
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (11): 1275-1282.DOI: 10.15541/jim20240075
Special Issue: 【生物材料】骨骼与齿类组织修复(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
CAI Hao( ), WANG Qihang(
), WANG Qihang( ), ZOU Zhaoyong(
), ZOU Zhaoyong( )
)
Received:2024-02-20
Revised:2024-03-30
Published:2024-11-20
Online:2024-05-31
Contact:
WANG Qihang, lecturer. E-mail: qhwang@whut.edu.cn;About author:CAI Hao (1999-), male, Master candidate. E-mail: c17596125882@163.com
Supported by:CLC Number:
CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion[J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282.
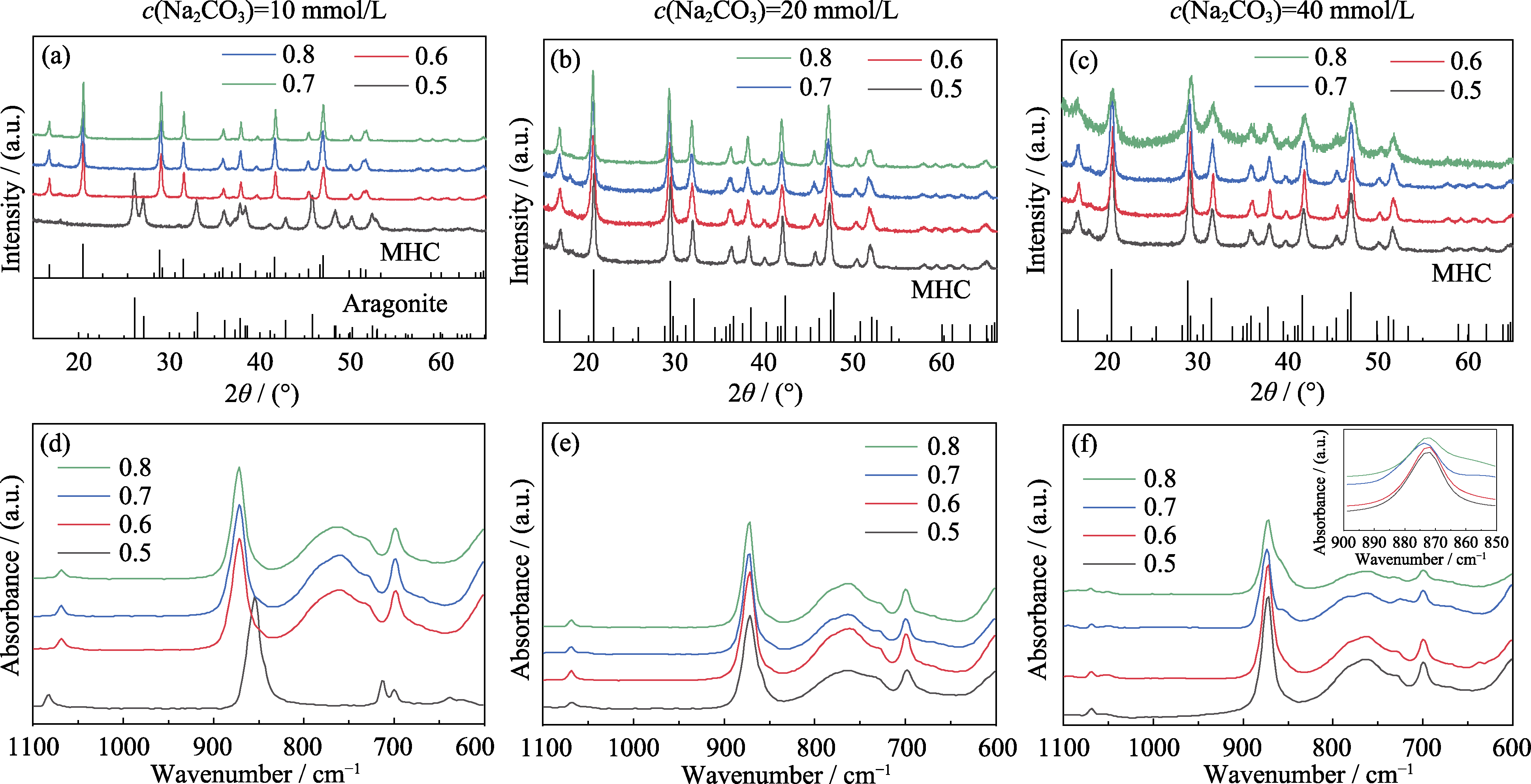
Fig. 1 (a-c) XRD patterns and (d-f) FT-IR spectra of crystallized products prepared at different carbonate concentrations of (a, d) 10, (b, e) 20, (c, f) 40 mmol/L and different ratios of Mg/(Mg+Ca) Colorful figures are available on website

