
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (5): 544-552.DOI: 10.15541/jim20220532
Special Issue: 【结构材料】热障与环境障涂层(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
FAN Dong1,2( ), ZHONG Xin1(
), ZHONG Xin1( ), WANG Yawen1, ZHANG Zhenzhong2(
), WANG Yawen1, ZHANG Zhenzhong2( ), NIU Yaran1, LI Qilian3, ZHANG Le3, ZHENG Xuebin1
), NIU Yaran1, LI Qilian3, ZHANG Le3, ZHENG Xuebin1
Received:2022-09-13
Revised:2022-10-06
Published:2022-10-28
Online:2022-10-28
Contact:
ZHONG Xin, assistant professor. E-mail: zhongxin@mail.sic.ac.cn;About author:FAN Dong (1998-), male, Master candidate. E-mail: fandong1998@126.com
Supported by:CLC Number:
FAN Dong, ZHONG Xin, WANG Yawen, ZHANG Zhenzhong, NIU Yaran, LI Qilian, ZHANG Le, ZHENG Xuebin. Corrosion Behavior and Mechanism of Aluminum-rich CMAS on Rare-earth Silicate Environmental Barrier Coatings:[J]. Journal of Inorganic Materials, 2023, 38(5): 544-552.
| Parameter | RE2SiO5 (RE=Gd, Y, Er) |
|---|---|
| Primary Ar/(L·min-1) | 43 |
| Secondary H2/(L·min-1) | 12 |
| Carrier Ar/(L·min-1) | 2.3 |
| Spray distance/mm | 230 |
Table 1 Technical parameters used for plasma spraying
| Parameter | RE2SiO5 (RE=Gd, Y, Er) |
|---|---|
| Primary Ar/(L·min-1) | 43 |
| Secondary H2/(L·min-1) | 12 |
| Carrier Ar/(L·min-1) | 2.3 |
| Spray distance/mm | 230 |
| XRF/(%,in mol) | CaO | MgO | AlO1.5 | SiO2 |
|---|---|---|---|---|
| CMAS | 27.87 | 8.79 | 26.22 | 38.52 |
Table 2 Chemical compositions of CMAS powders
| XRF/(%,in mol) | CaO | MgO | AlO1.5 | SiO2 |
|---|---|---|---|---|
| CMAS | 27.87 | 8.79 | 26.22 | 38.52 |
| EDS/ (%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 20.60 | — | — | 19.62 | 51.73 | 8.02 | — | — |
| Point 2 | — | 26.04 | — | 17.55 | 50.57 | 5.83 | — | — |
