
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (12): 1315-1320.DOI: 10.15541/jim20150213
• Orginal Article • Previous Articles Next Articles
WANG Yan-En1, WU Xiao-Jie1, HE Cheng-Lei1, ZHAO Jia-Ning1, TANG Ya-Wen2, LU Tian-Hong2
Received:2015-05-04
Revised:2015-07-20
Published:2015-12-20
Online:2015-11-24
Supported by:CLC Number:
WANG Yan-En, WU Xiao-Jie, HE Cheng-Lei, ZHAO Jia-Ning, TANG Ya-Wen, LU Tian-Hong. Carbon Supported Pd-Fe Catalyst with Uniform Alloy Structure Prepared with Direct Thermo-decomposition Method for Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2015, 30(12): 1315-1320.
| Catalyst | Pd-Pd bond distance/nm | Lattice constant, α/nm | Crystalline size /nm | Atomic fraction of Fe in the Pd3-Fe1/C catalyst/% | Atomic fraction of alloying Fe/% | Electrochemical area/ (m2·g-1) |
|---|---|---|---|---|---|---|
| Pd/C | 0.277 | 0.391 | 3.51 | 0 | 0 | 44.51 |
| Pd3-Fe1/C | 0.274 | 0.387 | 2.54 | 25 | 24.63 | 52.79 |
Table 1 Structure parameters of Pd/C and Pd3-Fe1/C catalysts
| Catalyst | Pd-Pd bond distance/nm | Lattice constant, α/nm | Crystalline size /nm | Atomic fraction of Fe in the Pd3-Fe1/C catalyst/% | Atomic fraction of alloying Fe/% | Electrochemical area/ (m2·g-1) |
|---|---|---|---|---|---|---|
| Pd/C | 0.277 | 0.391 | 3.51 | 0 | 0 | 44.51 |
| Pd3-Fe1/C | 0.274 | 0.387 | 2.54 | 25 | 24.63 | 52.79 |
| Catalyst | Speciation | Binding energy of Pd3d5/2/eV | Relative intensity/% |
|---|---|---|---|
| Pd/C | Pd0 | 335.26 | 51.1 |
| PdII | 336.90 | 48.9 | |
| Pd3-Fe1/C | Pd0 | 335.75 | 73.0 |
| PdII | 337.60 | 37.0 |
Table 2 Binding energy and relative intensities of Pd0 and PdII
| Catalyst | Speciation | Binding energy of Pd3d5/2/eV | Relative intensity/% |
|---|---|---|---|
| Pd/C | Pd0 | 335.26 | 51.1 |
| PdII | 336.90 | 48.9 | |
| Pd3-Fe1/C | Pd0 | 335.75 | 73.0 |
| PdII | 337.60 | 37.0 |

Fig.4 HRTEM and TEM images of the as-prepared catalysts and the catalysts EDS spectra. (a, b): HRTEM images of (a) Pd/C and (b) Pd3-Fe1/C catalysts; (c): TEM images of the Pd3-Fe1/C catalyst; (d): Overall EDS spectrum of large set of Pd3-Fe1/C particles in (c); (e): EDS spectrum of individual Pd3-Fe1/C particle
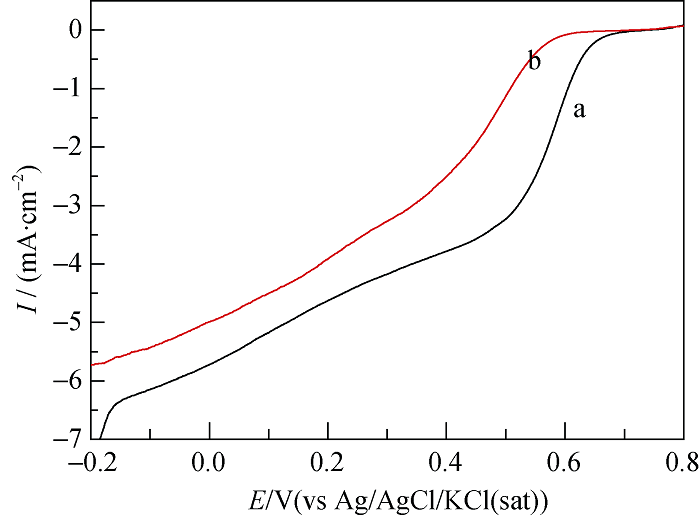
Fig. 6 Linear sweep voltammograms of (a) Pd/C and (b) Pd3- Fe1/C catalyst electrodes in 0.5 mol/L HClO4 solution with saturated oxygen. Scan rate: 5 mV/s, Rotating speed: 2000 r/min
| [1] | STAMENKOVIC V, FOWLER B, MUN B S, et al.Improved oxygen reduction activity on Pt3Ni(111) via increased surface site.Availability Science, 2007, 315(26): 493-497. |
| [2] | YANG H, VANTE N A, LEGER J M, et al.Tailoring, structure, and activity of carbon-supported nanosized Pt-Cr alloy electrocatalysts for oxygen reduction in pure and methanol-containing electrolytes.J. Phys. Chem. B, 2004, 108(6): 1938-1947. |
| [3] | LI X G, XING W, LU T H, et al.Studies on methanol-tolerant cathode electrocatalysts cobalt tetracarboxylic phthalocyanine supported on carbon black(CoPcTc/C) in direct methanol fuel cell.Chem. J. Chin. Univ., 2003, 7(24): 1246-1250. |
| [4] | PIRES F I, VILLULLAS H M.Pd-based catalysts: Influence of the second metal on their stability and oxygen reduction activity.Int. J. Hydrogen Energy, 2012, 37(22): 17052-17059. |
| [5] | ALEXEYEVA N, SARAPUU A, TAMMEVESKI K, et al.Electroreduction of oxygen on Vulcan carbon supported Pd nanoparticles and Pd-M nanoalloys in acid and alkaline solutions.Electrochim. Acta, 2011, 56(19): 6702-6708. |