Fig. 2 SEM images of crystallized products at carbonate concentration of 10 mmol/L and different ratios of Mg/(Mg+Ca) (a, b) 0.5; (c, d) 0.6; (e, f) 0.7; (g, h) 0.8
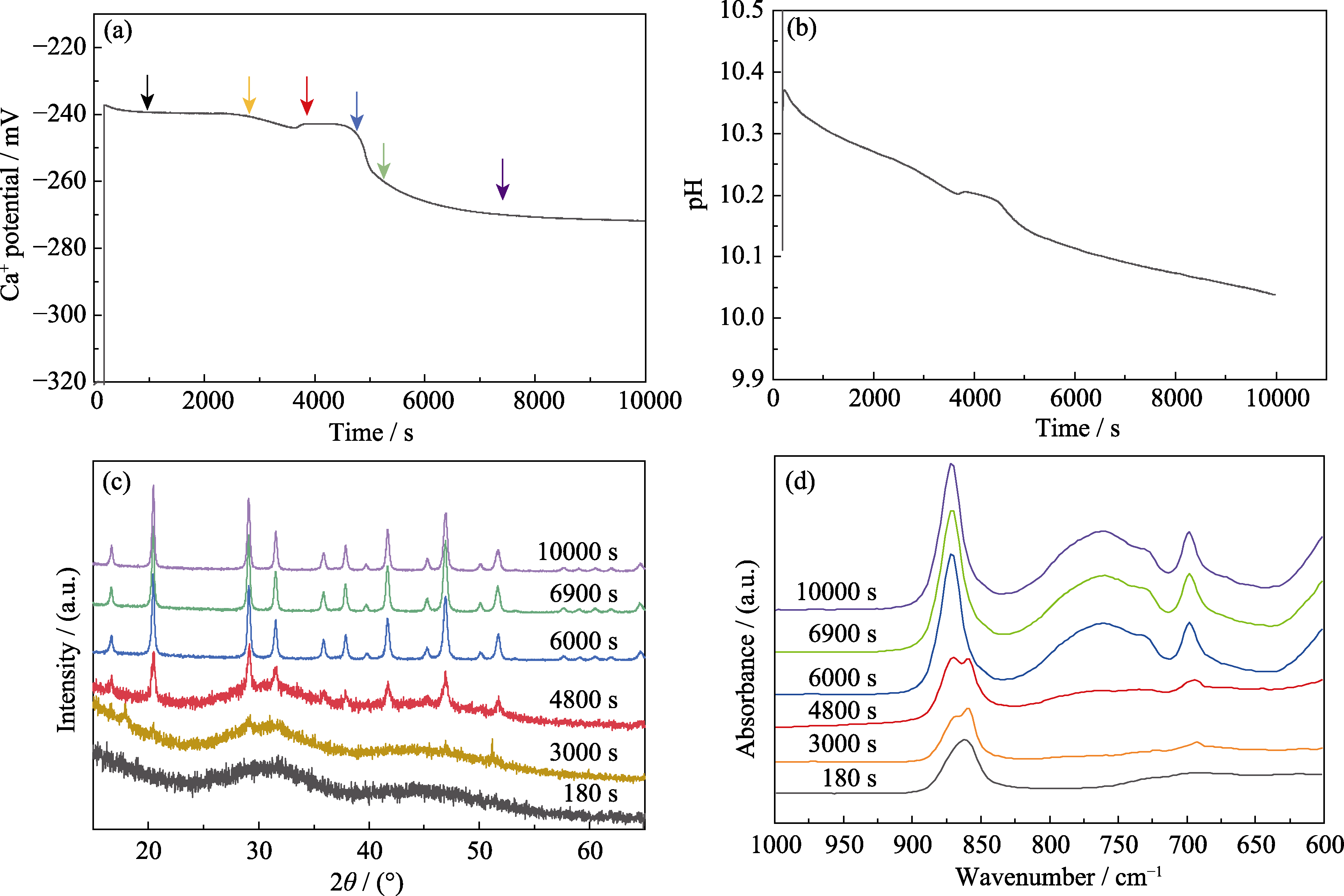
Fig. 3 In-situ monitoring of solution changes during crystallization of Mg-ACC and structural characterization of products at different time points (a) Ca2+ activity; (b) pH evolution; (c) XRD patterns and (d) FT-IR spectra of the crystallized products at different crystallization stages. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
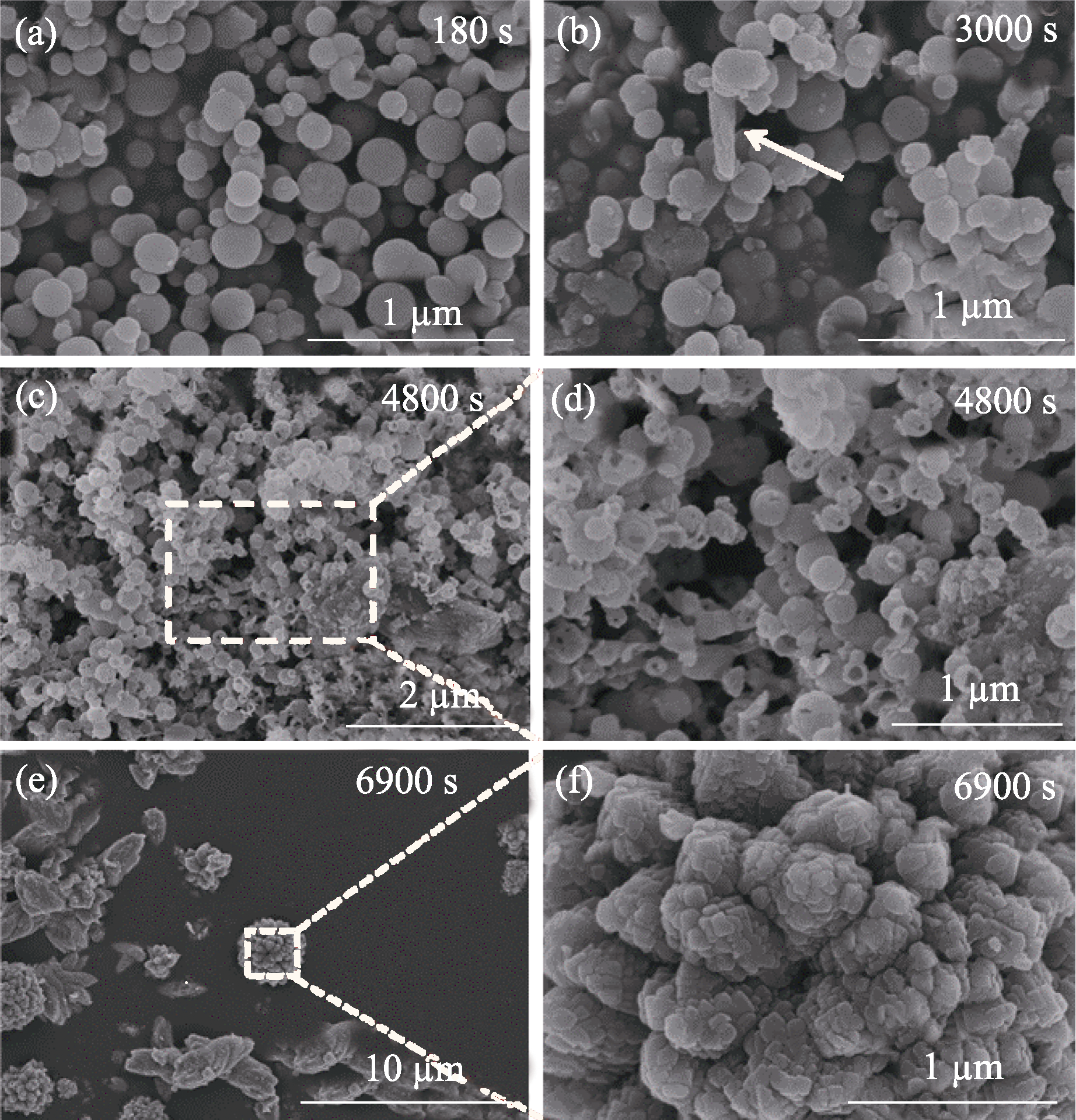
Fig. 4 SEM images of the products at different stages during ACC crystallization (a) 180 s; (b) 3000 s; (c, d) 4800 s; (e, f) 6900 s. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6