| Point 3 | — | — | 26.95 | 15.16 | 50.78 | 7.10 | — | — |
| Point 4 | — | 14.03 | — | 5.72 | 52.27 | 4.01 | 20.31 | 3.66 |
Table 3 EDS elemental compositions of the marked regions in Fig. 3
| EDS/ (%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 20.60 | — | — | 19.62 | 51.73 | 8.02 | — | — |
| Point 2 | — | 26.04 | — | 17.55 | 50.57 | 5.83 | — | — |
| Point 3 | — | — | 26.95 | 15.16 | 50.78 | 7.10 | — | — |
| Point 4 | — | 14.03 | — | 5.72 | 52.27 | 4.01 | 20.31 | 3.66 |
| EDS/(%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 19.21 | — | —- | 11.70 | 60.65 | 8.44 | — | — |
| Point 2 | 1.16 | — | — | 13.06 | 59.37 | 13.78 | 10.18 | 2.45 |
| Point 3 | — | 15.29 | — | 16.00 | 61.82 | 6.89 | — | — |
| Point 4 | — | 7.18 | — | 15.50 | 50.17 | 9.58 | 12.14 | 5.43 |
| Point 5 | — | 0.96 | — | 13.56 | 59.62 | 13.03 | 10.40 | 2.42 |
| Point 6 | — | — | 20.27 | 12.11 | 61.12 | 6.50 | — | — |
| Point 7 | — | — | 8.70 | 10.95 | 59.15 | 8.09 | 10.95 | 4.65 |
| Point 8 | — | — | 0.92 | 3.29 | 58.74 | 19.61 | 3.29 | 2.07 |
Table 4 EDS elemental compositions of the marked regions in Fig. 4
| EDS/(%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 19.21 | — | —- | 11.70 | 60.65 | 8.44 | — | — |
| Point 2 | 1.16 | — | — | 13.06 | 59.37 | 13.78 | 10.18 | 2.45 |
| Point 3 | — | 15.29 | — | 16.00 | 61.82 | 6.89 | — | — |
| Point 4 | — | 7.18 | — | 15.50 | 50.17 | 9.58 | 12.14 | 5.43 |
| Point 5 | — | 0.96 | — | 13.56 | 59.62 | 13.03 | 10.40 | 2.42 |
| Point 6 | — | — | 20.27 | 12.11 | 61.12 | 6.50 | — | — |
| Point 7 | — | — | 8.70 | 10.95 | 59.15 | 8.09 | 10.95 | 4.65 |
| Point 8 | — | — | 0.92 | 3.29 | 58.74 | 19.61 | 3.29 | 2.07 |
