
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (7): 687-694.DOI: 10.3724/SP.J.1077.2014.13494
• Orginal Article • Previous Articles Next Articles
XU Run1, LI Xin-Da1, LI Zhi-Qiang1, ZHAO Ren-Yu1, FAN Gen-Lian1, XIONG Ding-Bang1, TANG Jie1, XU Yong2, ZHANG Di1
Received:2013-09-29
Revised:2013-12-25
Published:2014-07-20
Online:2014-06-20
About author:XU Run. E-mail: x.r.my.mail@gmail.com
Supported by:CLC Number:
XU Run, LI Xin-Da, LI Zhi-Qiang, ZHAO Ren-Yu, FAN Gen-Lian, XIONG Ding-Bang, TANG Jie, XU Yong, ZHANG Di. Preparation and Reaction Kinetics of Carbon Nanotubes/Aluminum Composite Powders Using Polymer Pyrolysis Method[J]. Journal of Inorganic Materials, 2014, 29(7): 687-694.
Add to citation manager EndNote|Ris|BibTeX
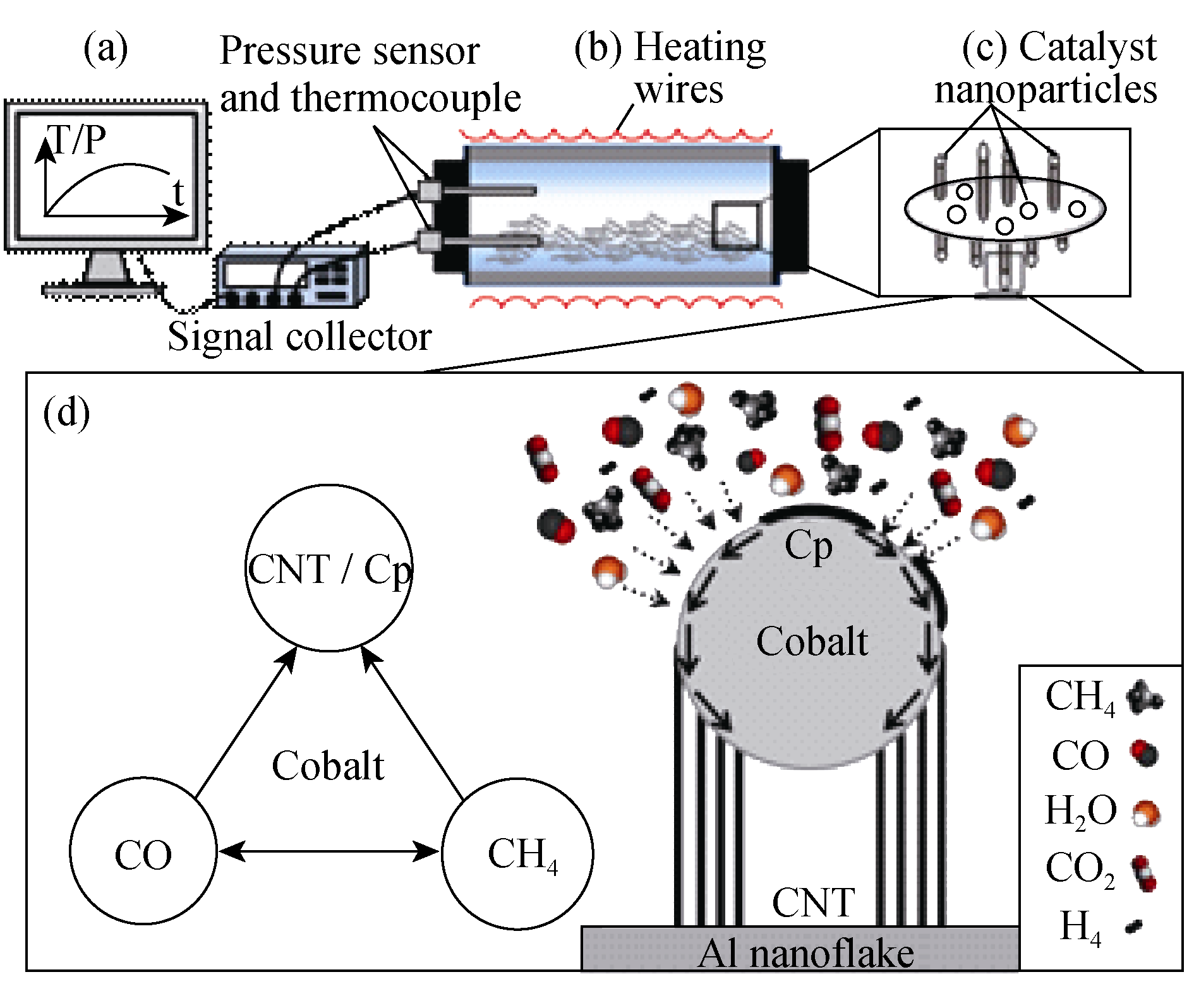
Fig. 1 Schematic drawing of CNTs grown by PP-CVD (a) The temperature and pressure monitoring system; (b) The closed batch reactor; (c) Al nanoflakes with Co nanoparticles and CNTs as grown; (d) The gas-solid reaction pathway and tip growth mode of CNTs. The dashed arrows represent collision of gaseous species and the solid arrows represent surface diffusion of carbon atoms around the Co nanoparticle
| Symbols | Definition |
|---|---|
| Cp | Encapsulating carbon |
| (S) | Surface species |
| C(S) | Active carbon atom |
| | The forward (f ) reaction rate constant of the i th reaction |
| | The reverse (r) reaction rate constant of the i th reaction |
| A | The Arrhenius pre-exponential factor |
| | The Arrhenius temperature coefficient |
| Ei | The activation energy of the i th reaction |
| R | The universal gas constant |
| | The change in the Gibbs free energy of formation as a result of the reaction |