Fig. 5 TEM images of the products at different stages during ACC crystallization (a) 180 s; (b) 3000 s; (c, d) 4800 s; (e, f) 6900 s. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
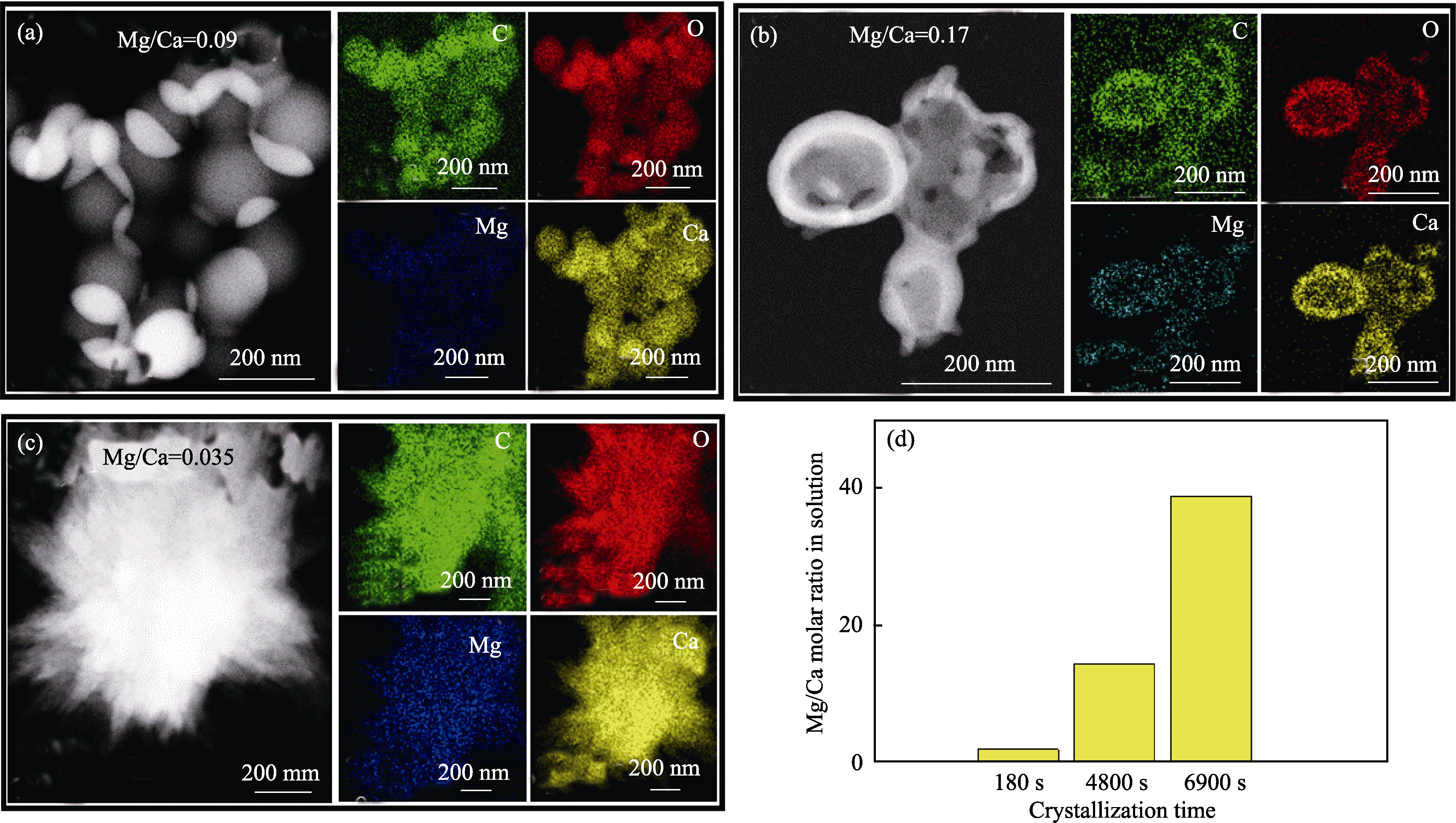
Fig. 6 Mg/Ca molar ratios of the products and solutions at different stages during ACC crystallization (a-c) HAADF images and their corresponding EDS mappings of products during crystallization for |(a) 180, (b) 4800 and (c) 6900 s; (d) Mg/Ca molar ratio in solution after different crystallization time. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
| [1] | FERMANI S, DŽAKULA B N, REGGI M, et al. Effects of magnesium and temperature control on aragonite crystal aggregation and morphology. CrystEngComm, 2017, 19(18): 2451. |
| [2] | RODRIGUEZ-BLANCO J D, SHAW S, BOTS P, et al. The role of Mg in the crystallization of monohydrocalcite. Geochimica et Cosmochimica Acta, 2014, 127: 204. |
