
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (5): 601-607.DOI: 10.15541/jim20190144
Special Issue: 生物材料论文精选(2020)
Previous Articles Next Articles
LIU Ziyang,GENG Zhen,LI Zhaoyang
Received:2019-04-04
Revised:2019-04-24
Published:2020-05-20
Online:2019-05-29
Supported by:CLC Number:
LIU Ziyang, GENG Zhen, LI Zhaoyang. Preparing Biomedical CaCO3/HA Composite with Oyster Shell[J]. Journal of Inorganic Materials, 2020, 35(5): 601-607.
| Element | Calcined product /(mg·kg-1) | Limit/(mg·kg-1) |
|---|---|---|
| Pb | <10 | 30 |
| Cd | <1.5 | 5 |
| Hg | <1 | 5 |
| As | <1 | 3 |
Table 1 Heavy metal content of the calcined products of oyster shells
| Element | Calcined product /(mg·kg-1) | Limit/(mg·kg-1) |
|---|---|---|
| Pb | <10 | 30 |
| Cd | <1.5 | 5 |
| Hg | <1 | 5 |
| As | <1 | 3 |
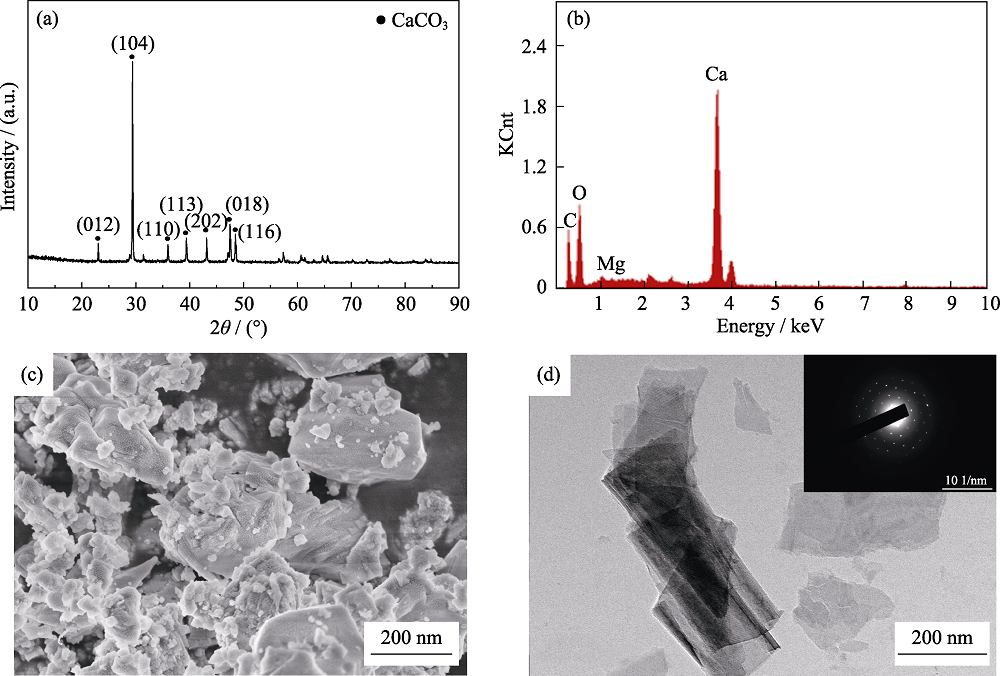
Fig. 1 (a) XRD pattern, (b) EDS analysis, (c) SEM micrograph and (d) TEM image and SAED pattern of the powders prepared by heating and washing the oyster shell
| 20%HA | 40%HA | 60%HA | |
|---|---|---|---|
| Concentration of Ca/(mg·L-1) | (19.91±0.72) | (20.00±1.08) | (20.02±0.52) |
| Concentration of P/(mg·L-1) | (1.62±0.17) | (3.19±0.22) | (4.02±0.19) |
| Degree of HA transition/% | (17.5±0.57) | (34.3±1.02) | (43.2±0.33) |
Table 2 Ca and P concentrations and HA transition degree of samples
| 20%HA | 40%HA | 60%HA | |
|---|---|---|---|
| Concentration of Ca/(mg·L-1) | (19.91±0.72) | (20.00±1.08) | (20.02±0.52) |
| Concentration of P/(mg·L-1) | (1.62±0.17) | (3.19±0.22) | (4.02±0.19) |
| Degree of HA transition/% | (17.5±0.57) | (34.3±1.02) | (43.2±0.33) |
| 20%HA | 40%HA | 60%HA | CaCO3 | |||||
|---|---|---|---|---|---|---|---|---|
| Degradation time/d | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% |
| 7 | (16.13±0.40) | 8.1 | (13.39±0.23) | 6.7 | (11.61±0.92) | 5.8 | (18.93±0.17) | 9.8 |
| 14 | (30.26±1.12) | 15.2 | (24.02±0.83) | 12.0 | (21.62±1.36) | 10.8 | (42.70±0.19) | 22.1 |
| 21 | (43.20±1.47) | 21.7 | (33.40±1.38) | 16.2 | (28.11±1.07) | 14.3 | (56.41±0.32) | 29.2 |
Table 3 Ca2+ concentration and degradation rate of samples
| 20%HA | 40%HA | 60%HA | CaCO3 | |||||
|---|---|---|---|---|---|---|---|---|
| Degradation time/d | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% | Concentration of Ca2+/(mg·L-1) | Degradation rate/% |
| 7 | (16.13±0.40) | 8.1 | (13.39±0.23) | 6.7 | (11.61±0.92) | 5.8 | (18.93±0.17) | 9.8 |
| 14 | (30.26±1.12) | 15.2 | (24.02±0.83) | 12.0 | (21.62±1.36) | 10.8 | (42.70±0.19) | 22.1 |
| 21 | (43.20±1.47) | 21.7 | (33.40±1.38) | 16.2 | (28.11±1.07) | 14.3 | (56.41±0.32) | 29.2 |
| [1] |
MEHDI S S, KHORASANI M T, EHSAN D K , et al. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater., 2013; 9(8):7591-7621.
