
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (5): 553-560.DOI: 10.15541/jim20220513
Special Issue: 【信息功能】纪念殷之文先生诞辰105周年虚拟学术专辑
• RESEARCH ARTICLE • Previous Articles Next Articles
LUO Shuwen1,2( ), MA Mingsheng2(
), MA Mingsheng2( ), LIU Feng2, LIU Zhifu2(
), LIU Feng2, LIU Zhifu2( )
)
Received:2022-09-02
Revised:2022-10-10
Published:2022-12-27
Online:2022-12-27
Contact:
MA Mingsheng, associate professor. E-mail: mamingsheng@mail.sic.ac.cn;About author:LUO Shuwen (1998-), female, Master candidate. E-mail: 1000497469@smail.shnu.edu.cn
Supported by:CLC Number:
LUO Shuwen, MA Mingsheng, LIU Feng, LIU Zhifu. Corrosion Behavior and Mechanism of LTCC Materials in Ca-B-Si System[J]. Journal of Inorganic Materials, 2023, 38(5): 553-560.
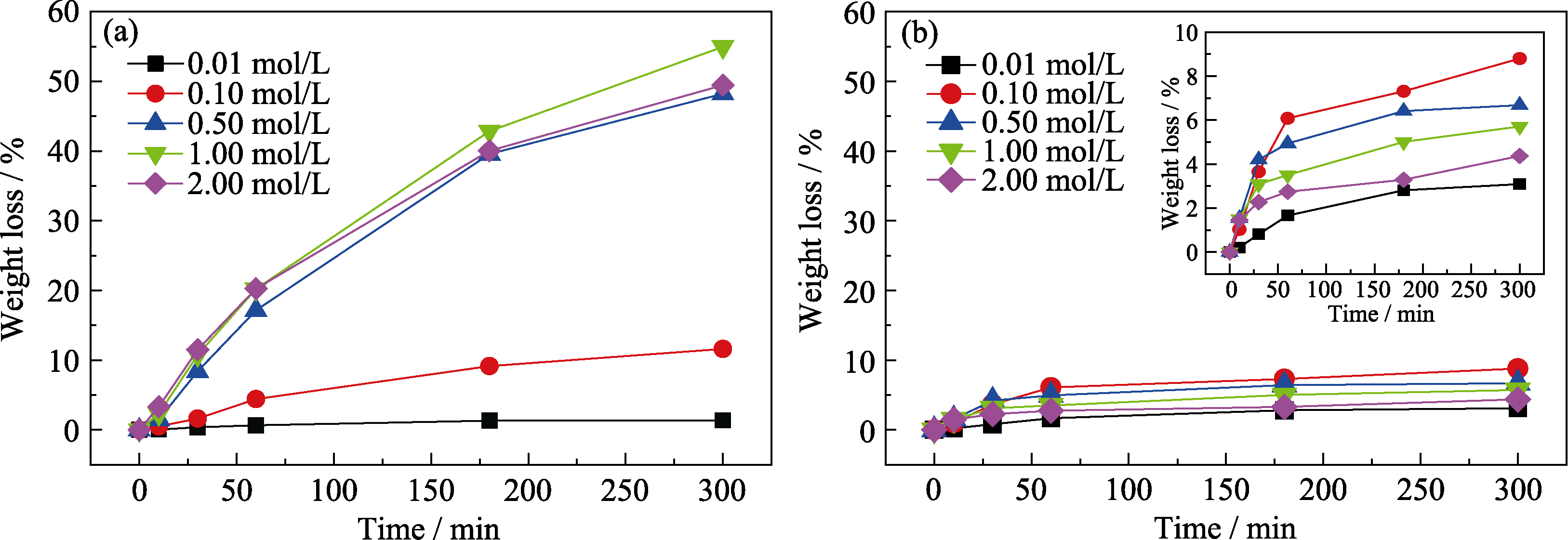
Fig. 1 Variation of weight loss with corrosion time after corrosion of samples in different concentrations of acid solutions (a) HCl solution; (b) H2SO4 solution
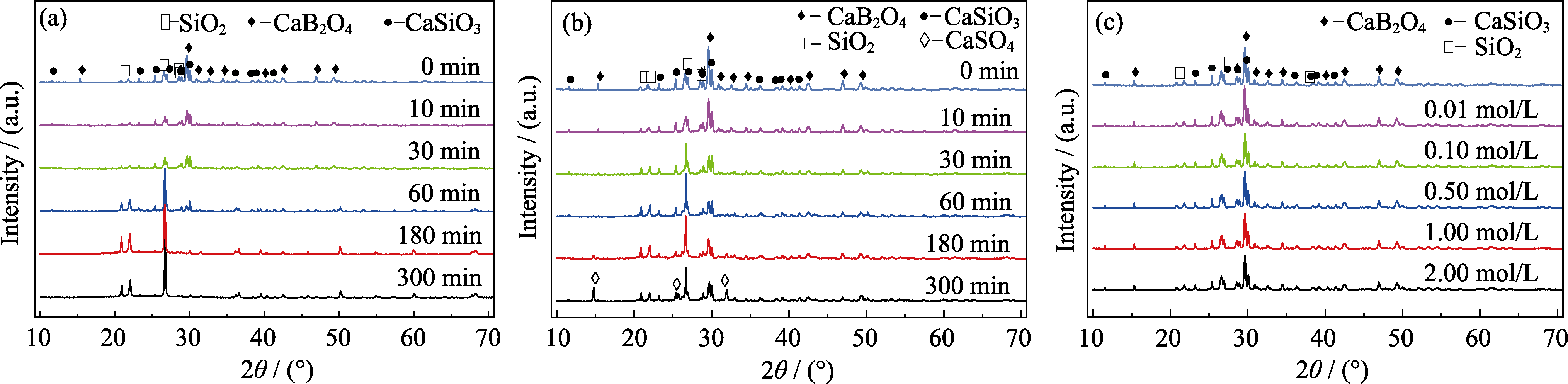
Fig. 2 XRD patterns of samples before and after corrosion for different time in various solutions (a) 1.00 mol/L HCl solution; (b) 0.10 mol/L H2SO4 solution; (c) NaOH solutions at different concentrations