| [3] | WANG C Y, XU Y, LIU Y L, et al. Synthesis and characterization of lamellar aragonite with hydrophobic property. Materials Science & Engineering: C, 2009, 29(3): 843. |
| [4] |
GOWER L B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chemical Reviews, 2008, 108(11): 4551.
DOI PMID |
| [5] | WANG Q, YUAN B, HUANG W, et al. Bioprocess inspired formation of calcite mesocrystals by cation-mediated particle attachment mechanism. National Science Review, 2023, 10(4): nwad014. |
| [6] | SU J T, ZHU F J, ZHANG G Y, et al. Transformation of amorphous calcium carbonate nanoparticles into aragonite controlled by ACCBP. CrystEngComm, 2016, 18(12): 2125. |
| [7] | KARTHIKA S, RADHAKRISHNAN T K, KALAICHELVI P. A review of classical and nonclassical nucleation theories. Crystal Growth & Design, 2016, 16(11): 6663. |
| [8] | WANG Q, HU L, WANG X, et al. Expanding from materials to biology inspired by biomineralization. Interdisciplinary Materials, 2024, 3(2): 165. |
| [9] |
ZOU Z, HABRAKEN W J E M, MATVEEVA G, et al. A hydrated crystalline calcium carbonate phase: calcium carbonate hemihydrate. Science, 2019, 363(6425): 396.
DOI PMID |
| [10] | 解晶晶, 邹朝勇, 傅正义. 无定形碳酸钙的稳定性和结晶转化过程研究进展. 中国材料进展, 2020(4): 261. |
| [11] | LU Z, RICKABY R E M, KENNEDY H, et al. An ikaite record of late holocene climate at the antarctic peninsula. Earth and Planetary Science Letters, 2012, 325: 108. |
| [12] | POLITI Y, ARAD T, KLEIN E, et al. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science, 2004, 306(5699): 1161. |
| [13] |
POLITI Y, METZLER R A, ABRECHT M, et al. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(45): 17362.