| | The net stoichiometric coefficient for species j in i th reaction |
| qi | The rate of progress of the i th reaction |
| Rj | The net rate of production of species j |
| Cj | The volumetric concentration of species j |
| Vdeposited | The volume of deposited carbon atoms |
| | The volume of encapsulating carbon |
| VC | The volume of a carbon atom |
| | The density of Co nanoparticles |
| | CNTs growth rate |
| d | The diameter of Co nanoparticles |
| NA | Avogadro constant |
| Do | The surface diffusion coefficient of carbon atoms |
Table 1 Nomenclature of the symbols used in the modeling
| Symbols | Definition |
|---|---|
| Cp | Encapsulating carbon |
| (S) | Surface species |
| C(S) | Active carbon atom |
| | The forward (f ) reaction rate constant of the i th reaction |
| | The reverse (r) reaction rate constant of the i th reaction |
| A | The Arrhenius pre-exponential factor |
| | The Arrhenius temperature coefficient |
| Ei | The activation energy of the i th reaction |
| R | The universal gas constant |
| | The change in the Gibbs free energy of formation as a result of the reaction |
| | The net stoichiometric coefficient for species j in i th reaction |
| qi | The rate of progress of the i th reaction |
| Rj | The net rate of production of species j |
| Cj | The volumetric concentration of species j |
| Vdeposited | The volume of deposited carbon atoms |
| | The volume of encapsulating carbon |
| VC | The volume of a carbon atom |
| | The density of Co nanoparticles |
| | CNTs growth rate |
| d | The diameter of Co nanoparticles |
| NA | Avogadro constant |
| Do | The surface diffusion coefficient of carbon atoms |
