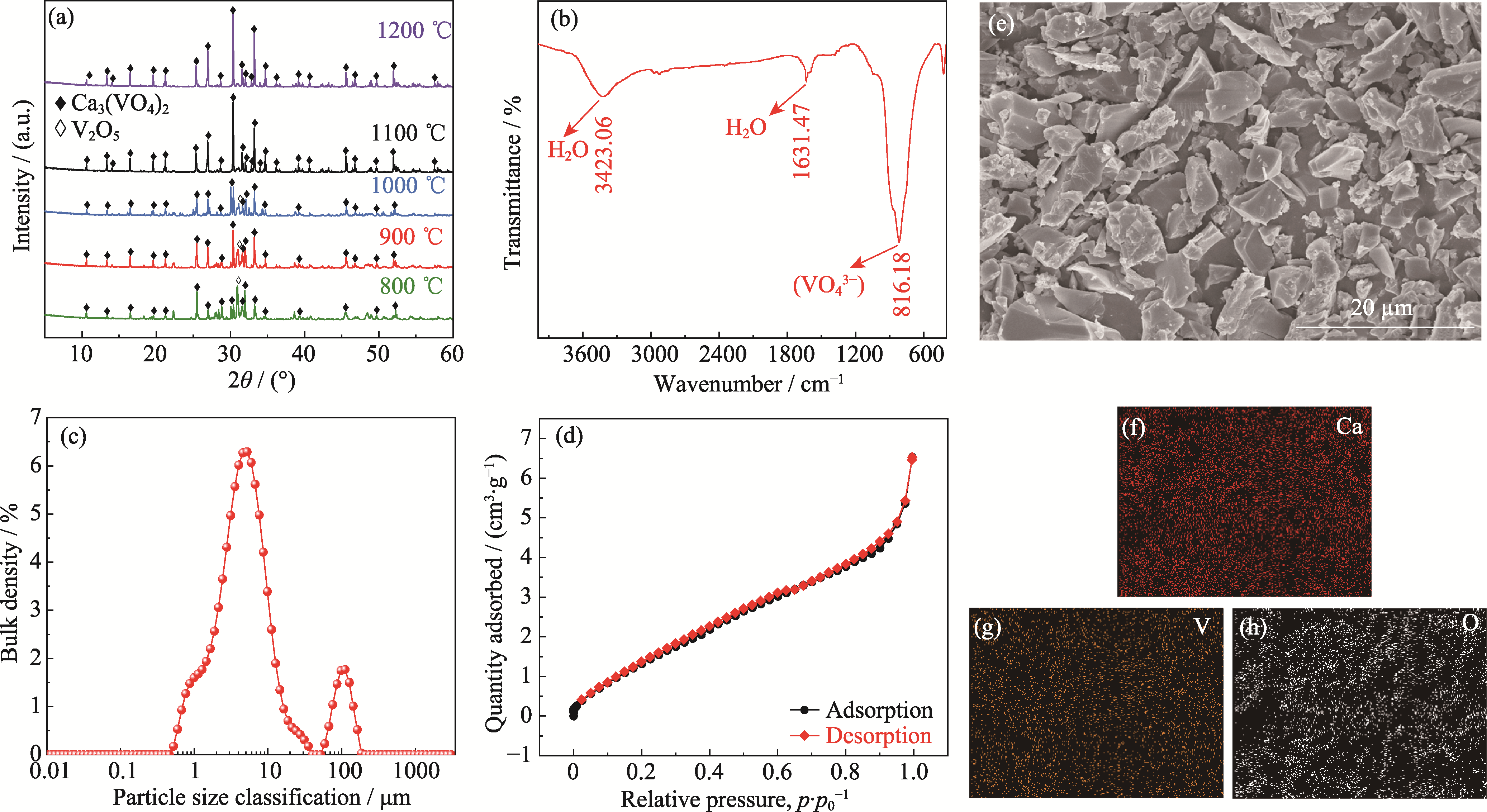
Fig. 1 Characterization results of Ca3(VO4)2 powder (a) XRD patterns of Ca3(VO4)2 prepared at different temperatures for 4 h; (b-h) FT-IR spectrum (b), particle size distribution (c), nitrogen adsorption-desorption curves (d), SEM image (e) and EDS mappings (f-h) of Ca3(VO4)2 prepared at 1100 ℃ for 4 h
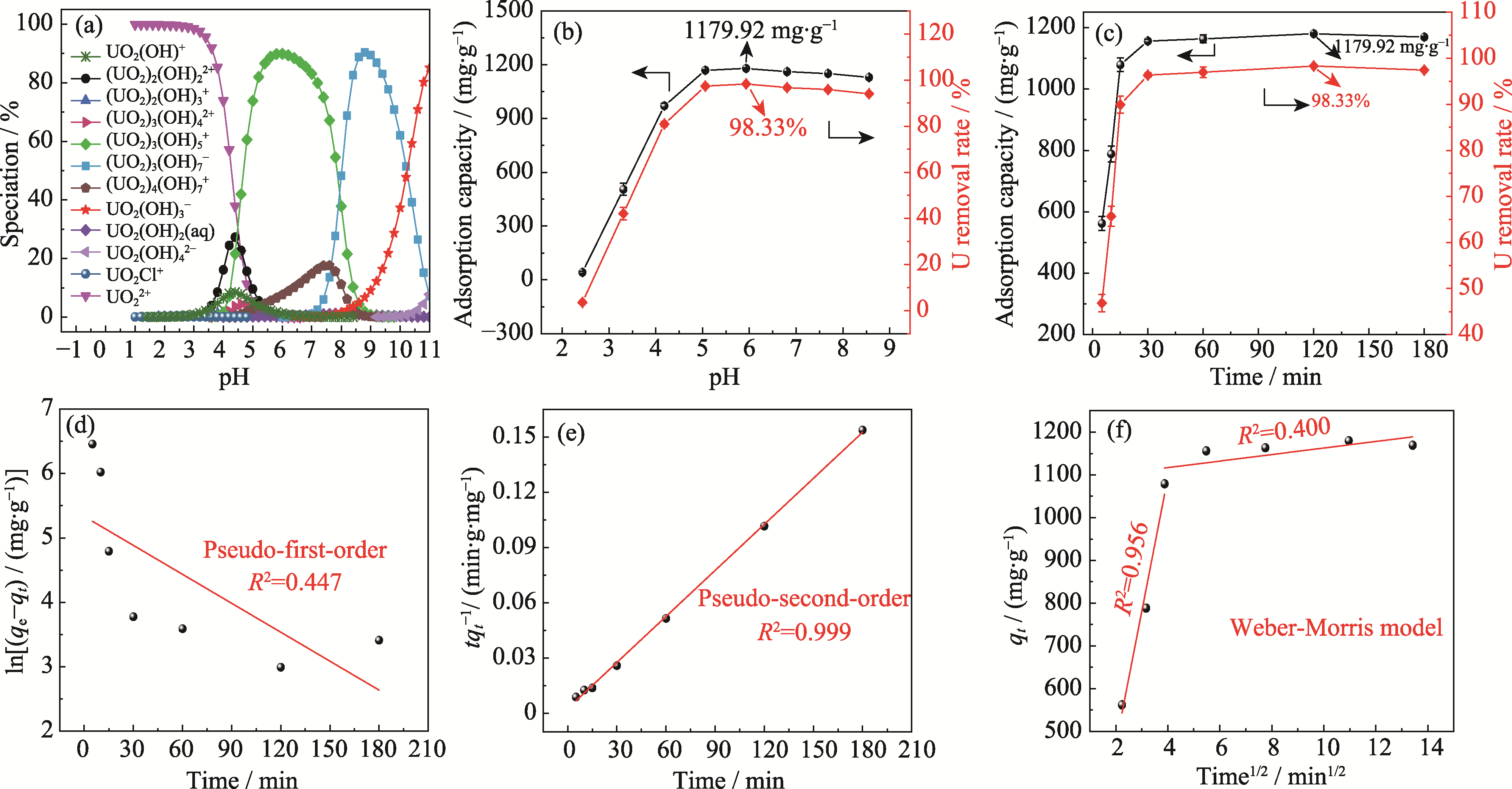
Fig. 2 Effect of pH and adsorption time on removal of U(VI) by Ca3(VO4)2 (a) Species distribution of U(VI) with different pH (U(VI) at mass concentration of 120 mg·L-1 under 308 K); (b, c) Adsorption capacity and removal rate of U(VI) with different pH (b) and durations (c); (d-f) Pseudo-first-order (d), pseudo-second-order (e) and Weber-Morris (f) models fitting (temperature at 308 K, initial U(VI) mass concentration at 120 mg·L-1, adsorbent dosage at 0.1 g·L-1)
| Material | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| k1/min-1 | R2 | k2/(g·mg-1·min-1) | R2 | |
| Ca3(VO4)2 | 0.01 | 0.447 | 2.62×10-4 | 0.999 |
Table 2 Fitting parameters of pseudo-first-order and pseudo-second-order kinetic models
| Material | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| k1/min-1 | R2 | k2/(g·mg-1·min-1) | R2 | |
| Ca3(VO4)2 | 0.01 | 0.447 | 2.62×10-4 | 0.999 |