DOI PMID |
| [14] |
AIZENBERG J, LAMBERT G, WEINER S, et al. Factors involved in the formation of amorphous and crystalline calcium carbonate: a study of an ascidian skeleton. Journal of the American Chemical Society, 2002, 124(1): 32.
PMID |
| [15] | KHAIROUN I, MAGNE D, GAUTHIER O, et al. In vitro characterization and in vivo properties of a carbonated apatite bone cement. Journal of Biomedical Materials Research, 2002, 60(4): 633. |
| [16] | HABRAKEN W J E M, MASIC A, BERTINETTI L, et al. Layered growth of crayfish gastrolith: about the stability of amorphous calcium carbonate and role of additives. Journal of Structural Biology, 2014, 189(1): 28. |
| [17] | KRAUSS F, SCHRIEVER W. Die hydrate des calcium carbonats. Zeitschrift Für Anorganische Und Allgemeine Chemie, 2004, 188(1): 259. |
| [18] | DAHL K, BUCHARDT B. Monohydrocalcite in the arctic Ikka Fjord, SW Greenland: first reported marine occurrence. Journal of Sedimentary Research, 2006, 76(3): 460. |
| [19] | SWAINSON I P. The structure of monohydrocalcite and the phase composition of the beachrock deposits of Lake Butler and Lake Fellmongery, South Australia. American Mineralogist, 2008, 93(7): 1014. |
| [20] | YAMAMOTO G, ATSUSHI K, SATORU O. Structural variations of amorphous magnesium carbonate during nucleation, crystallization, and decomposition of nesquehonite MgCO3·3H2O. Physics and Chemistry of Minerals, 2022, 50(5): 305. |
| [21] | SON S, LI W Q, LEE J Y, et al. On the coordination of Mg2+ in aragonite: ab-initio absorption spectroscopy and isotope fractionation study. Geochimica et Cosmochimica Acta, 2020, 286: 324. |
| [22] | PURGSTALLER B, KONRAD F, DIETZEL M, et al. Control of Mg2+/Ca2+ activity ratio on the formation of crystalline carbonate minerals via an amorphous precursor. Crystal Growth & Design, 2017, 17(3): 1069. |
| [23] | MATSUMOTO M, FUKUNAGA T, ONOE K. Polymorph control of calcium carbonate by reactive crystallization using microbubble technique. Chemical Engineering Research and Design, 2010, 88(12): 1624. |
| [24] | TADIER S, ROKIDI S, REY C, et al. Crystal growth of aragonite in the presence of phosphate. Journal of Crystal Growth, 2017, 458: 44. |
| [25] | WILLINGER M G, POLLEUX J, ANTONIETTI M, et al. Structural evolution of aragonite superstructures obtained in the presence of the siderophore deferoxamine. CrystEngComm, 2015, 17(21): 3927. |
| [26] | ZHU F J, NISHIMURA T, SAKAMOTO T, et al. Tuning the stability of CaCO3 crystals with magnesium ions for the formation of aragonite thin films on organic polymer templates. Chemistry-An Asian Journal, 2013, 8(12): 3002. |
| [27] | SUZUKI M, KOGURE T, WEINER S, et al. Formation of aragonite crystals in the crossed lamellar microstructure of limpet shells. Crystal Growth & Design, 2011, 11(11): 4850. |
| [28] | PARK W K, KO S J, LEE S W, et al. Effects of magnesium chloride and organic additives on the synthesis of aragonite precipitated calcium carbonate. Journal of Crystal Growth, 2008, 310(10): 2593. |