| The initial pressure /(1.013×105, Pa) | The initial partial pressure /(1.013×105, Pa) | ||||
|---|---|---|---|---|---|
| CH4 | CO | CO2 | H2O | H2 | |
| 22 | 1.61 | 6.24 | 6.18 | 5.65 | 1.53 |
Table 2 Composition of the PEG-CA-Co(II) pyrolysis gases in the batch reactor
| The initial pressure /(1.013×105, Pa) | The initial partial pressure /(1.013×105, Pa) | ||||
|---|---|---|---|---|---|
| CH4 | CO | CO2 | H2O | H2 | |
| 22 | 1.61 | 6.24 | 6.18 | 5.65 | 1.53 |
| Species | CH4 | CO | CO2 |
|---|---|---|---|
| Projected areaa | 2.43×10-2 | 2.01×10-2 | 3.45×10-2 |
| Ratiob | 3656 | 4388 | 2531 |
| Active site density c | 1.72×10-9 | 2.06×10-9 | 1.19×10-9 |
Table 3 The ratio of Co nanoparticle surface area to the projected area of carbon source gas molecules
| Species | CH4 | CO | CO2 |
|---|---|---|---|
| Projected areaa | 2.43×10-2 | 2.01×10-2 | 3.45×10-2 |
| Ratiob | 3656 | 4388 | 2531 |
| Active site density c | 1.72×10-9 | 2.06×10-9 | 1.19×10-9 |
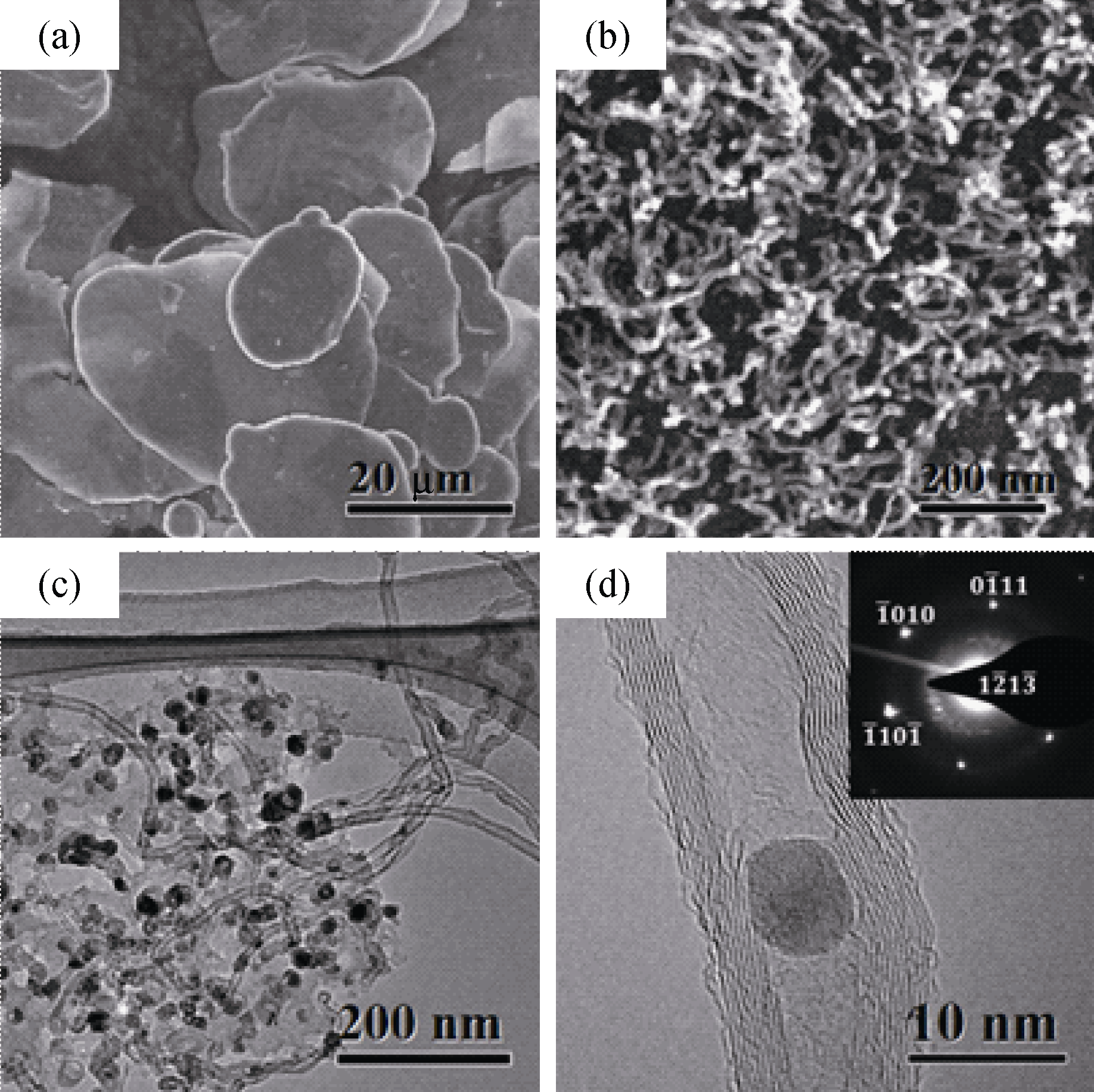
Fig. 3 Morphologies of CNTs at different scales (a) SEM image of the Al flake substrates; (b) SEM image of the CNTs; (c) TEM image of the CNTs; (d) HRTEM image of the CNTs and SEAD pattern of catalyst particle
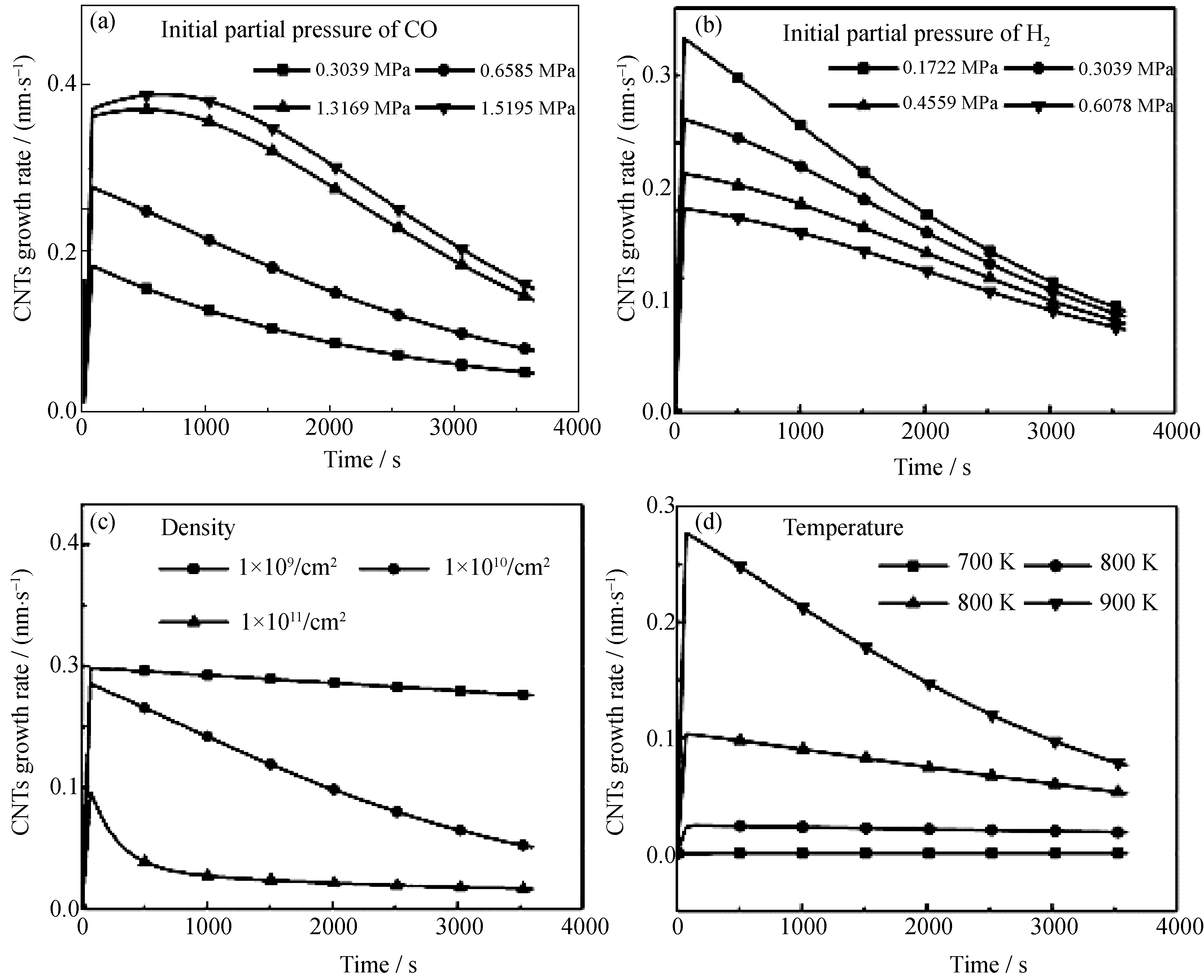
Fig.4 Effects of different vatiables in the modeling on the CNT growth rates in 60 min (a) Partial pressure of CO; (b) Partial pressure of H2; (c) Co nanoparticles density; (c) Reaction temperature
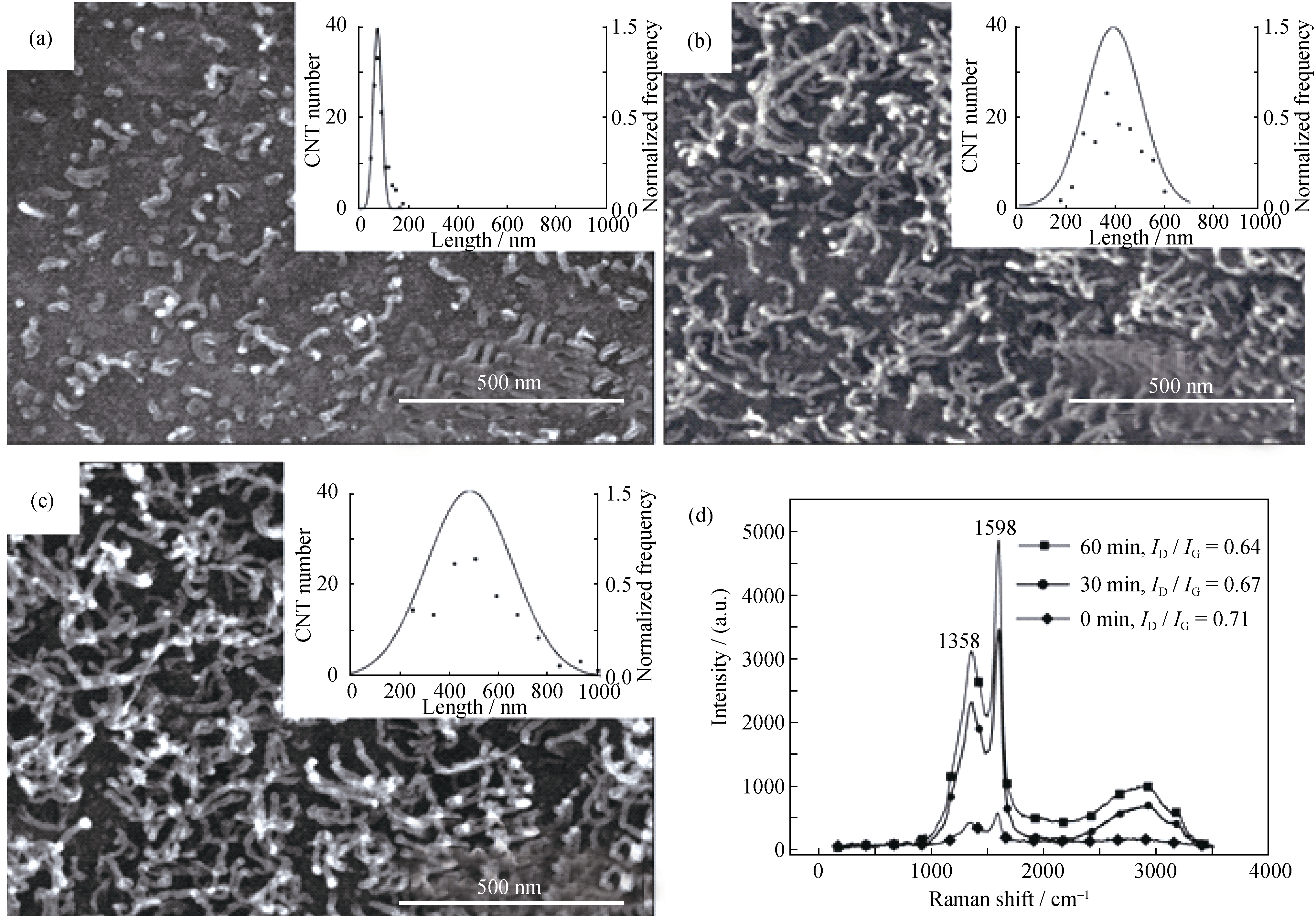
Fig.6 SEM images with length and normalized frequency statistic (inset) of the CNTs after grown at 873 K for (a) 0 min, (b) 30 min and (c) 60 min. (d) Raman spectra of the CNT/Al composite powders The lengths of CNTs were measured on the corresponding SEM images randomly, and 120 CNTs were counted at each reaction time