| EDS/ (%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 24.48 | — | — | 16.22 | 51.61 | 7.68 | — | — |
| Point 2 | 1.05 | — | — | 12.62 | 59.63 | 14.25 | 9.78 | 2.66 |
| Point 3 | — | 26.70 | — | 15.78 | 50.12 | 7.40 | — | — |
| Point 4 | — | 17.10 | — | 8.23 | 51.25 | 2.92 | 16.41 | 4.09 |
| Point 5 | — | 0.94 | — | 14.23 | 57.36 | 13.94 | 11.98 | 1.54 |
| Point 6 | — | — | 28.80 | 12.77 | 52.22 | 6.22 | — | — |
| Point 7 | — | — | 7.12 | 13.05 | 49.26 | 7.08 | 18.26 | 5.24 |
Table 5 EDS elemental compositions of the marked regions in Fig. 5
| EDS/ (%, in atom) | Gd | Y | Er | Si | O | Ca | Al | Mg |
|---|---|---|---|---|---|---|---|---|
| Point 1 | 24.48 | — | — | 16.22 | 51.61 | 7.68 | — | — |
| Point 2 | 1.05 | — | — | 12.62 | 59.63 | 14.25 | 9.78 | 2.66 |
| Point 3 | — | 26.70 | — | 15.78 | 50.12 | 7.40 | — | — |
| Point 4 | — | 17.10 | — | 8.23 | 51.25 | 2.92 | 16.41 | 4.09 |
| Point 5 | — | 0.94 | — | 14.23 | 57.36 | 13.94 | 11.98 | 1.54 |
| Point 6 | — | — | 28.80 | 12.77 | 52.22 | 6.22 | — | — |
| Point 7 | — | — | 7.12 | 13.05 | 49.26 | 7.08 | 18.26 | 5.24 |
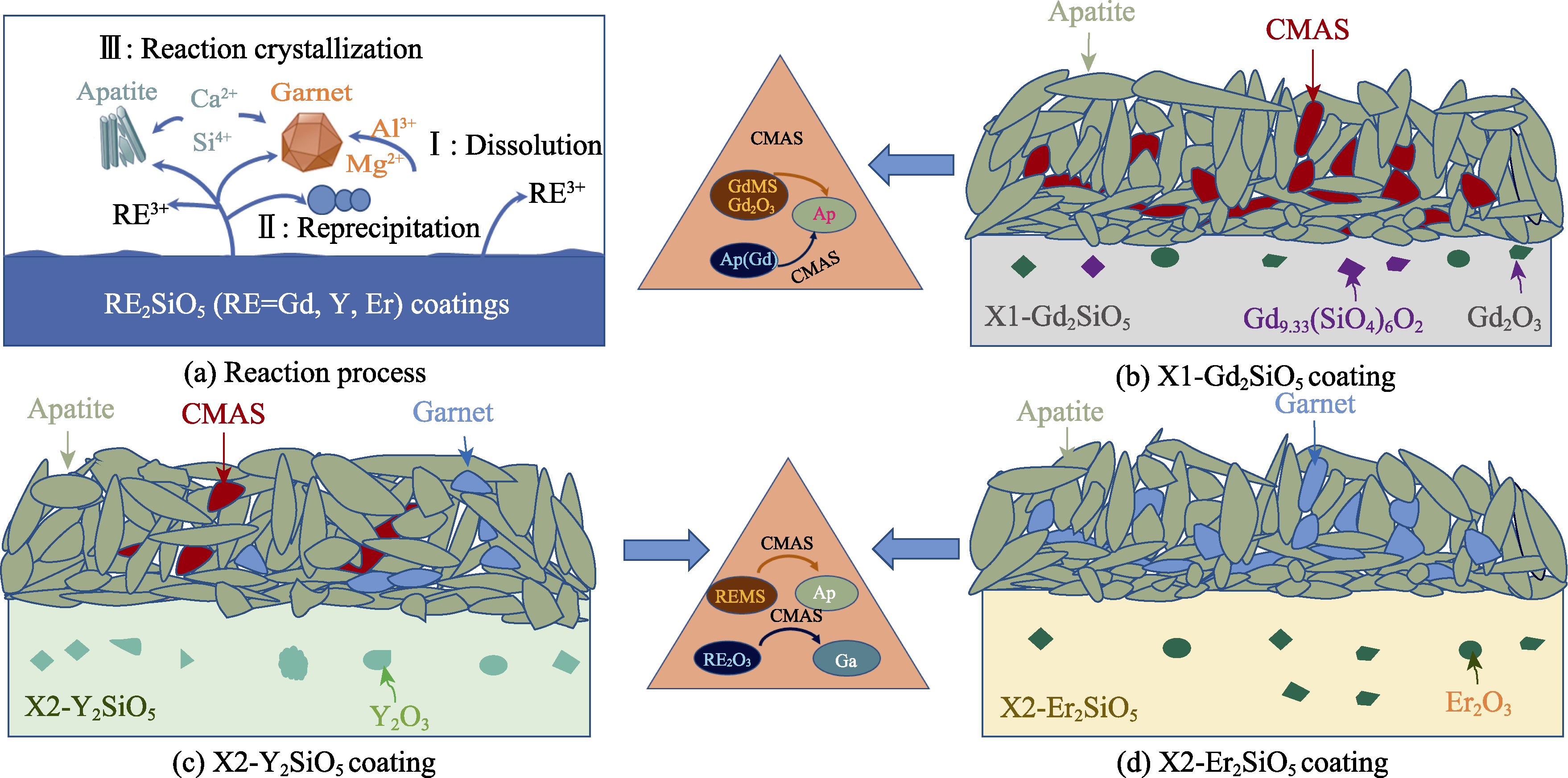
Fig. 6 Schematic diagrams of different coatings under CMAS molten salt corrosion at 1400 ℃ (a) Reaction process; (b) X1-Gd2SiO5; (c) X2-Y2SiO5; (d) X2-Er2SiO5
| [1] |
PADTURE N P. Advanced structural ceramics in aerospace propulsion. Nature Materials, 2016, 15(8): 804.
DOI PMID |
| [2] |
RAJ R. Fundamental research in structural ceramics for service near 2000 ℃. Journal of the American Ceramic Society, 1993, 76(9): 2147.
DOI URL |
| [3] |
EATON H E, LINSEY G D. Accelerated oxidation of SiC CMC's by water vapor and protection via environmental barrier coating approach. Journal of the European Ceramic Society, 2002, 22(14-15): 2741.