| [29] | HEYWOOD B R, MANN S. Molecular construction of oriented inorganic materials: controlled nucleation of calcite and aragonite under compressed langmuir monolayers. Chemistry of Materials, 1994, 6(3): 311. |
| [30] | LEVI-KALISMAN Y, RAZ S, WEINER S, et al. X-ray absorption spectroscopy studies on the structure of a biogenic “amorphous” calcium carbonate phase. Journal of the Chemical Society, Dalton Transactions, 2000(21): 3977. |
| [31] | LAM R S K, CHARNOCK J M, LENNIE A, et al. Synthesis-dependant structural variations in amorphous calcium carbonate. CrystEngComm, 2007, 9(12): 1226. |
| [32] | SUN W, JAYARAMAN S, CHEN W, et al. Nucleation of metastable aragonite CaCO3 in seawater. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(11): 3199. |
| [33] | HUANG Z, ZHANG G. Biomimetic synthesis of aragonite nanorod aggregates with unusual morphologies using a novel template of natural fibrous proteins at ambient condition. Crystal Growth & Design, 2012, 12(4): 1816. |
| [34] | MUNEMOTO T, FUKUSHI K. Transformation kinetics of monohydrocalcite to aragonite in aqueous solutions. Journal of Mineralogical and Petrological Sciences, 2008, 103(5): 345. |
| [35] | NEUMANN M, EPPLE M. Monohydrocalcite and its relationship to hydrated amorphous calcium carbonate in biominerals. European Journal of Inorganic Chemistry, 2007, 2007(14): 1953. |
| [36] | CHEN T, NEVILLE A, YUAN M. Assessing the effect of Mg2+ on CaCO3 scale formation-bulk precipitation and surface deposition. Journal of Crystal Growth, 2005, 275(1): e1341. |
| [37] | BOTS P, BENNING L G, RODRIGUEZ-BLANCO J D, et al. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Crystal Growth & Design, 2012, 12(7): 3806. |
| [38] | ANDERSEN F A, BREČEVIĆ L J C. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chemica Scandinavica, 1991, 45: 1018. |
| [39] | COLEYSHAW E E, CRUMP G, GRIFFITH W P. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2003, 59(10): 2231. |
| [40] | LOSTE E, WILSON R M, SESHADRI R, et al. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. Journal of Crystal Growth, 2003, 254(1): 206. |
| [41] | JU Y M, HUANG F, DING X, et al. Phase transformation-induced Mg isotope fractionation in Mg-mediated CaCO3 mineralization. Nano Research, 2023, 16(2): 3597. |
| [42] | POLITI Y, BATCHELOR D R, ZASLANSKY P, et al. Role of magnesium ion in the stabilization of biogenic amorphous calcium carbonate: a structure-function investigation. Chemistry of Materials, 2010, 22(1): 161. |
| [43] | RODRIGUEZ-BLANCO J D, SHAW S, BOTS P, et al. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. Journal of Alloys and Compounds, 2012, 536: S477. |
| [44] | YAGI S, FUKUSHI K. Phosphate sorption on monohydrocalcite. Journal of Mineralogical and Petrological Sciences, 2011, 106(2): 109. |
| [45] |
DI TOMMASO D, DE LEEUW N H. Structure and dynamics of the hydrated magnesium ion and of the solvated magnesium carbonates: insights from first principles simulations. Physical Chemistry Chemical Physics, 2010, 12(4): 894.