| Material | Weber-Morris | |||
|---|---|---|---|---|
| kp,1/(mg·g-1·min-1/2) | R12 | kp,2/(mg·g-1·min-1/2) | R22 | |
| Ca3(VO4)2 | 312.25 | 0.956 | 7.65 | 0.400 |
Table 3 Fitting parameters of Weber-Morris dynamic model
| Material | Weber-Morris | |||
|---|---|---|---|---|
| kp,1/(mg·g-1·min-1/2) | R12 | kp,2/(mg·g-1·min-1/2) | R22 | |
| Ca3(VO4)2 | 312.25 | 0.956 | 7.65 | 0.400 |
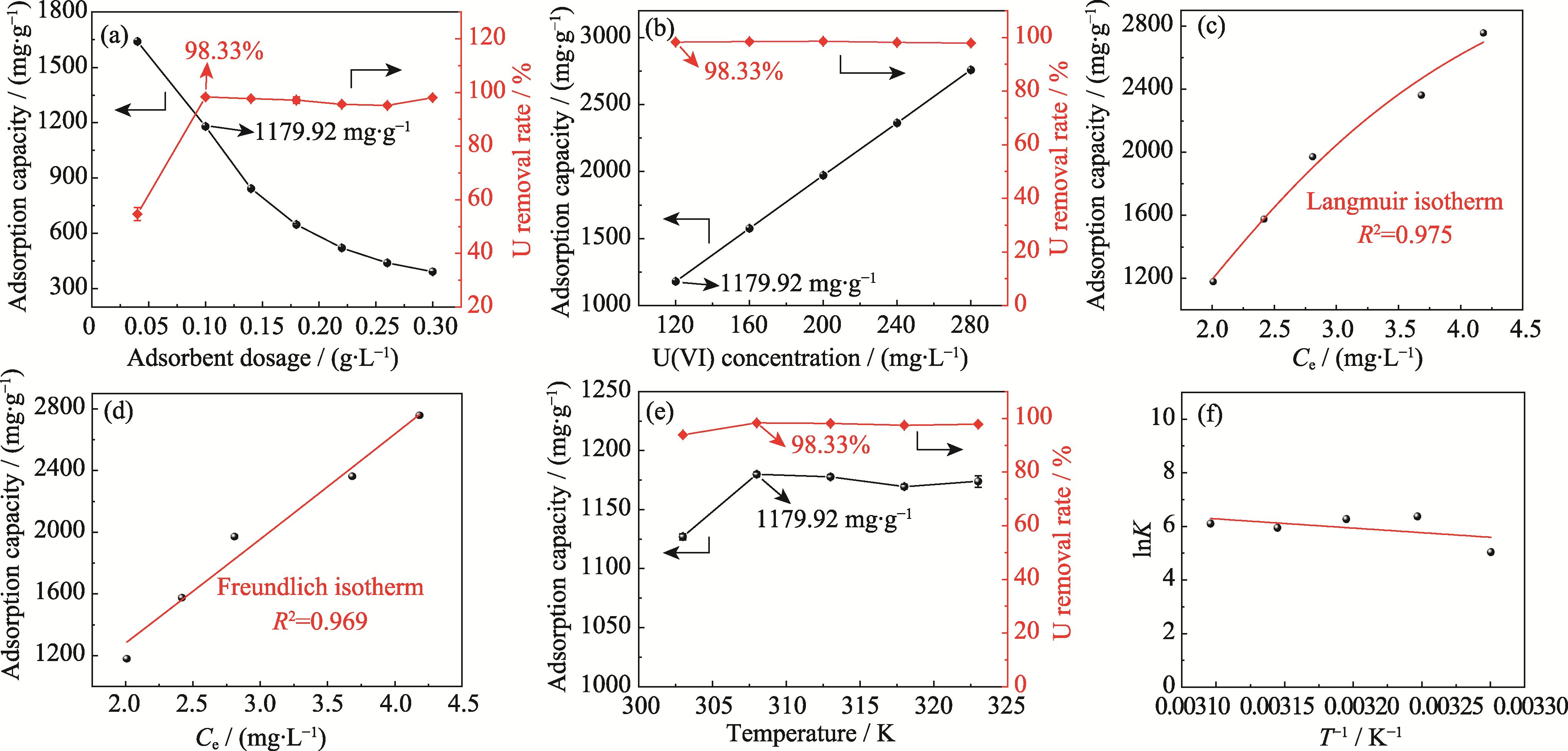
Fig. 3 Effects of adsorbent dosage, initial U(VI) mass concentration, and temperature on removal of U(VI) by Ca3(VO4)2 (a, b) Adsorption capacity and removal rate of U(VI) with different adsorbent dosages (a) and initial U(VI) mass concentrations (b);(c, d) Langmuir (c) and Freundlich (d) isothermal adsorption models fitting; (e) Adsorption capacity and removal rate of U(VI) at different temperatures; (f) Relationship between lnK and 1/T (temperature at 308 K, pH 6, adsorption time at 2 h)
| Material | Freundlich | Langmuir | ||
|---|---|---|---|---|
| kF/(mg·g-1)·(mg·L-1)-1/n | R2 | kL/(L·mg-1) | R2 | |
| Ca3(VO4)2 | 623.24 | 0.969 | 0.09 | 0.975 |
Table 4 Fitting parameters of Freundlich and Langmuir isothermal adsorption models
| Material | Freundlich | Langmuir | ||