| [6] | NEERGAT M, GUNASEKAR V, RAHUL R.Carbon-supported Pd-Fe electrocatalysts for oxygen reduction reaction (ORR) and their methanol tolerance.J. Electroanal. Chem., 2011, 658(1/2): 25-32. |
| [7] | XU J, LV X S, LI J D, et al.Simultaneous adsorption and dechlorination of 2, 4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support.J. Hazard. Mater., 2012, 225(7): 36-45. |
| [8] | WANG C, MARKOVIC N M, STAMENKOVIC V R.Advanced platinum alloy electrocatalysts for the oxygen reduction reaction.ACS Catal., 2012, 2(5): 891-898. |
| [9] | VONDROVA M, BURGESS C M, BOCARSLY A B.Cyanogel coordination polymers as precursors to transition metal alloys and intermetallics-from traditional heating to microwave processing.Chem. Mater., 2007, 19(9): 2203-2212. |
| [10] | WANG R, LIAO S, FU Z, et al.Platinum free ternary electrocatalysts prepared via organic colloidal method for oxygen reduction.Electrochem. Commun., 2008, 10(4): 523-526. |
| [11] | GREELEY J, MAVRIKAKIS M.Alloy catalysts designed from first principles.Nature Materials, 2004, 3(11): 810-815. |
| [12] | WHITE J H, SAMMELLS A F.Perovskite anode electrocatalysis for direct methanol fuel cells.J. Electrochem. Soc., 1993, 140(8): 2167-2176. |
| [13] | ANTOLINI E, CARDELLINI F.Formation of carbon supported PtRu alloys: an XRD analysis.J. Alloys Compounds, 2001, 315(1-2): 118-122. |
| [14] | ZHANG L, LEE K, ZHANG J.The effect of heat treatment on nanoparticle size and ORR activity for carbon-supported Pd-Co alloy electrocatalysts.Electrochim. Acta, 2007, 52(9): 3088-3094. |
| [15] | WANG W M, ZHENG D, DU C, et al.Carbon-supported Pd-Co bimetallic nanoparticles as electrocatalysts for the oxygen reduction reaction.J. Power Sources, 2007, 167(2): 243-249. |
| [16] | TANG Y W, ZHANG L L, WANG Y E, et al.Preparation of a carbon supported Pt catalyst using an improved organic sol method and its electrocatalytic activity for methanol oxidation.J. Power Sources, 2006, 162(1): 124-131. |
| [17] | TOMINAKA S, MOMMAB T, OSAKA T.Electrodeposited Pd-Co catalyst for direct methanol fuel cell electrodes: preparation and characterization.Electrochim. Acta, 2008, 53(14): 4679-4686. |
| [18] | DUMBUYA K, DENECKE R, STEINRUCK H P.Surface analysis of Pd/ZnO catalysts dispersed on micro-channeled Al-foils by XPS.Appl. Catal. A: Gen., 2008, 348(2): 209-213. |
| [19] | ZHANG L L, TANG Y W, BAO J C, et al.A carbon-supported Pd-P catalyst as the anodic catalyst in a direct formic acid fuel cell.J. Power Sources, 2006, 162(1): 177-179. |
| [20] | PERSSON K, ERSSON A, JANSSON K, et al.Influence of co-metals on bimetallic palladium catalysts for methane combustion.J. Catal., 2005, 31(1): 139-150. |
| [21] | DIMITRATOS N, VILLA A, WANG D, et al.Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols.J. Catal., 2006, 244(1): 113-121. |
| [22] | SUO Y, ZHUANG L, LU J.First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction.Angew. Chem. Int. Ed., 2007, 46(16): 2862-2864. |
| [23] | LI X W, HUANG Q H, ZOU Z Q, et al.Low temperature preparation of carbon-supported Pd-Co alloy electrocatalysts for methanol-tolerant oxygen reduction reaction.Electrochim. Acta, 2008, 53(22): 6662-6667. |