DOI URL |
| [4] |
LIU P P, ZHONG X, ZHANG L, et al. Molten salt corrosion behaviors and mechanisms of ytterbium silicate environmental barrier coating. Journal of Inorganic Materials, 2022, 37(12): 1267.
DOI |
| [5] |
OPILA E J. Oxidation and volatilization of silica formers in water vapor. Journal of the American Ceramic Society, 2003, 86(8): 1238.
DOI URL |
| [6] |
ZHANG X F, SONG J B, DENG Z Q, et al. Interface evolution of Si/Mullite/Yb2SiO5 PS-PVD environmental barrier coatings under high temperature. Journal of the European Ceramic Society, 2020, 40(4): 1478.
DOI URL |
| [7] |
TIAN Z L, ZHENG L Y, WANG J M, et al. Theoretical and experimental determination of the major thermo-mechanical properties of RE2SiO5 (RE= Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) for environmental and thermal barrier coating applications. Journal of the European Ceramic Society, 2016, 36(1): 189.
DOI URL |
| [8] |
ZHANG X F, ZHOU K S, LIU M, et al. Preparation of Si/Mullite/Yb2SiO5environment barrier coating (EBC) by plasma spray-physical vapor deposition (PS-PVD). Journal of Inorganic Materials, 2018, 33(3): 325.
DOI URL |
| [9] | SUMMERS W D, POERSCHKE D L, TAYLOR A A, et al. Reactions of molten silicate deposits with yttrium monosilicate. Journal of the European Ceramic Society, 2020, 103(4): 2919. |
| [10] |
STOLZENBURG F, KENESEI P, ALMER J, et al. The influence of calcium-magnesium-aluminosilicate deposits on internal stresses in Yb2Si2O7 multilayer environmental barrier coatings. Acta Materialia, 2016, 105: 189.
DOI URL |
| [11] | WANG C, ZHANG X F, ZHOU K S, et al. Nano-composite structured environmental barrier coatings prepared by plasma spray- physical vapor deposition and their thermal cycle performance. Rare Metal Materials and Engineering, 2019, 48(11): 3455. |
| [12] |
LI G, QIN L, CAO X Q, et al. Water vapor corrosion resistance and failure mechanism of SiCf/SiC composites completely coated with plasma sprayed tri-layer EBCs. Ceramics International, 2022, 48(5): 7082.
DOI URL |
| [13] |
LEE K N. Yb2Si2O7 Environmental barrier coatings with reduced bond coat oxidation rates via chemical modifications for long life. Journal of the American Ceramic Society, 2019, 102(3): 1507.
DOI URL |
| [14] |
WANG J G, TIAN S J, LI G B, et al. Preparation and X-ray characterization of low-temperature phases of R2SiO5 (R=rare earth elements). Materials of Research Bulletin, 2001, 36: 1855.
DOI URL |
| [15] |
WOLF M, MACK D E, GUILLO O, et al. Resistance of pure and mixed rare earth silicates against calcium-magnesium- aluminosilicate (CMAS): a comparative study. Journal of the American Ceramic Society, 2020, 103(12): 7056.
DOI URL |
| [16] |
JIANG F R, CHENG L F, WANG Y G. Hot corrosion of RE2SiO5 with different cation substitution under calcium-magnesium- aluminosilicate attack. Ceramics International, 2017, 43(12): 9019.
DOI URL |
| [17] |
ZHONG X, WANG Y W, LIU P P, et al. Effects of microstructure on corrosion behaviors for RE2SiO5 (RE=Gd, Y, Er) environmental barrier coatings against calcium-magnesium-alumino-silicate melts. Corrosion Science, 2022, 199: 110174.
DOI URL |
| [18] |
TIAN Z L, REN X M, LEI Y M, et al. Corrosion of RE2Si2O7 (RE = Y, Yb, and Lu) environmental barrier coating materials by molten calcium-magnesium-alumino-silicate glass at high temperatures. Journal of the European Ceramic Society, 2019, 39(14): 4245.