|---|---|---|---|---|
| kF/(mg·g-1)·(mg·L-1)-1/n | R2 | kL/(L·mg-1) | R2 | |
| Ca3(VO4)2 | 623.24 | 0.969 | 0.09 | 0.975 |
| Material | ∆H/(kJ·mol-1) | ∆S/(J·mol-1·K-1) | ∆G/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Ca3(VO4)2 | 28.55 | 140.74 | -14.09 | -14.79 | -15.50 | -16.20 | -16.91 |
Table 5 Thermodynamic fitting parameters
| Material | ∆H/(kJ·mol-1) | ∆S/(J·mol-1·K-1) | ∆G/(kJ·mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Ca3(VO4)2 | 28.55 | 140.74 | -14.09 | -14.79 | -15.50 | -16.20 | -16.91 |
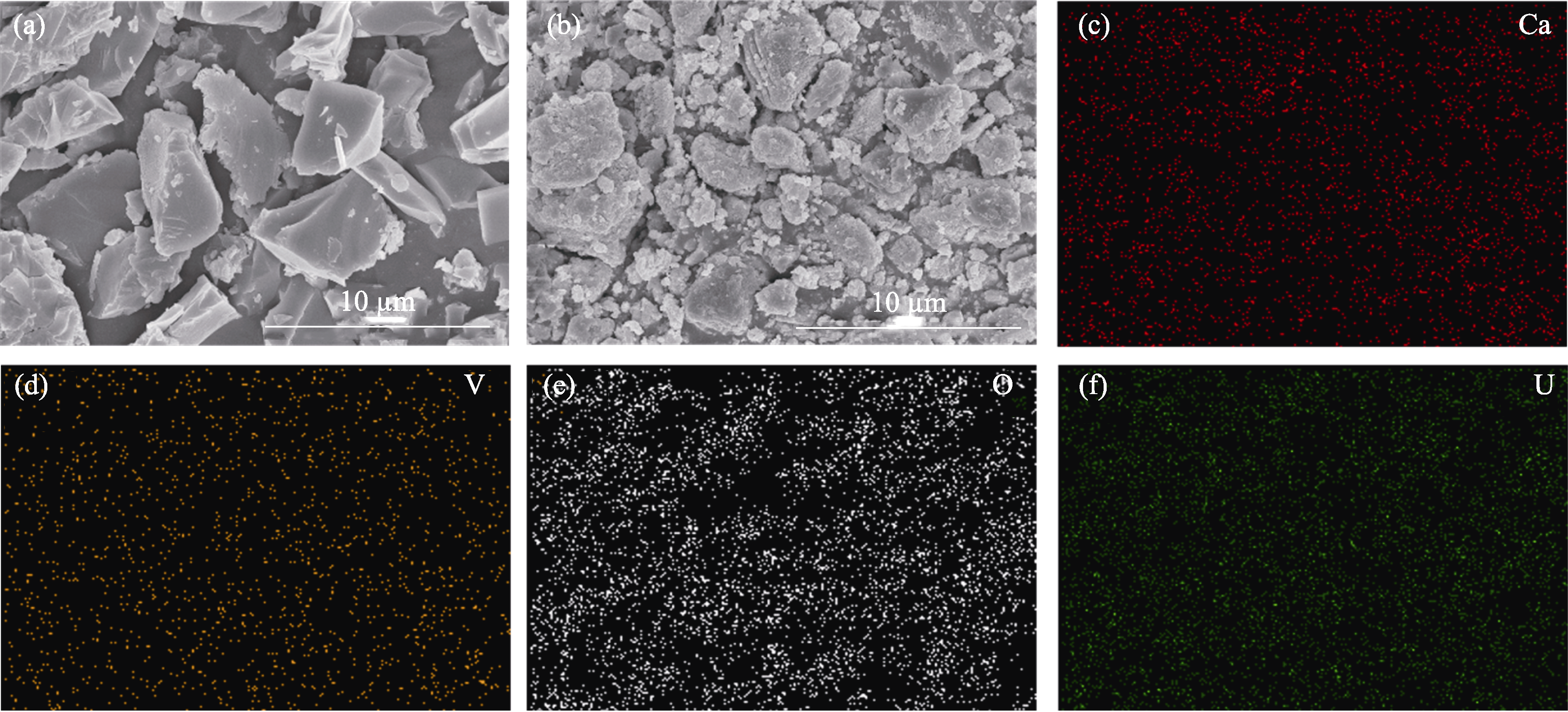
Fig. 4 SEM images and surface element distributions of Ca3(VO4)2 before and after adsorption of U(VI) (a, b) SEM images of Ca3(VO4)2 before (a) and after (b) adsorption of U(VI); (c-f) EDS mappings of Ca3(VO4)2 after adsorption of U(VI)
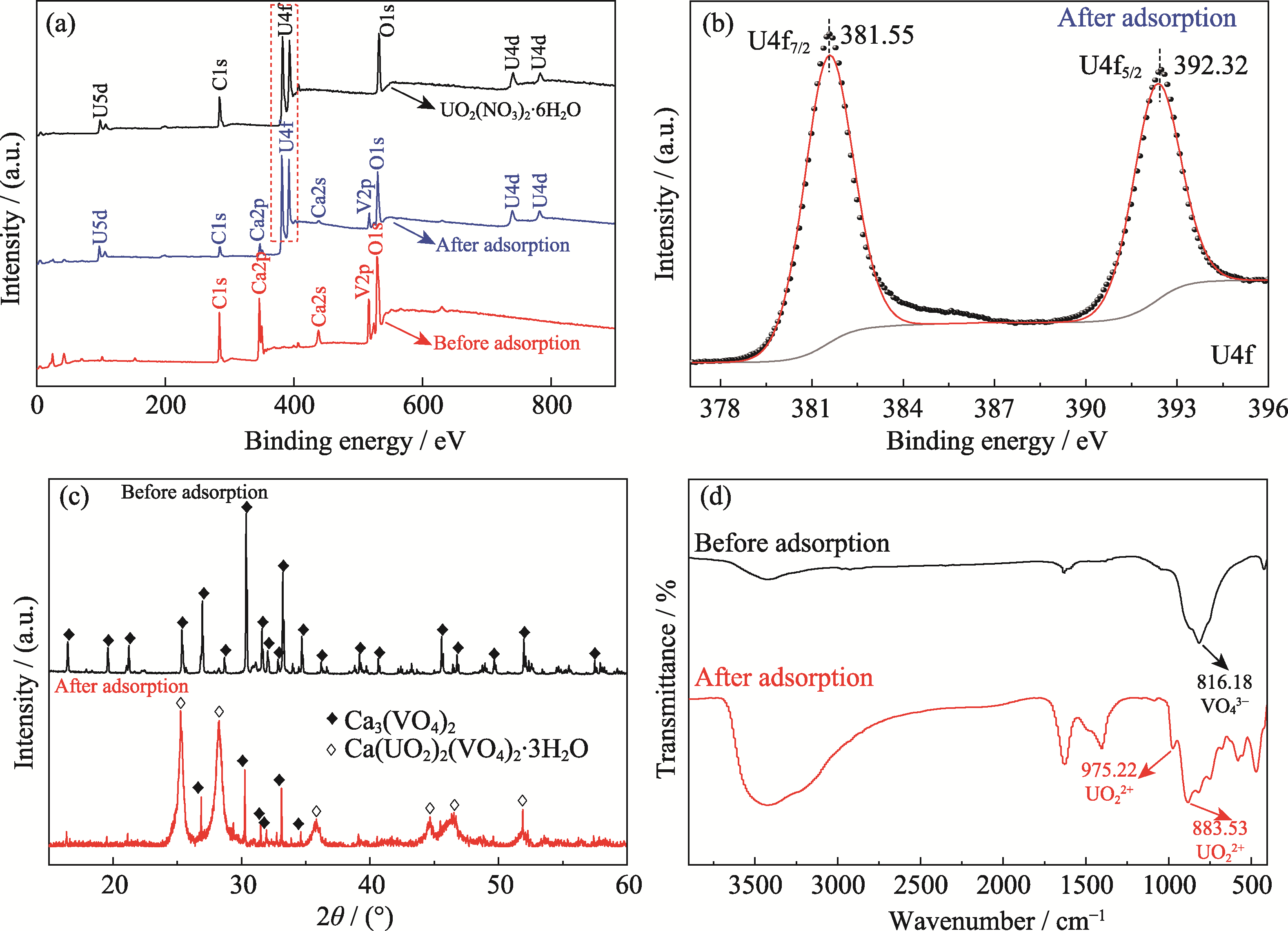
Fig. 5 Characterization results of Ca3(VO4)2 before and after adsorption of U(VI) (a) XPS spectra of Ca3(VO4)2 and UO2(NO3)2·6H2O; (b) U4f XPS spectrum of Ca3(VO4)2 after adsorption of U(VI); (c, d) XRD patterns (c) and FT-IR spectra (d) of Ca3(VO4)2
| Metal ion | ρ0/(mg·L-1) | Metal ion | ρ0/(mg·L-1) |
|---|---|---|---|
| Zn2+ | 588.55 | Co2+ | 63.66 |
| Cr3+ | 0.01 | Ba2+ | 60.51 |