| [1] | LIU Lei, GUO Ruihua, WANG Li, WANG Yan, ZHANG Guofang, GUAN Lili. Oxygen Reduction Reaction on Pt3Co High-index Facets by Density Functional Theory [J]. Journal of Inorganic Materials, 2025, 40(1): 39-46. |
| [2] | YANG Daihui, SUN Tian, TIAN Hexin, SHI Xiaofei, MA Dongwei. Iron-nitrogen-codoped Mesoporous Carbon: Facile Synthesis and Catalytic Performance of Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2023, 38(11): 1309-1315. |
| [3] | SUN Lian, GU Quanchao, YANG Yaping, WANG Honglei, YU Jinshan, ZHOU Xingui. Two-dimensional Transition Metal Dichalcogenides for Electrocatalytic Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2022, 37(7): 697-709. |
| [4] | JIANG Lili, XU Shuaishuai, XIA Baokai, CHEN Sheng, ZHU Junwu. Defect Engineering of Graphene Hybrid Catalysts for Oxygen Reduction Reactions [J]. Journal of Inorganic Materials, 2022, 37(2): 215-222. |
| [5] | LIU Ziruo, LIU Wei, HAO Ce, HU Jinwen, SHI Yantao. Honeycomb-like Carbon-supported Fe Single Atom Catalyst: Preparation and Electrocatalytic Performance in Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(9): 943-949. |
| [6] | HAO Ce, LIU Ziruo, LIU Wei, SHI Yantao. Research Progress of Carbon-supported Metal Single Atom Catalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2021, 36(8): 820-834. |
| [7] | ZHU Yong, GU Jun, YU Tao, HE Haitong, YAO Rui. Synthesis and Property of Platinum-cobalt Alloy Nano Catalyst [J]. Journal of Inorganic Materials, 2021, 36(3): 299-305. |
| [8] | DING Sheng, NING Kai, YUAN Binxia, PAN Weiguo, YIN Shibin, LIU Jianfeng. Durability of Fe-N/C Catalysts with Different Nanostructures for Electrochemical Oxygen Reduction in Alkaline Solution [J]. Journal of Inorganic Materials, 2020, 35(8): 953-958. |
| [9] | HE Wang-Tao, MA Ru-Guang, ZHU Yu-Fang, YANG Ming-Jie, WANG Jia-Cheng. Renewable Porous Carbons Prepared by KOH Activation as Oxygen Reduction Electrocatalysts [J]. Journal of Inorganic Materials, 2019, 34(10): 1115-1122. |
| [10] | XIE Yang-En, WANG Ding-Ling, MA Zhao-Kun, SONG Huai-He, XU Pei. Fe-N Modified Carbon Black as a High-performance and Cost-effective Cathode Catalyst in Microbial Fuel Cells [J]. Journal of Inorganic Materials, 2018, 33(3): 295-300. |
| [11] | CAO Zhao-Xia, DING Yan-Min, WANG Zhi-Chao, MAO Xin-Xin, YIN Yan-Hong, YANG Shu-Ting. Porous Calcium Manganese Oxide: Preparation and Electrocatalytic Activity of Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2017, 32(5): 535-542. |
| [12] | LI Shu-Ling, YUAN Xian-Xia, KONG Hai-Chuan, XU Jin, MA Zi-Feng. Fe-PPy-TsOH/C as Cathode Catalyst for Proton Exchange Membrane Fuel Cells [J]. Journal of Inorganic Materials, 2017, 32(4): 393-399. |
| [13] | XU Hong-Mei, ZHANG Hua, LI Heng, JIAN Yao-Yong, XIE Wu, WANG Yi-Ping, XU Ming-Ze. Preparation and Oxygen-reduction Mechanism Investigation of Nanostructure LSCF-SDC Composite Cathodes [J]. Journal of Inorganic Materials, 2017, 32(4): 379-385. |
| [14] | SHI Qi, LEI Yong-Peng, WANG Ying-De, WANG Zhong-Min. In-situ Preparation and Electrocatalytic Oxygen Reduction Performance of N-doped Graphene@CNF [J]. Journal of Inorganic Materials, 2016, 31(4): 351-357. |
| [15] | ZHANG Yu-Hui, YI Qing-Feng, LIU Xiao-Ping, XIANG Bai-Lin. Carbonizing Products of the Fe/Co Doped Polypyrrole as Efficient Electrocatalysts for Oxygen Reduction Reaction [J]. Journal of Inorganic Materials, 2014, 29(3): 269-274. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||