Fig. 3 Kinds and contents of elements entering the solutions after the samples being corroded in different concentrations of HCl, H2SO4 and NaOH solutions for 300 min Colorful figures are available on website

Fig. 4 SEM images of samples in different concentrations of HCl solutions before corrosion and after corrosion for different time (a) 0.50 mol/L HCl solution; (b) 1.00 mol/L HCl solution; (c) 2.00 mol/L HCl solution
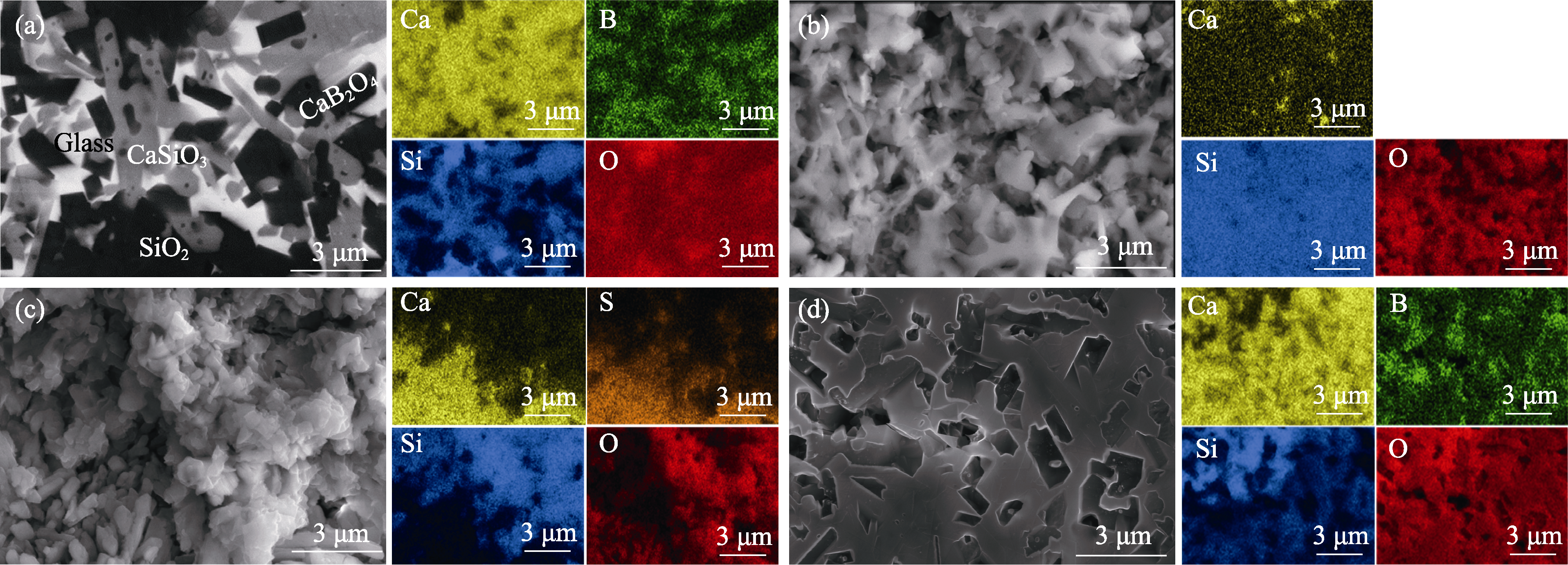
Fig. 5 Phase compositions and elemental distributions of sample surfaces before and after corrosion in different solutions (a) No corrosion; (b) Corrosion in 1.00 mol/L HCl solution for 300 min; (c) Corrosion in 0.10 mol/L H2SO4 solution for 300 min; (d) Corrosion in 2.00 mol/L NaOH solution for 300 min

Fig. 7 Raman spectra of samples before and after corrosion in different acid solutions for different time (a) 1.00 mol/L HCl solution; (b) 0.10 mol/L H2SO4 solution
| [1] | IMANAKA Y. Multilayered Low Temperature Cofired Ceramics (LTCC) Technology. New York: Springer Science & Business Media, 2005: 1-17. |
| [2] | WANG Y H, ZHOU J, CUI X M, et al. Development of low temperature cofired ceramic technology in material field. Journal of Inorganic Materials, 2006, 21(2): 267. |
| [3] | WANG Z Y. Reliability analysis of mixed conductor LTCC substrate. Electronic Components & Materials, 2003, 22(2): 7. |