| Cu2+ | 0.90 | U(VI) | 0.10 |
| Ni2+ | 126.60 |
Table 6 Mass concentrations of several metal ions in coexisting ionic solution after adsorption
| Metal ion | ρ0/(mg·L-1) | Metal ion | ρ0/(mg·L-1) |
|---|---|---|---|
| Zn2+ | 588.55 | Co2+ | 63.66 |
| Cr3+ | 0.01 | Ba2+ | 60.51 |
| Cu2+ | 0.90 | U(VI) | 0.10 |
| Ni2+ | 126.60 |
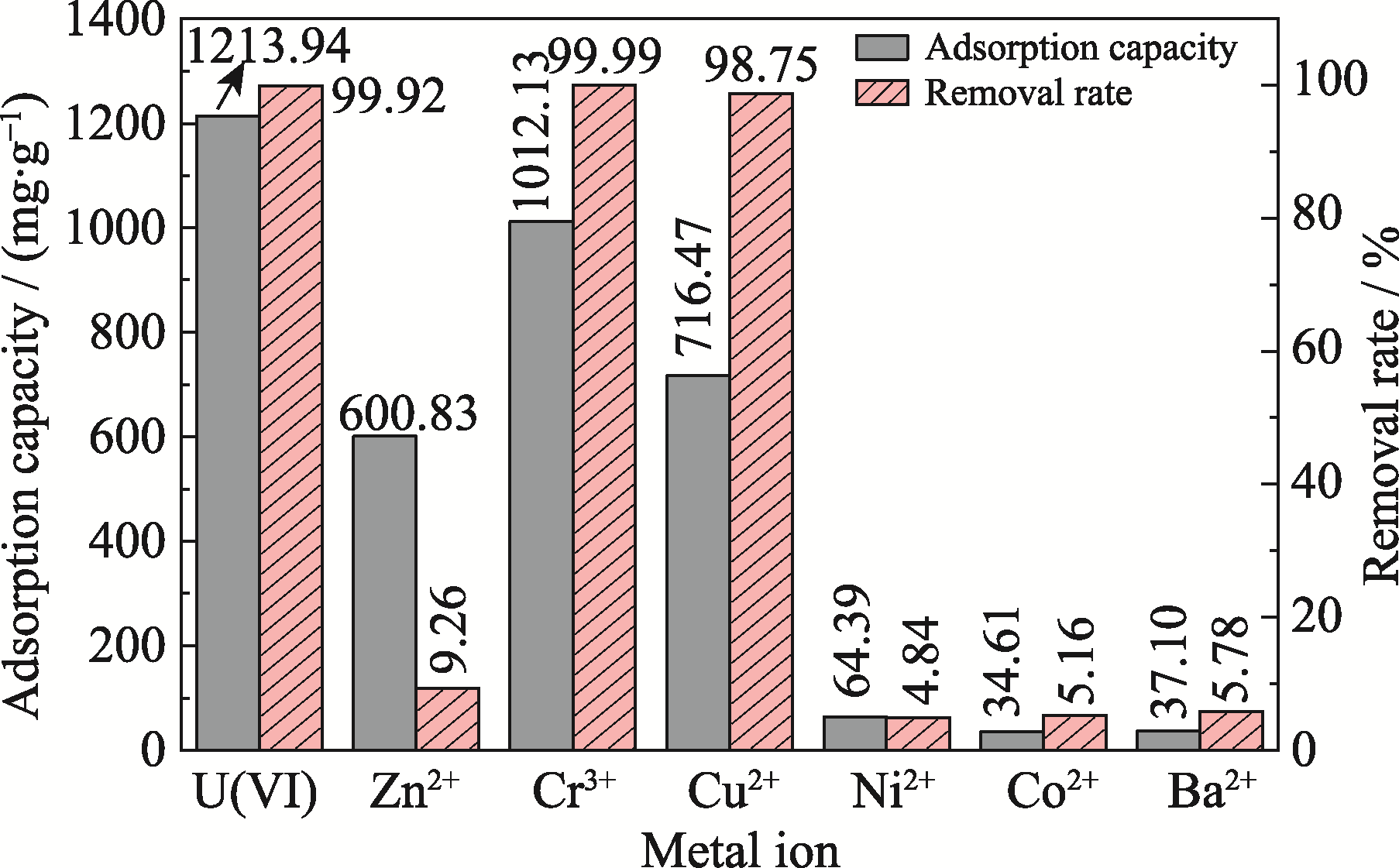
Fig. 6 Adsorption capacity and removal rate of Ca3(VO4)2 for several metal ions in coexisting ionic solution pH 6; Adsorption time: 2 h; Adsorbent dosage: 0.1 g·L-1; Temperature: 308 K
| Metal ion | Kd/(L·g-1) | k | Metal ion | Kd/(L·g-1) | k |
|---|---|---|---|---|---|
| U(VI) | 12039.43 | 1.00 | Ni2+ | 0.51 | 23673.64 |
| Zn2+ | 1.02 | 11793.32 | Co2+ | 0.54 | 22142.30 |
| Cr3+ | 168687.73 | 0.07 | Ba2+ | 0.61 | 19636.28 |
| Cu2+ | 791.97 | 15.20 |
Table 7 Selective adsorption parameters of Ca3(VO4)2 for several metal ions in coexisting ionic solution
| Metal ion | Kd/(L·g-1) | k | Metal ion | Kd/(L·g-1) | k |
|---|---|---|---|---|---|
| U(VI) | 12039.43 | 1.00 | Ni2+ | 0.51 | 23673.64 |
| Zn2+ | 1.02 | 11793.32 | Co2+ | 0.54 | 22142.30 |
| Cr3+ | 168687.73 | 0.07 | Ba2+ | 0.61 | 19636.28 |
| Cu2+ | 791.97 | 15.20 |
| Material | Adsorption capacity/(mg·g-1) | pH | Ref. |