| [1] | TREACY M M J, EBBESEN T W, GIBSON J M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature, 1996, 381: 678-680. |
| [2] | ESAWI A M K, MORSI K, SAYED A, et al. Effect of carbon nanotube (CNT) content on the mechanical properties of CNT-reinforced aluminium composites. Composites Science and Technology, 2010, 70(16): 2237-2241. |
| [3] | GEORGE R, KASHYAP K T, RAHUL R, et al. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scripta Materialia, 2005, 53(10): 1159-1163. |
| [4] | JIANG L, LI Z, FAN G, et al. The use of flake powder metallurgy to produce carbon nanotube (CNT)/aluminum composites with a homogenous CNT distribution. Carbon, 2012, 50(5): 1993-1998. |
| [5] | DENG C, ZHANG X X, WANG D, et al. Preparation and characterization of carbon nanotubes/aluminum matrix composites. Materials Letters, 2007, 61(8): 1725-1728. |
| [6] | BAKSHI S R, AGARWAL A. An analysis of the factors affecting strengthening in carbon nanotube reinforced aluminum composites. Carbon, 2011, 49(2): 533-544. |
| [7] | BAKSHI S R, LAHIRI D, AGARWAL A. Carbon nanotube reinforced metal matrix composites: a review. International Materials Reviews, 2010, 55(1): 41-64. |
| [8] | WANG L, CHOI H, MYOUNG J M, et al. Mechanical alloying of multi-walled carbon nanotubes and aluminium powders for the preparation of carbon/metal composites. Carbon, 2009, 47(15): 3427-3433. |
| [9] | POIRIER D, GAUVIN R, DREW R A L. Structural characterization of a mechanically milled carbon nanotube/aluminum mixture. Composites Part A: Applied Science and Manufacturing, 2009, 40(9): 1482-1489. |
