
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (11): 1229-1236.DOI: 10.15541/jim20250092
• RESEARCH ARTICLE • Previous Articles Next Articles
HU Xuemin1,2( ), ZHANG Xingjian1, JIANG Zhihao1, HUANG Liwen1, DING Kaining3, ZHANG Shengli2(
), ZHANG Xingjian1, JIANG Zhihao1, HUANG Liwen1, DING Kaining3, ZHANG Shengli2( )
)
Received:2025-03-03
Revised:2025-05-18
Published:2025-11-20
Online:2025-06-03
Contact:
ZHANG Shengli, professor. E-mail: zhangslvip@njust.edu.cnAbout author:HU Xuemin (1986-), female, PhD. E-mail: huxm@jit.edu.cn
Supported by:CLC Number:
HU Xuemin, ZHANG Xingjian, JIANG Zhihao, HUANG Liwen, DING Kaining, ZHANG Shengli. First-principles Study on Oxygen Evolution Reaction Activity of CoPS3 Quantum Dots Edge States Modified with Oxygen[J]. Journal of Inorganic Materials, 2025, 40(11): 1229-1236.
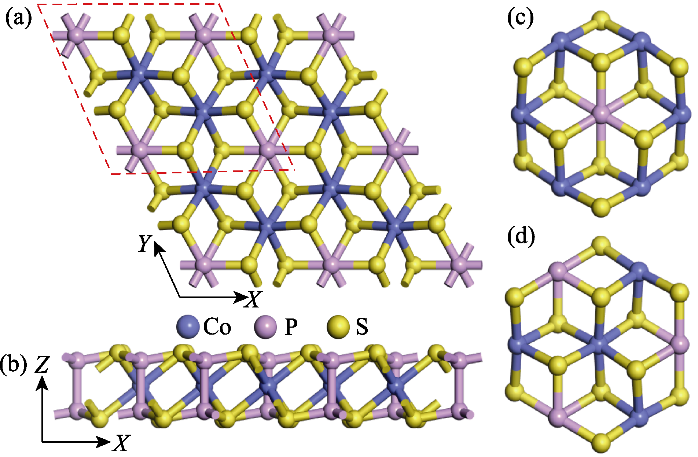
Fig. 1 Monolayer crystal structure model and quantum dots model of CoPS3 (a) Top and (b) side views of the monolayer CoPS3 structure model; (c, d) Top views of (c) CoPS3-QDs1 and (d) CoPS3-QDs2; Red dashed line in (a) indicating unit cell of CoPS3
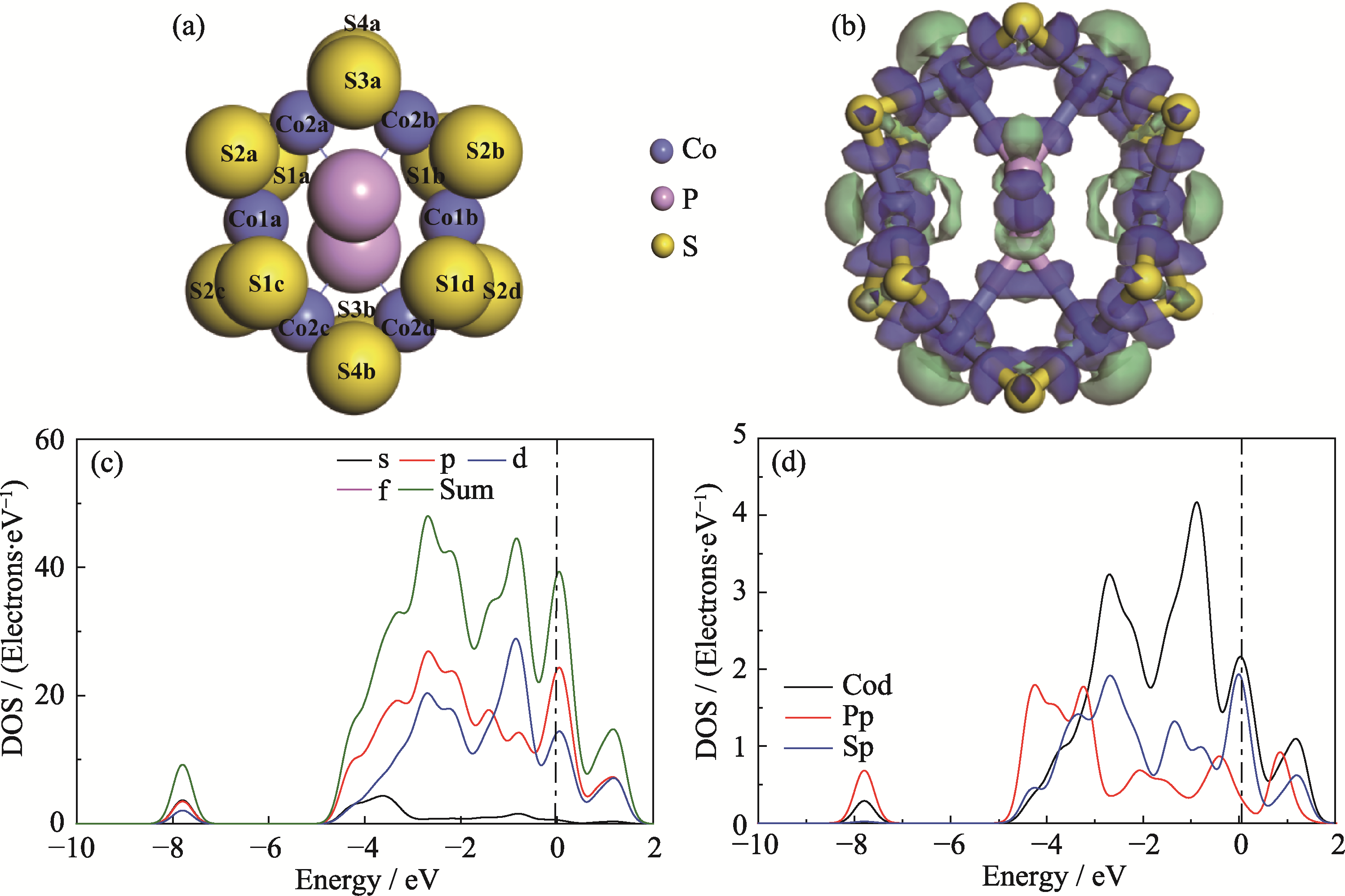
Fig. 2 Optimized structure model and electronic property data of CoPS3-QDs1 (a) Top view of the optimized CoPS3-QDs1 structure model, where S1-S4, Co1, and Co2 represent six inequivalent edge atomic positions obtained from Mulliken charge calculations; (b) Charge density difference map of