|---|---|---|---|
| Mg2CO3(OH)2 | 370.00 | 5.0 | [ |
| Crab carapace | 1.38 | 8.0 | [ |
| PG/SFA | 84.60 | 6.0 | [ |
| HAP | 130.08 | 5.0 | [ |
| Kaolinite | 9.17 | 4.5 | [ |
| ZSM-12 zeolite | 12.00 | 3.0 | [ |
| H11Al2V6O23.2 | 917.00 | 5.0 | [ |
| EuVO4 | 276.16 | 4.5 | [ |
| Ca3(VO4)2 | 1179.92 | 6.0 | This study |
Table 8 Adsorption capacity of Ca3(VO4)2 and other adsorbents for uranium (VI) removal[11-12,15,33-37]
| Material | Adsorption capacity/(mg·g-1) | pH | Ref. |
|---|---|---|---|
| Mg2CO3(OH)2 | 370.00 | 5.0 | [ |
| Crab carapace | 1.38 | 8.0 | [ |
| PG/SFA | 84.60 | 6.0 | [ |
| HAP | 130.08 | 5.0 | [ |
| Kaolinite | 9.17 | 4.5 | [ |
| ZSM-12 zeolite | 12.00 | 3.0 | [ |
| H11Al2V6O23.2 | 917.00 | 5.0 | [ |
| EuVO4 | 276.16 | 4.5 | [ |
| Ca3(VO4)2 | 1179.92 | 6.0 | This study |
| [1] |
XIE Y H, YU L, CHEN L, et al. Recent progress of radionuclides separation by porous materials. Science China Chemistry, 2024, 67(11): 3515.
DOI |
| [2] |
ZHANG X Y, YAN M J, CHEN P, et al. Emerging MOFs, COFs, and their derivatives for energy and environmental applications. The Innovation, 2025, 6(2): 100778.
DOI URL |
| [3] | 谢永波, 曾涛涛, 王国华, 等. 铀矿山生态环境修复. 北京: 科学出版社, 2021. |
| [4] |