DOI URL PMID |
| [2] |
BASHA R Y, SAMPATH K T S, DOBLE M . Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C, 2015,57:452-463.
DOI URL PMID |
| [3] |
FACCA S, LAHIRI D, FIORETTI F , et al. In vivo osseointegration of nanodesigned composite coatings on titanium implants. ACS Nano, 2011,5(6):4790-4799.
DOI URL PMID |
| [4] | RHAITI H, LAGHZIZIL A, SAOIABI A , et al. Surface properties of porous hydroxyapatite derived from natural phosphate. Mater. Chem. Phys., 2012,136(2):1022-1026. |
| [5] | ZHANG C Y, ZHANG S Y, LIU X P , et al. Degradation behavior of MgF2/HA composite coating on magnesium alloy in vitro. The Chinese Journal of Nonferrous Metals, 2018,28(4):766-773. |
| [6] | LEMOS A F, ROCHA J H G, FERREIRA J M F , et al. Hydroxyapatite nano-powders produced hydrothermally from nacreous material.Journal of the European Ceramic Society. 2006, 26(16):3639-3646. |
| [7] |
VECCCHIO K S, ZHANG X, MASSIE J B , et al. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomaterials, 2007,3(6):910-918.
DOI URL PMID |
| [8] | LANDI E, CELOTTI G, LOGROSCINO G , et al. Carbonated hydroxyapatite as bone substitute. Journal of the European Ceramic Society, 2003,23(15):2931-2937. |
| [9] | LI L, ZHU Y J, CAO S W , et al. Preparation and drug release properties of nanostructured CaCO3 porous hollow microspheres. Journal of Inorganic Materials, 2009,24(1):166-170. |
| [10] | GUAN J K, XU Z Y, CHEN B , et al. Preparation and properties of nanometer calcium carbonate/poly (L-lactide) composite materials. Journal of Clinical Rehabilitative Tissue Engineering Research, 2008,12(10):1889-1891. |
| [11] | ZHAO H, CHEN Y, LI Z M , et al. Preparation of nanoribbon hydroxyapatite from discarded shells. Gansu Science and Technology, 2016,32(17):50-52. |
| [12] | XUE Q H, XING Y Z, ZHANG Q Q . Preparation technology of oyster hydroxyapatite porous material for bone repair. Int. J. Biomed. Eng., 2018,41(4):291-295. |
| [13] |
YANG S L, GAO Q, WANG H T . Theoretical prediction of ring strain energies of several carbon-monocyclic and carbon-dicyclic compounds. Journal of Natural Science of Heilongjiang University, 2010,27(4):495-499.
DOI URL PMID |
| [14] | RUJITANAPANICH S, KUMPAPAN P, WANJANOI P , et al. Synthesis of hydroxyapatite from oyster shell via precipitation method. Energy Proc., 2014, ( 56):112-117. |
| [15] | AKRAM M, AHMED R, SHAKIR I , et al. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014; 49(4):1461-1475. |
| [16] | NAYAR S, GUHA A . Waste utilization for the controlled synthesis of nanosized hydroxyapatite. Mater. Sci. Eng., 2009,29(4):1326-1329. |
| [17] |
LANDI E, SPRIO S, SANDRI M , et al. Development of Sr and CO3 co-substituted hydroxyapatites for biomedical applications. Acta Biomaterialia, 2008,4(3):656-663.
DOI URL PMID |
| [18] |
MEEJOO S, MANEEPRAKORN W, WINOTAI P . Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochimica Acta, 2006,447(1):115-120.