| [10] | CHA S I, KIM K T, ARSHAD S N, et al. Extraordinary strengthening effect of carbon nanotubes in metal-matrix nanocomposites processed by molecular-level mixing. Advanced Materials, 2005, 17(11): 1377-1381. |
| [11] | HE C, ZHAO N, SHI C, et al. An approach to obtaining homogeneously dispersed carbon nanotubes in al powders for preparing reinforced al-matrix composites. Advanced Materials, 2007, 19(8): 1128-1132. |
| [12] | CAO L, LI Z, FAN G, et al. The growth of carbon nanotubes in aluminum powders by the catalytic pyrolysis of polyethylene glycol. Carbon, 2012, 50(3): 1057-1062. |
| [13] | TANG J, FAN G, LI Z, et al. Synthesis of carbon nanotube/ aluminium composite powders by polymer pyrolysis chemical vapor deposition. Carbon, 2013, 55(1): 202-208. |
| [14] | GRUJICIC M, CAO G, GERSTEN B. Optimization of the chemical vapor deposition process for carbon nanotubes fabrication. Applied Surface Science, 2002, 191(1): 223-239. |
| [15] | GRUJICIC M, CAO G, GERSTEN B. An atomic-scale analysis of catalytically-assisted chemical vapor deposition of carbon nanotubes. Materials Science and Engineering: B, 2002, 94(2): 247-259. |
| [16] | LOUCHEV O A, LAUDE T, SATO Y, et al. Diffusion-controlled kinetics of carbon nanotube forest growth by chemical vapor deposition. Journal of Chemical Physics, 2003, 118(16): 7622-7634. |
| [17] | MA H, PAN L, NAKAYAMA Y. Modelling the growth of carbon nanotubes produced by chemical vapor deposition. Carbon, 2011, 49(3): 854-861. |
| [18] | CHEN D, LØDENG R, ANUNDSKÅS A, et al. Deactivation during carbon dioxide reforming of methane over Ni catalyst: microkinetic analysis. Chemical Engineering Science, 2001, 56(4): 1371-1379. |