KHARE D, ACHARYA C. Uranium biomineralization by immobilized Chryseobacterium sp. strain PMSZPI cells for efficient uranium removal. Journal of Hazardous Materials, 2024, 465: 133503.
DOI URL |
| [5] |
MEZA I, HUA H, GAGNON K, et al. Removal of aqueous uranyl and arsenate mixtures after reaction with limestone, PO43-, and Ca2+. Environmental Science & Technology, 2023, 57(49): 20881.
DOI URL |
| [6] |
YU Y, LIU J Y, CHEN S S, et al. Bioinspired electrostatic layer-by-layer assembly membranes constructed based on mild strategy for uranium extraction from seawater. Chemical Engineering Journal, 2024, 486: 149783.
DOI URL |
| [7] |
LI X, LIU Z R, HUANG M. Purification of uranium-containing wastewater by adsorption: a review of research on resin materials. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(7): 3043.
DOI |
| [8] |
YU C X, JIANG W, LEI M, et al. Fabrication of carboxylate- functionalized 2D MOF nanosheet with caged cavity for efficient and selective extraction of uranium from aqueous solution. Small, 2024, 20(23): 2308910.
DOI URL |
| [9] |
ZHONG X, TAN Y B, WU S Y, et al. Efficient and rapid capture of uranium(VI) in wastewater via multi-amine modified β-cyclodextrin porous polymer. Chinese Journal of Chemical Engineering, 2024, 68: 144.