| [4] | CHEN X Y, JIA S X, WANG Y L, et al. Research on the process of LTCC substrates based on ENEPIG. Printed Circuit Information, 2021, 29(6): 52. |
| [5] | BEIKMOHAMADI A, STEWART S, PARISI J, et al. Electroplating and electroless plating process development for DuPont™ GreenTape™ 9K7 LTCC. Additional Papers and Presentations, 2013, 2013(CICMT): 00283. |
| [6] | WANG Y L, LI J. The technology of Enepig of low temperature Co- fired ceramic substitute. Printed Circuit Information, 2020, 28(7): 49. |
| [7] | NAIR K M, SKURSKI M A, VOULTOS J D. Nickel-gold Plateable Thick Film Silver Paste: US8609256B2. 2013-12-17. |
| [8] | THOMAS S, BALAKRISHNAN P, SREEKALA M S. Fundamental Biomaterials:Ceramics. Cambridge: Woodhead Publishing, 2018: 223-250. |
| [9] |
LEE S M, LIM W B, CHO Y S. Corrosion behavior of highly- crystallizable BaO-Nd2O3-TiO2-B2O3 glass-based composites. Corrosion Science, 2013, 66: 399.
DOI URL |
| [10] |
LIM W B, SHIN D W, MOHANTY B C, et al. Chemical durability of anorthite-based low temperature co-fired ceramics. Journal of the Ceramic Society of Japan, 2009, 117(1370): 1138.
DOI URL |
| [11] |
JO Y H, KANG M S, CHUNG K W, et al. Chemical stability and dielectric properties of RO-La2O3-B2O3 (R= Ca, Mg, Zn)-based ceramics. Materials Research Bulletin, 2008, 43(2): 361.