CoPS3-QDs1, where blue regions indicate electron accumulation and green regions indicate electron depletion, with isovalue=0.048 e/Å3; (c) Orbital-resolved partial density of states (PDOS) and (d) atom-resolved PDOS of CoPS3-QDs1. Colorful figures are available on website
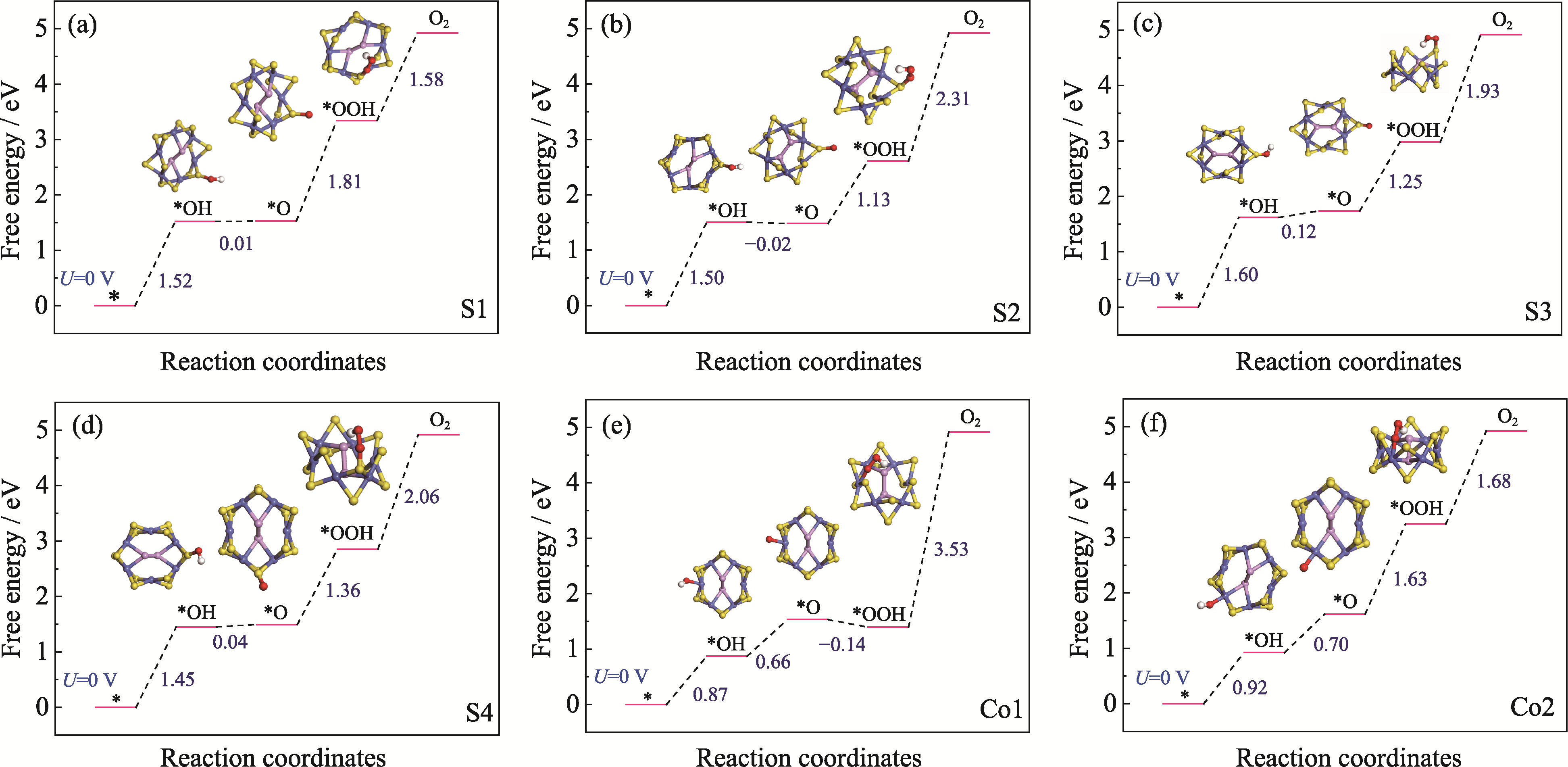
Fig. 3 Step diagrams of Gibbs free energy for the OER at six adsorption sites of CoPS3-QDs1 and schematic illustrations of the adsorbed intermediate models (a) S1 site; (b) S2 site; (c) S3 site; (d) S4 site; (e) Co1 site; (f) Co2 site
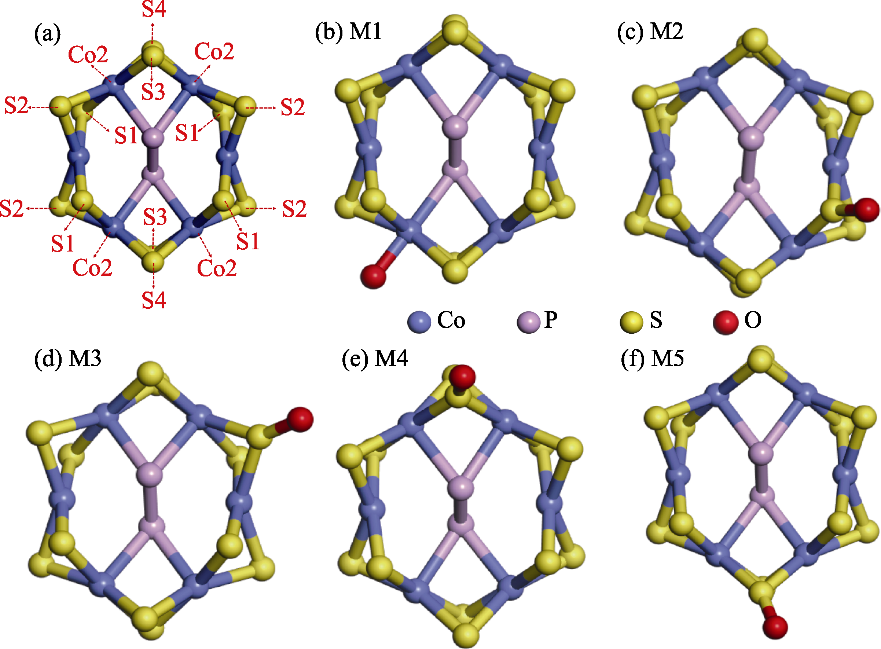
Fig. 4 Structural models of O modification at the Co2 site of CoPS3-QDs1 and its surrounding four inequivalent S atoms (a) Distribution of Co2 