DOI URL |
| [10] | 王浩, 陈枫, 柯倩, 等. 氮化硼负载磷钨酸铁对U(VI)的吸附及其机理研究. 中国科学: 化学, 2019, 49(1): 123. |
| [11] |
FENG S W, FENG L J, WANG M, et al. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chemical Engineering Journal, 2022, 430: 133038.
DOI URL |
| [12] | 夏雪, 周磊, 杨国辉, 等. 磷石膏/固硫灰渣复合材料对铀(VI)的吸附行为及机理研究. 金属矿山, 2023(11): 81. |
| [13] |
WU T N, WANG M B, ZHONG T T, et al. Behavior of uranium immobilization with hydroxyapatite and dissolution stability of the immobilization product. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(3): 647.
DOI |
| [14] |
DAN H, CHEN L Y, XIAN Q, et al. Tailored synthesis of SBA-15 rods using different types of acids and its application in adsorption of uranium. Separation and Purification Technology, 2019, 210: 491.
DOI URL |
| [15] |
LIN Y L, LIU Y H, ZHANG S, et al. Electrochemical synthesis of EuVO4 for the adsorption of U(VI): performance and mechanism. Chemosphere, 2021, 273: 128569.
DOI URL |
| [16] | LAUF R J. Mineralogy of uranium and thorium. Atglen: Schiffer Publishing, 2016. |
| [17] |
BANERJEE C, DUDWADKAR N, TRIPATHI S C, et al. Nano-cerium vanadate: a novel inorganic ion exchanger for removal of americium and uranium from simulated aqueous nuclear waste. Journal of Hazardous Materials, 2014, 280: 63.
DOI PMID |
| [18] |
KARYAKIN N V, CHERNORUKOV N G, SULEIMANOV E V, et al. Thermodynamics of alkali metal uranovanadates. Russian Journal of General Chemistry, 2001, 71(9): 1333.
DOI |
| [19] |
KARYAKIN N V, CHERNORUKOV N G, SULEIMANOV E V, et al. Chemical thermodynamics of alkaline-earth metal uranovanadates. Radiochemistry, 2003, 45(5): 457.
DOI |
| [20] |
杜浪, 李玉香, 马雪, 等. 偶氮胂Ⅲ分光光度法测定微量铀. 冶金分析, 2015, 35(1): 68.
DOI |
| [21] | 门倩, 唐振平, 刘江, 等. 湘南某铀矿山周边水体放射性金属及重金属污染特征. 湖北农业科学, 2019, 58(15): 39. |
| [22] |
YUE H R, XUE X X. Evolution of generated calcium vanadates at different locations in the vicinity of the V2O5/CaO interface with annealing parameters. Metallurgical and Materials Transactions B, 2020, 51(5): 2358.
DOI |
| [23] |
PARHI P, MANIVANNAN V, KOHLI S, et al. Synthesis and characterization of M3V2O8 (M=Ca, Sr and Ba) by a solid-state metathesis approach. Bulletin of Materials Science, 2008, 31(6): 885.
DOI URL |
| [24] |
CHEN J L, YANG X, WANG L Y, et al. Graphene oxide wrapped Cu-MOF as an efficient adsorbent for uranium extraction from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 2024, 333(1): 263.
DOI |
| [25] |
LIAO J, HE X S, ZHANG Y, et al. The construction of magnetic hydroxyapatite-functionalized pig manure-derived biochar for the efficient uranium separation. Chemical Engineering Journal, 2023, 457: 141367.
DOI URL |
| [26] |
LIU Y F, NI S, WANG W J, et al. Facile and scalable synthesis of functionalized hierarchical porous polymers for efficient uranium adsorption. Water Research, 2024, 257: 121683.
DOI URL |
| [27] |
GUO X, FENG Y R, MA L, et al. Phosphoryl functionalized mesoporous silica for uranium adsorption. Applied Surface Science, 2017, 402: 53.
DOI URL |
| [28] |
PENG T Q, WANG Y F, XU Y F, et al. Synthesis, characterization and uranium (VI) adsorption mechanism of novel adsorption material poly(tetraethylenepentamine-trimesoyl chloride). Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(2): 409.
DOI |
| [29] |
WANG Q, WANG Y, WU Y Q, et al. Salicylhydroxamic acid intercalated layered double hydroxide for efficient uranium uptake from seawater. Journal of Environmental Chemical Engineering, 2025, 13(1): 115055.
DOI URL |
| [30] |
CHERNORUKOV N G, NIPRUK O V, KNYAZEV A V, et al. Uranyl orthovanadate of composition (UO2)3(VO4)2·4H2O: synthesis and characterization. Russian Journal of Inorganic Chemistry, 2013, 58(5): 506.
DOI URL |
| [31] | MOLLICK S, SAURABH S, MORE Y D, et al. Benchmark uranium extraction from seawater using an ionic macroporous metal-organic framework. Energy & Environmental Science, 2022, 15(8): 3462. |
| [32] |
赵凯鑫, 高琼, 田强, 等. 磷酸三钙去除U(VI)的性能与机理研究. 核化学与放射化学, 2023, 45(4): 364.
DOI |
| [33] |
ZHANG L, JING X Y, LI R M, et al. Magnesium carbonate basic coating on cotton cloth as a novel adsorbent for the removal of uranium. RSC Advances, 2015, 5(30): 23144.