DOI URL |
| [12] |
STEINHÄUßER F, TALAI A, GÖLTL G, et al. Concentration and temperature dependent selectivity of the LTCC porosification process with phosphoric acid. Ceramics International, 2017, 43(1): 714.
DOI URL |
| [13] |
KANG J, WANG J, ZHOU X, et al. Effects of alkali metal oxides on crystallization behavior and acid corrosion resistance of cordierite-based glass-ceramics. Journal of Non-Crystalline Solids, 2018, 481: 184.
DOI URL |
| [14] |
HAJIAN A, STÖGER-POLLACH M, SCHNEIDER M, et al. Porosification behaviour of LTCC substrates with potassium hydroxide. Journal of the European Ceramic Society, 2018, 38(5): 2369.
DOI URL |
| [15] |
SALAMA S N, SALMAN S M. Characterization of glass-ceramic corrosion and durability. Journal of the European Ceramic Society, 1994, 13(6): 521.
DOI URL |
| [16] |
GHOSH S, DATTA S. Surface degradation behaviour of MgO- Al2O3-TiO2-SiO2 based glass-ceramics. Transactions of the Indian Ceramic Society, 2014, 73(3): 216.
DOI URL |
| [17] |
SEBASTIAN M T, JANTUNEN H. Low loss dielectric materials for LTCC applications: a review. International Materials Reviews, 2008, 53(2): 57.
DOI URL |
| [18] | WANG T, WANG Y, YANG H, et al. Structure, dielectric properties of low-temperature-sintering BaTiO3-based glass-ceramics for energy storage. Journal of Advanced Dielectrics, 2018, 8(6): 1850041. |
| [19] |
ĆURKOVIĆ L, JELAČA M F, KURAJICA S. Corrosion behavior of alumina ceramics in aqueous HCl and H2SO4solutions. Corrosion Science, 2008, 50(3): 872.
DOI URL |
| [20] | CLARK D E, ZOITOS B K. Corrosion of Glass, Ceramics and Ceramic Superconductor: Principles, Testing, Characterization and Applications. New Jersey: Noyes Publications, 1992: 2-28. |
| [21] | SPEIGHT J G. Lange's Handbook of Chemistry. New York: McGraw-hill, 2005: 237-279. |
| [22] |
OSIPOV A A, OSIPOVA L M. Structural studies of Na2O-B2O3 glasses and melts using high-temperature Raman spectroscopy. Physica B: Condensed Matter, 2010, 405(23): 4718.
DOI URL |
| [23] | STEFANOVSKY S V, FOX K M, MARRA J C. Infrared and raman spectroscopic study of glasses in the Al2O3-B2O3-Fe2O3- Na2O-SiO2 system. MRS Online Proceedings Library (OPL), 2013, 1518:53. |
| [24] | 王晨. 硅酸盐及含铝硅酸盐矿物的拉曼光谱研究. 北京: 中国地质大学(北京)硕士学位论文, 2005. |
| [25] | 尤静林. 高温拉曼光谱创新技术、光谱计算和在无机化合物微结构研究中的应用. 上海: 上海大学博士学位论文, 2006. |
| [26] |
YIN C D, OKUNO M, MORIKAWA H, et al. Structural analysis of CaSiO3 glass by X-ray diffraction and Raman spectroscopy. Journal of Non-crystalline Solids, 1986, 80(1/2/3): 167.
DOI URL |
| [27] |
WANG Z, SHU Q, CHOU K. Structure of CaO-B2O3-SiO2-TiO2 glasses: a Raman spectral study. ISIJ International, 2011, 51(7): 1021.
DOI URL |
| [28] |
NEUVILLE D R, DE LIGNY D, HENDERSON G S. Advances in Raman spectroscopy applied to earth and material sciences. Reviews in Mineralogy and Geochemistry, 2014, 78(1): 509.
DOI URL |
| [29] |
GIN S, ABDELOUAS A, CRISCENTI L J, et al. An international initiative on long-term behavior of high-level nuclear waste glass. Materials Today, 2013, 16(6): 243.