DOI URL |
| [19] |
HU GF, XIAO LW, FU H , et al. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. Journal of Materials Science: Materials in Medicine, 2010,21(2):627-634.
DOI URL PMID |
| [20] |
QI X, YE J, WANG Y . Improved injectability and in vitro degradation of a calcium phosphate cement containing poly (lactide-co- glycolide) microspheres. Acta Biomaterialia, 2008,4(6):1837-1845.
DOI URL PMID |
| [21] |
SRINATH PALAKURTHY, VENU GOPAL REDDY K, RAJ KUMAR SAMUDRALA , et al. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Materials Science & Engineering, 2019,98:109-117.
DOI URL PMID |
| [1] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [2] | LI Chengyu, DING Ziyou, HAN Yingchao. In vitro Antibacterial and Osteogenic Properties of Manganese Doped Nano Hydroxyapatite [J]. Journal of Inorganic Materials, 2024, 39(3): 313-320. |
| [3] | CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion [J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282. |
| [4] | LIU Yan, ZHANG Yufan, WANG Ximan, LI Ting, MA Wenting, YANG Fuwei, CHEN Liang, ZHAO Dongyue, YAN Xiaoqin. Consolidation of Fragile Weathered Bone Relics Using Hydroxyapatite Material as Consolidant [J]. Journal of Inorganic Materials, 2023, 38(11): 1345-1354. |
| [5] | CHEN Yaling, SHU Song, WANG Shaoxin, LI Jianjun. Mn-HAP SCR Catalyst: Preparation and Sulfur Resistance [J]. Journal of Inorganic Materials, 2022, 37(10): 1065-1072. |
| [6] | ZHU Yutong, TAN Peijie, LIN Hai, ZHU Xiangdong, ZHANG Xingdong. Injectable Hyaluronan/Hydroxyapatite Composite: Preparation, Physicochemical Property and Biocompatibility [J]. Journal of Inorganic Materials, 2021, 36(9): 981-990. |
| [7] | LIN Ziyang, CHANG Yuchen, WU Zhangfan, BAO Rong, LIN Wenqing, WANG Deping. Different Simulated Body Fluid on Mineralization of Borosilicate Bioactive Glass-based Bone Cement [J]. Journal of Inorganic Materials, 2021, 36(7): 745-752. |
| [8] | WU Zhongcao, HUAN Zhiguang, ZHU Yufang, WU Chengtie. 3D Printing and Characterization of Microsphere Hydroxyapatite Scaffolds [J]. Journal of Inorganic Materials, 2021, 36(6): 601-607. |
| [9] | WU Yonghao, LI Xiangfeng, ZHU Xiangdong, ZHANG Xingdong. Construction of Hydroxyapatite Nanoceramics with High Mechanical Strength and Efficiency in Promoting the Spreading and Viability of Osteoblasts [J]. Journal of Inorganic Materials, 2021, 36(5): 552-560. |
| [10] | SONG Keke, HUANG Hao, LU Mengjie, YANG Anchun, WENG Jie, DUAN Ke. Hydrothermal Preparation and Characterization of Zn, Si, Mg, Fe Doped Hydroxyapatite [J]. Journal of Inorganic Materials, 2021, 36(10): 1091-1096. |
| [11] | SHAO Yueting, ZHU Yingjie, DONG Liying, CAI Anyong. Nanocomposite “Xuan Paper” Made from Ultralong Hydroxyapatite Nanowires and Cellulose Fibers and Its Anti-mildew Properties [J]. Journal of Inorganic Materials, 2021, 36(1): 107-112. |
| [12] | SUN Tuanwei,ZHU Yingjie. One-step Solvothermal Synthesis of Strontium-doped Ultralong Hydroxyapatite Nanowires [J]. Journal of Inorganic Materials, 2020, 35(6): 724-728. |
| [13] | DU Xudong, TANG Chengyuan, YANG Xiaoli, CHENG Jianbo, JIA Yuke, YANG Shubin. High-efficiency Biogenic Calcium Carbonate for Adsorption of Pb(II) and Methyl Orange from Wastewater [J]. Journal of Inorganic Materials, 2020, 35(3): 315-323. |
| [14] | DAI Zhao,WANG Ming,WANG Shuang,LI Jing,CHEN Xiang,WANG Da-Lin,ZHU Ying-Chun. Zirconia Reinforced Trace Element Co-doped Hydroxyapatite Coating [J]. Journal of Inorganic Materials, 2020, 35(2): 179-186. |
| [15] | FU Ya-Kang,WENG Jie,LIU Yao-Wen,ZHANG Ke-Hong. hBMP-2 Contained Composite Coatings on Titanium Mesh Surface: Preparation and hBMP-2 Release [J]. Journal of Inorganic Materials, 2020, 35(2): 173-178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||