DOI URL |
| [34] |
LI L X, ZHOU Z K, WANG G H, et al. Adsorption performance and its mechanism of uranium using rod-like hydroxyapatite by one- step hydrothermal method. Physica Scripta, 2024, 99(8): 085944.
DOI |
| [35] |
ISSA R A M, AMARI A O E, ALHANASH H B, et al. Removal of uranium (VI) ion from aqueous solution using kaolinite. Kuwait Journal of Science, 2023, 50(4): 609.
DOI URL |
| [36] |
KHEMAISSIA S, ABAIDIA R, HOUHOUNE F, et al. Synthesis and characterization of ZSM-12 zeolite for uranium(VI) adsorption: isotherm, kinetic, and thermodynamic investigations. Comptes Rendus Chimie, 2025, 28: 79.
DOI URL |
| [37] |
LUO J Q, CHEN J L, CHEN J, et al. Aluminum vanadate microspheres is a simple but effective material for uranium extraction: performance and mechanism. Journal of Solid State Chemistry, 2022, 312: 123237.
DOI URL |
| [1] | CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion [J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282. |
| [2] | WU Rui, ZHANG Minhui, JIN Chenyun, LIN Jian, WANG Deping. Photothermal Core-Shell TiN@Borosilicate Bioglass Nanoparticles: Degradation and Mineralization [J]. Journal of Inorganic Materials, 2023, 38(6): 708-716. |
| [3] | MA Lei, HUANG Yi, DENG Hao, YIN Hang, TIAN Qiang, YAN Minghao. Removal of Uranium (VI) from Acidic Aqueous Solution by Fluorapatite [J]. Journal of Inorganic Materials, 2022, 37(4): 395-403. |
| [4] | ZHU Zimin, ZHANG Minhui, ZHANG Xuanyu, YAO Aihua, LIN Jian, WANG Deping. In Vitro Mineralization Property of Borosilicate Bioactive Glass under DC Electric Field [J]. Journal of Inorganic Materials, 2021, 36(9): 1006-1012. |
| [5] | YU Xiangkun, LIU Kun, LI Zhipeng, ZHAO Yulu, SHEN Jinyou, MAO Ping, SUN Aiwu, JIANG Jinlong. Efficient Adsorption of Radioactive Iodide by Copper/Palygorskite Composite [J]. Journal of Inorganic Materials, 2021, 36(8): 856-864. |
| [6] | XU Hongyi, ZHAI Dong, CAO Wanting, CHEN Zhenhua, QIAN Wenhao, CHEN Lei. Mineralization Activity of Li2Ca2Si2O7 Bioceramics [J]. Journal of Inorganic Materials, 2021, 36(7): 753-760. |
| [7] | JIANG Li, GAO Huihui, CAO Ruya, ZHANG Shouwei, LI Jiaxing. Construction of Novel Three Dimensionally Macroporous g-C3N4 for Efficient Adsorption/Photocatalytic Reduction of U(VI) [J]. Journal of Inorganic Materials, 2020, 35(3): 359-366. |
| [8] | PANG Hongwei, TANG Hao, WANG Jiaqi, WANG Xiangxue, YU Shujun. Ternary Layered Double Hydroxide Supported Sulfide NZVI: Efficient U(VI) Elimination and Mechanism [J]. Journal of Inorganic Materials, 2020, 35(3): 381-389. |
| [9] | ZHANG Zhibin, ZHOU Runze, DONG Zhimin, CAO Xiaohong, LIU Yunhai. Adsorption of U(VI)-CO3/Ca-U(VI)-CO3 by Amidoxime-functionalized Hydrothermal Carbon [J]. Journal of Inorganic Materials, 2020, 35(3): 352-358. |
| [10] | LIU Ji-Tao, CHUAN Ding-Ze, YANG Ze-Bin, CHEN Xi-Liang, YAN Ting-Ting, CHEN Qing-Hua. In Vitro Remineralization of Acid-etched Bovine Enamel with Amino Acids/Hydroxyapatite Composite [J]. Journal of Inorganic Materials, 2019, 34(11): 1222-1230. |
| [11] | DONG Zhi-Hong, NIE Zhi-Ping, ZHOU Chang-Chun. Bionic Remineralization of Acidic Etched Enamel Induced by Using Mesoporous Bioactive Glass in Natural Oral Saliva [J]. Journal of Inorganic Materials, 2016, 31(1): 88-94. |
| [12] | XU Jin-Mei, LIU Xin-Ling, GAO Yan-Feng. Effect of Dopamine on Hydroxyapatite Deposition for Dental Restoration [J]. Journal of Inorganic Materials, 2016, 31(1): 95-99. |
| [13] | MA Yu-Fei, QIAO Li, FENG Qing-Ling. Research Progress on Biomineralization Mechanism of Freshwater Pearl [J]. Journal of Inorganic Materials, 2013, 28(1): 109-116. |
| [14] | ZHU Yun-Rong, CHEN Yu-Yun, XU Guo-Hua, YE Xiao-Jian, ZHONG Jian, HE Dan-Nong. Effect of Silk Fibroin Content on the Bionic Mineralization and In Vitro Cellular Compatibility of Silk Fibroin/Hydroxyapaptite Nanocomposites [J]. Journal of Inorganic Materials, 2012, 27(8): 883-886. |
| [15] | LI Zhi-Hong, WU Ji-Min, HUANG Shu-Jie, GUAN Jing, ZHANGXi-Zheng. Effect of Pretreatment on Fabrication of Natural FibroinFiber/Apatite Composites Using Alternate Soaking Method [J]. Journal of Inorganic Materials, 2011, 26(1): 43-48. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||