| [19] | KEE R J, RUPLEY F M, MILLER J A, et al. Chemkin Release 4.1, Reaction Design, San Diego, CA (2006). |
| [20] | CHASE M W. NIST-JANAF. Thermochemical Tables. 4th ed. Gaithersburg: ACS & AIP, 1998. |
| [21] | COLTRIN M E, DANDY D S. Analysis of diamond growth in subatmospheric dc plasma-gun reactors. Journal of Applied Physics, 1993, 74(9): 5803-5820. |
| [22] | FOGLER H. Elements of Chemical Reaction Engineering. 4th edn. New York: Prentice Hall, 2006. |
| [23] | TESSONNIER J P, SU D S. Recent progress on the growth mechanism of carbon nanotubes: a review. Chem. Sus. Chem., 2011, 4(7): 824-847. |
| [24] | KUMAR M, ANDO Y. Chemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass production. Journal of Nanoscience and Nanotechnology, 2010, 10(6): 3739-3758. |
| [25] | LYSAGHT A C, CHIU W K S. Modeling of the carbon nanotube chemical vapor deposition process using methane and acetylene precursor gases. Nanotechnology, 2008, 19(16): 165607. |
| [26] | STORSÆTER S, CHEN D, HOLMEN A. Microkinetic modelling of the formation of C1 and C2 products in the Fischer-Tropsch synthesis over cobalt catalysts. Surface Science, 2006, 600(10): 2051-2063. |
| [27] | ZADEH J S M, SMITH K J. Kinetics of CH4 decomposition on supported cobalt catalysts. Journal of Catalysis, 1998, 176: 115-124. |
| [28] | PURETZKY A A, GEOHEGAN D B, JESSE S, et al. In situ measurements and modeling of carbon nanotube array growth kinetics during chemical vapor deposition. Applied Physics A, 2005, 81(2): 223-240. |
| [29] | YOKOYAMA H, NUMAKURA H, KOIWA M. The solubility and diffusion of carbon in palladium. Acta Materialia, 1998, 46(8): 2823-2830. |