DOI PMID |
| [46] | MOOMAW A S, MAGUIRE M E. The unique nature of Mg2+channels. Physiology, 2008, 23: 275. |
| [47] | ZOU Z, XIE J, MACÍAS-SÁNCHEZ E, et al. Nonclassical crystallization of amorphous calcium carbonate in the presence of phosphate ions. Crystal Growth Design, 2021, 21(1): 414. |
| [48] | ZOU Z, BERTINETTI L, POLITI Y, et al. Control of polymorph selection in amorphous calcium carbonate crystallization by poly (aspartic acid): two different mechanisms. Small, 2017, 13(21): 1603100. |
| [49] | HUANG W, WANG Q, CHI W, et al. Multiple crystallization pathways of amorphous calcium carbonate in the presence of poly (aspartic acid) with a chain length of 30. CrystEngComm, 2022, 24(26): 4809. |
| [1] | LI Xianke, ZHANG Chaoyi, HUANG Lin, SUN Peng, LIU Bo, XU Jun, TANG Huili. High-quality Indium-doped Gallium Oxide Single Crystal Growth by Floating Zone Method [J]. Journal of Inorganic Materials, 2024, 39(12): 1384-1390. |
| [2] | HAO Yongxin, QIN Juan, SUN Jun, YANG Jinfeng, LI Qinglian, HUANG Guijun, XU Jingjun. Impact of Crucible Bottom Shape on the Growth of Congruent Lithium Niobate Crystals by Czochralski Method [J]. Journal of Inorganic Materials, 2024, 39(10): 1167-1174. |
| [3] | QIN Juan, LIANG Dandan, SUN Jun, YANG Jinfeng, HAO Yongxin, LI Qinglian, ZHANG Ling, XU Jingjun. Flat Shoulder Congruent Lithium Niobate Crystals Grown by the Czochralski Method [J]. Journal of Inorganic Materials, 2023, 38(8): 978-986. |
| [4] | LIN Siqi, LI Airan, FU Chenguang, LI Rongbing, JIN Min. Crystal Growth and Thermoelectric Properties of Zintl Phase Mg3X2 (X=Sb, Bi) Based Materials: a Review [J]. Journal of Inorganic Materials, 2023, 38(3): 270-279. |
| [5] | YANG Jiaxue, LI Wen, WANG Yan, ZHU Zhaojie, YOU Zhenyu, LI Jianfu, TU Chaoyang. Spectroscopic and Yellow Laser Features of Dy3+: Y3Al5O12 Single Crystals [J]. Journal of Inorganic Materials, 2023, 38(3): 350-356. |
| [6] | WU Zhen, LI Huifang, ZHANG Zhonghan, ZHANG Zhen, LI Yang, LAN Jianghe, SU Liangbi, WU Anhua. Growth and Characterization of CeF3 Crystals for Magneto-optical Application [J]. Journal of Inorganic Materials, 2023, 38(3): 296-302. |
| [7] | QI Xuejun, ZHANG Jian, CHEN Lei, WANG Shaohan, LI Xiang, DU Yong, CHEN Junfeng. Macroscopic Defects of Large Bi12GeO20 Crystals Grown Using Vertical Bridgman Method [J]. Journal of Inorganic Materials, 2023, 38(3): 280-287. |
| [8] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [9] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| [10] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| [11] | WANG Haidong, WANG Yan, ZHU Zhaojie, LI Jianfu, LAKSHMINARAYANA Gandham, TU Chaoyang. Crystal Growth and Structural, Optical, and Visible Fluorescence Traits of Dy3+-doped SrGdGa3O7 Crystal [J]. Journal of Inorganic Materials, 2023, 38(12): 1475-1482. |
| [12] | MING Yue, HU Yue, MEI Anyi, RONG Yaoguang, HAN Hongwei. Application of Lead Acetate Additive for Printable Perovskite Solar Cell [J]. Journal of Inorganic Materials, 2022, 37(2): 197-203. |
| [13] | XU Jiayue, LI Zhichao, PAN Yunfang, ZHOU Ding, WEN Feng, MA Wenjun. Research Progress of Hyperstoichiometric UO2 Crystals [J]. Journal of Inorganic Materials, 2020, 35(11): 1183-1192. |
| [14] | Rong-Hui LI, Yi-Zheng JIA, Nan-Nan HU. 3D Hierarchical Flower Like Alumina Nanomaterials: Preparation and Arsenic Removal Performance [J]. Journal of Inorganic Materials, 2019, 34(5): 553-559. |
| [15] | WANG Dong-Hai, XUE Yan-Yan, LI Na, ZHOU Shi-Ming, XU Xiao-Dong, LI Dong-Zhen, XU Jun, WANG Qing-Guo. Micro-tube Sapphire Crystal Grown by the Edge-defined-film Fed Method [J]. Journal of Inorganic Materials, 2019, 34(12): 1290-1294. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||