DOI URL |
| [30] |
FRANKEL G S, VIENNA J D, LIAN J, et al. A comparative review of the aqueous corrosion of glasses, crystalline ceramics, and metals. npj Materials Degradation, 2018, 2(1): 15.
DOI |
| [31] |
FRANKEL G S, VIENNA J D, LIAN J, et al. Recent advances in corrosion science applicable to disposal of high-level nuclear waste. Chemical Reviews, 2021, 121(20): 12327.
DOI PMID |
| [32] |
RAHIMI R A, SADRNEZHAAD S K, RAISALI G. Chemical durability of lead silicate glass in HNO3, HCl and H2SO4 aqueous acid solutions. Journal of Non-crystalline Solids, 2009, 355(3): 169.
DOI URL |
| [33] |
SALDI G D, KÖHLER S J, MARTY N, et al. Dissolution rates of talc as a function of solution composition, pH and temperature. Geochimica et Cosmochimica Acta, 2007, 71(14): 3446.
DOI URL |
| [34] |
WANG J. Thermodynamic equilibrium and kinetic fundamentals of oxide dissolution in aqueous solution. Journal of Materials Research, 2020, 35(8): 898.
DOI URL |
| [35] |
CARTLEDGE G H. Studies on the periodic system. I. The ionic potential as a periodic function. Journal of the American Chemical Society, 1928, 50(11): 2855.
DOI URL |
| [36] |
CARTLEDGE G H. Studies on the periodic system. II. The ionic potential and related properties. Journal of the American Chemical Society, 1928, 50(11): 2863.
DOI URL |
| [37] |
BUNKER B C. Molecular mechanisms for corrosion of silica and silicate glasses. Journal of Non-Crystalline Solids, 1994, 179: 300.
DOI URL |
| [38] |
COLLIN M, FOURNIER M, FRUGIER P, et al. Structure of international simple glass and properties of passivating layer formed in circumneutral pH conditions. npj Materials Degradation, 2018, 2(1): 4.
DOI |
| [39] |
OELKERS E H, SCHOTT J. An experimental study of enstatite dissolution rates as a function of pH, temperature, and aqueous Mg and Si concentration, and the mechanism of pyroxene/pyroxenoid dissolution. Geochimica et Cosmochimica Acta, 2001, 65(8): 1219.
DOI URL |
| [40] |
GIN S, NEILL L, FOURNIER M, et al. The controversial role of inter-diffusion in glass alteration. Chemical Geology, 2016, 440: 115.
DOI URL |
| [41] |
SCHOTT J, POKROVSKY O S, SPALLA O, et al. Formation, growth and transformation of leached layers during silicate minerals dissolution: the example of wollastonite. Geochimica et Cosmochimica Acta, 2012, 98: 259.
DOI URL |
| [42] |
ZAPOL P, HE H, KWON K D, et al. First-principles study of hydrolysis reaction barriers in a sodium borosilicate glass. International Journal of Applied Glass Science, 2013, 4(4): 395.
DOI URL |
| [43] | BRANTLEY S L. Kinetics of Water-rock Interaction. New York: Springer, 2008: 151-210. |
| [44] |
CAILLETEAU C, ANGELI F, DEVREUX F, et al. Insight into silicate- glass corrosion mechanisms. Nature Materials, 2008, 7(12): 978.