| [30] | HUANG C W, LI Y Y. In situ synthesis of platelet graphite nanofibers from thermal decomposition of poly (ethylene glycol). The Journal of Physical Chemistry B, 2006, 110(46): 23242-23246. |
| [31] | 宋玉强, 李世春, 杨泽亮. Al/Co相界面的扩散溶解层. 焊接学报, 2008, 29(12): 8-12. |
| [1] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [2] | SUN Jing, LI Xiang, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Effect of Lauric Acid Modifier on the Hydrolysis Resistance of Aluminum Nitride Powders [J]. Journal of Inorganic Materials, 2025, 40(7): 826-832. |
| [3] | XIONG Siyu, MO Chen, ZHU Xiaowei, ZHU Guobin, CHEN Deqin, LIU Laijun, SHI Xiaodong, LI Chunchun. Low-temperature Sintering of LiBxAl1-xSi2O6 Microwave Dielectric Ceramics with Ultra-low Permittivity [J]. Journal of Inorganic Materials, 2025, 40(5): 536-544. |
| [4] | XIN Zhenyu, GUO Ruihua, WUREN Tuoya, WANG Yan, AN Shengli, ZHANG Guofang, GUAN Lili. Pt-Fe/GO Nanocatalysts: Preparation and Electrocatalytic Performance on Ethanol Oxidation [J]. Journal of Inorganic Materials, 2025, 40(4): 379-387. |
| [5] | FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders [J]. Journal of Inorganic Materials, 2025, 40(1): 104-110. |
| [6] | CHENG Bo, AN Xiaohang, LI Dinghua, YANG Rongjie. Flame-retardant Properties and Transformation of Flame-retardant Mechanisms of EVA: Effect of ATH/ADP Ratio [J]. Journal of Inorganic Materials, 2024, 39(5): 509-516. |
| [7] | ZHAO Yawen, QU Fajin, WANG Yanyi, WANG Zhiwen, CHEN Chusheng. Preparation and Properties of Aluminum Silicate Fiber Supported PtTFPP-PDMS Flexible Oxygen Sensing Components [J]. Journal of Inorganic Materials, 2024, 39(10): 1084-1090. |