DOI URL |
| [19] |
LIU P P, ZHONG X, NIU Y R, et al. Reaction behaviors and mechanisms of tri-layer Yb2SiO5/Yb2Si2O7/Si environmental barrier coatings with molten calcium-magnesium-alumino-silicate. Corrosion Science, 2022, 197: 110069.
DOI URL |
| [20] |
STOKES J L, HARDER B J, WIESNER V L, et al. Effects of crystal structure and cation size on molten silicate reactivity with environmental barrier coating materials. Journal of the American Ceramic Society, 2019, 103(1): 622.
DOI URL |
| [21] |
SUMMERS W D, POERSCHKE D L, PARK D, et al. Roles of composition and temperature in silicate deposit-induced recession of yttrium disilicate. Acta Materialia, 2018, 160: 34.
DOI URL |
| [22] |
LEVI C G, JOHN W H, MARIE V S, et al. Environmental degradation of thermal barrier coatings by molten deposits. MRS Bulletin, 2012, 37: 932.
DOI URL |
| [23] |
BONDAR I A, Rare-earth silicates. Ceramics International, 1982, 8: 83.
DOI URL |
| [24] | FELSCHE J. The crystal chemistry of the rare-earth silicates. Materials Science and Chemistry, 1973, 13: 99. |
| [25] |
ZHONG X, NIU Y R, LI H, et al. Microstructure evolution and thermomechanical properties of plasma-sprayed Yb2SiO5 coating during thermal aging. Journal of the American Ceramic Society. 2017, 100(5): 1896.
DOI URL |
| [26] |
POERSCHKE D L, JACKSON R W, LEVI C G. Silicate deposit degradation of engineered coatings in gas turbines: progress toward models and materials solutions. Annual Review of Materials Research, 2017, 47: 297.
DOI URL |
| [27] |
LI Y R, WANG J M, WANG J Y. Theoretical investigation of phonon contributions to thermal expansion coefficients for rare earth monosilicates RE2SiO5 (RE = Dy, Ho, Er, Tm, Yb and Lu). Journal of the European Ceramic Society, 2020, 40(7): 2658.
DOI URL |
| [1] | LIANG Ruihui, ZHONG Xin, HONG Du, HUANG Liping, NIU Yaran, ZHENG Xuebin. High-temperature Water Vapor Corrosion Behaviors of Environmental Barrier Coatings with Yb2O3-modified Silicon Bond Layer [J]. Journal of Inorganic Materials, 2025, 40(4): 425-432. |
| [2] | FAN Wenkai, YANG Xiao, LI Honghua, LI Yong, LI Jiangtao. Pressureless Sintering of (Y0.2Gd0.2Er0.2Yb0.2Lu0.2)2Zr2O7 High-entropy Ceramic and Its High Temperature CMAS Corrosion Resistance [J]. Journal of Inorganic Materials, 2025, 40(2): 159-167. |
| [3] | LI Liuyuan, HUANG Kaiming, ZHAO Xiuyi, LIU Huichao, WANG Chao. Influence of RE-Si-Al-O Glass Phase on Microstructure and CMAS Corrosion Resistance of High Entropy Rare Earth Disilicates [J]. Journal of Inorganic Materials, 2024, 39(7): 793-802. |
| [4] | LI Jie, LUO Zhixin, CUI Yang, ZHANG Guangheng, SUN Luchao, WANG Jingyang. CMAS Corrosion Resistance of Y3Al5O12/Al2O3 Ceramic Coating Deposited by Atmospheric Plasma Spraying [J]. Journal of Inorganic Materials, 2024, 39(6): 671-680. |