DOI |
| [1] | YUE Zihao, YANG Xiaotu, ZHANG Zhengliang, DENG Ruixiang, ZHANG Tao, SONG Lixin. Effect of Pb2+ on the Luminescent Performance of Borosilicate Glass Coated CsPbBr3 Perovskite Quantum Dots [J]. Journal of Inorganic Materials, 2024, 39(4): 449-456. |
| [2] | FAN Dong, ZHONG Xin, WANG Yawen, ZHANG Zhenzhong, NIU Yaran, LI Qilian, ZHANG Le, ZHENG Xuebin. Corrosion Behavior and Mechanism of Aluminum-rich CMAS on Rare-earth Silicate Environmental Barrier Coatings: [J]. Journal of Inorganic Materials, 2023, 38(5): 544-552. |
| [3] | PANG Libin, WANG Deping. Drug Carrier Based on Mesoporous Borosilicate Glass Microspheres: Preparation and Performance [J]. Journal of Inorganic Materials, 2022, 37(7): 780-786. |
| [4] | LIU Pingping, ZHONG Xin, ZHANG Le, LI Hong, NIU Yaran, ZHANG Xiangyu, LI Qilian, ZHENG Xuebin. Molten Salt Corrosion Behaviors and Mechanisms of Ytterbium Silicate Environmental Barrier Coating [J]. Journal of Inorganic Materials, 2022, 37(12): 1267-1274. |
| [5] | ZHANG Xiao-Yang, PENG Hai-Bo, LIU Feng-Fei, ZHAO Yan, SUN Meng-Li, GUAN Ming, ZHANG Bing-Tao, DU Xin, YUAN Wei, WANG Tie-Shan. Mechanical Properties of Borosilicate Glass with Different Irradiation of Heavy Ions [J]. Journal of Inorganic Materials, 2019, 34(7): 741-747. |
| [6] | ZHANG Xiao-Feng, ZHOU Ke-Song, SONG Jin-Bing, DENG Chun-Ming, NIU Shao-Peng, DENG Zi-Qian. Deposition and CMAS Corrosion Mechanism of 7YSZ Thermal Barrier Coatings Prepared by Plasma Spray-Physical Vapor Deposition [J]. Journal of Inorganic Materials, 2015, 30(3): 287-293. |
| [7] | YIN De-Wu, LIU Zhen, YANG Xin-Yu, ZHANG Xi-Yan, XIANG Wei-Dong. Preparation and Optical Properties of AgIn Alloy Quantum Dots Doped Glass [J]. Journal of Inorganic Materials, 2014, 29(10): 1034-1038. |
| [8] | ZHAO Xiu-Li, LIANG Xiao-Juan, LUO Hong-Yan, CHEN Zhao-Ping, XIANG Wei-Dong. Third-order Nonlinear Optical Properties of Silver Quantum Dots Doped in Sodium Borosilicate Glass [J]. Journal of Inorganic Materials, 2013, 28(9): 1003-1008. |
| [9] | YANG Xin-Yu, XIANG Wei-Dong, ZHANG Xi-Yan, LIU Hai-Tao, ZHAO Hai-Jun, LIANG Xiao-Juan. Study on the Third-order Optical Nonlinear Absorption Properties of Bi2O3 Nanocrystals Glass [J]. Journal of Inorganic Materials, 2012, 27(3): 317-322. |
| [10] | YANG Xin-Yu, , XIANG Wei-Dong, , ZHAO Hai-Jun,ZHANG Xi-Yan, LIANG Xiao-Juan, LIU Hai-Tao. Third-order Nonlinear Optical Properties of Bi2S3 Nanocrystals Embedded inSodium Borosilicate Glass [J]. Journal of Inorganic Materials, 2011, 26(3): 290-294. |
| [11] |
GUAN Yong-Jun,XIA Yuan.
Electrochemical Impedance Spectroscopy of PEO Coating on Aluminum Alloy in NaCl Solution [J]. Journal of Inorganic Materials, 2008, 23(4): 784-788. |
| [12] | WANG Yue-Hui,ZHOU Ji,CUI Xue-Min,SHEN Jian-Hong. Development of Low Temperature Cofired Ceramic Technology in Material Field [J]. Journal of Inorganic Materials, 2006, 21(2): 267-276. |
| [13] | LUO Ling-Hong,ZHOU He-Ping,PENG Rong,QIAO Liang. Low-T Sintering, Low-Dielectric Materials for High Frequency Multilayer Chip Inductors [J]. Journal of Inorganic Materials, 2002, 17(3): 497-503. |
| [14] | CHEN Hongbing,ZHU Congshan,GAN Fuxi. Preparation and Electroinduced Second Order Nonlinear Optical Properties of CuI Microcrystal Doped Borosilicate Glasses [J]. Journal of Inorganic Materials, 1997, 12(4): 487-493. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||