| [8] | WU Jun, XU Peifei, JING Rui, ZHANG Dahai, FEI Qingguo. Experimental Study on Low-velocity Impact and Residual Strength of SiC/SiC Composite Laminates [J]. Journal of Inorganic Materials, 2024, 39(1): 51-60. |
| [9] | SHI Weigang, ZHANG Chao, LI Mei, WANG Jing, ZHANG Chengyu. 2D-SiCf/SiC Interlaminar Mode I Fracture Testing and Characterization [J]. Journal of Inorganic Materials, 2024, 39(1): 45-50. |
| [10] | CHEN Haiyan, TANG Zhipeng, YIN Liangjun, ZHANG Linbo, XU Xin. Low-frequency Microwave Absorption of CIPs@Mn0.8Zn0.2Fe2O4-CNTs Composites [J]. Journal of Inorganic Materials, 2024, 39(1): 71-80. |
| [11] | WANG Bo, YU Jian, LI Cuncheng, NIE Xiaolei, ZHU Wanting, WEI Ping, ZHAO Wenyu, ZHANG Qingjie. Service Stability of Gd/Bi0.5Sb1.5Te3 Thermo-electro-magnetic Gradient Composites [J]. Journal of Inorganic Materials, 2023, 38(6): 663-670. |
| [12] | ZHANG Shuo, FU Qiangang, ZHANG Pei, FEI Jie, LI Wei. Influence of High Temperature Treatment of C/C Porous Preform on Friction and Wear Behavior of C/C-SiC Composites [J]. Journal of Inorganic Materials, 2023, 38(5): 561-568. |
| [13] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| [14] | JIA Yuna, CAO Xu, JIAO Xiuling, CHEN Dairong. Preparation of Alumina Ceramic Continuous Fibers with Inorganic Acidic Aluminum Sol as Precursor [J]. Journal of Inorganic Materials, 2023, 38(11): 1257-1264. |
| [15] | SUN Ming, SHAO Puzhen, SUN Kai, HUANG Jianhua, ZHANG Qiang, XIU Ziyang, XIAO Haiying, WU Gaohui. First-principles Study on Interface of Reduced Graphene Oxide Reinforced Aluminum Matrix Composites [J]. Journal of Inorganic Materials, 2022, 37(6): 651-659. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||