| [5] | FANG Guangwu, XIE Haoyuan, ZHANG Huajun, GAO Xiguang, SONG Yingdong. Progress of Damage Coupling Mechanism and Integrated Design Method for CMC-EBC [J]. Journal of Inorganic Materials, 2024, 39(6): 647-661. |
| [6] | TAO Shunyan, YANG Jiasheng, SHAO Fang, WU Yingchen, ZHAO Huayu, DONG Shaoming, ZHANG Xiangyu, XIONG Ying. Thermal Spray Coatings for Aircraft CMC Hot-end Components: Opportunities and Challenges [J]. Journal of Inorganic Materials, 2024, 39(10): 1077-1083. |
| [7] | LUO Shuwen, MA Mingsheng, LIU Feng, LIU Zhifu. Corrosion Behavior and Mechanism of LTCC Materials in Ca-B-Si System [J]. Journal of Inorganic Materials, 2023, 38(5): 553-560. |
| [8] | LIU Pingping, ZHONG Xin, ZHANG Le, LI Hong, NIU Yaran, ZHANG Xiangyu, LI Qilian, ZHENG Xuebin. Molten Salt Corrosion Behaviors and Mechanisms of Ytterbium Silicate Environmental Barrier Coating [J]. Journal of Inorganic Materials, 2022, 37(12): 1267-1274. |
| [9] | SUN Luchao, REN Xiaomin, DU Tiefeng, LUO Yixiu, ZHANG Jie, WANG Jingyang. High Entropy Engineering: New Strategy for the Critical Property Optimizations of Rare Earth Silicates [J]. Journal of Inorganic Materials, 2021, 36(4): 339-346. |
| [10] | FAN Jia-Feng,ZHANG Xiao-Feng,ZHOU Ke-Song,LIU Min,DENG Chang-Guang,DENG Chun-Ming,NIU Shao-Peng,DENG Zi-Qian. Influence of Al-modification on CMAS Corrosion Resistance of PS-PVD 7YSZ Thermal Barrier Coatings [J]. Journal of Inorganic Materials, 2019, 34(9): 938-946. |
| [11] | WANG Peng, WANG Qing-Lei, ZHANG Xiang-Yu, YANG Jin-Shan, ZHOU Hai-Jun, HU Jian-Bao, DING Yu-Sheng, DONG Shao-Ming. Oxidation Behavior of SiCf/SiC Composites Modified by Layered-Y2Si2O7 in Wet Oxygen Environment [J]. Journal of Inorganic Materials, 2019, 34(8): 904-908. |
| [12] | ZHANG Xiao-Feng, ZHOU Ke-Song, LIU Min, DENG Chun-Ming, NIU Shao-Peng, XU Shi-Ming. Preparation of Si/Mullite/Yb2SiO5 Environment Barrier Coating (EBC) by Plasma Spray-Physical Vapor Deposition (PS-PVD) [J]. Journal of Inorganic Materials, 2018, 33(3): 325-330. |
| [13] | ZHANG Xiao-Feng, ZHOU Ke-Song, SONG Jin-Bing, DENG Chun-Ming, NIU Shao-Peng, DENG Zi-Qian. Deposition and CMAS Corrosion Mechanism of 7YSZ Thermal Barrier Coatings Prepared by Plasma Spray-Physical Vapor Deposition [J]. Journal of Inorganic Materials, 2015, 30(3): 287-293. |
| [14] | LU Lin-Jing, CHENG Lai-Fei, HONG Zhi-Liang, WANG Yi-Guang, ZHANG Li-Tong. Fabrication and Water-vapor Corrosion Resistance of Ba0.25Sr0.75Al2Si2O8 Environmental Barrier Coating [J]. Journal of Inorganic Materials, 2011, 26(7): 701-706. |
| [15] | WU Jiang,LIN Hong,LI Jian-Bao,LI Jun-Feng. Corrosion Behavior of AlNbO4/Mullite Composite as Environmental Barrier Coating in Water Vapor Environment [J]. Journal of Inorganic Materials, 2010, 25(4): 445-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||