site and its surrounding four S atomic sites; (b) Model M1 with O modification at the Co2 site; (c) Model M2 with O modification at the S1 site; (d) Model M3 with O modification at the S2 site; (e) Model M4 with O modification at the S3 site; (f) Model M5 with O modification at the S4 site. Colorful figures are available on website
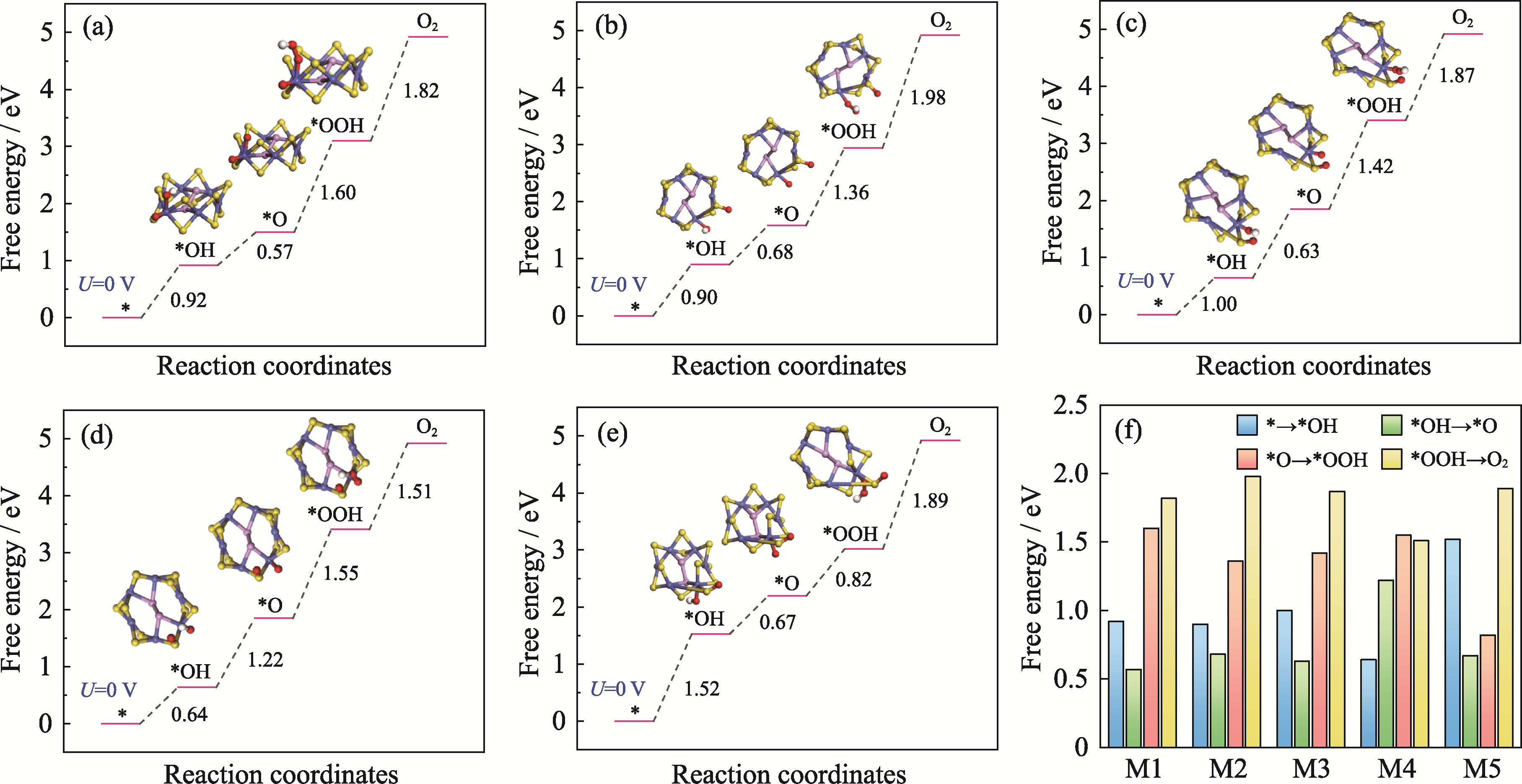
Fig. 5 OER activity comparisons of M1-M5 models with O modification (a-e) Gibbs free energy diagrams for OER on (a) M1, (b) M2, (c) M3, (d) M4, and (e) M5 models with O modification; (f) Comparisons of the Gibbs free energy differences for the four electron transfer steps in the OER process for M1-M5 models. Colorful figures are available on website
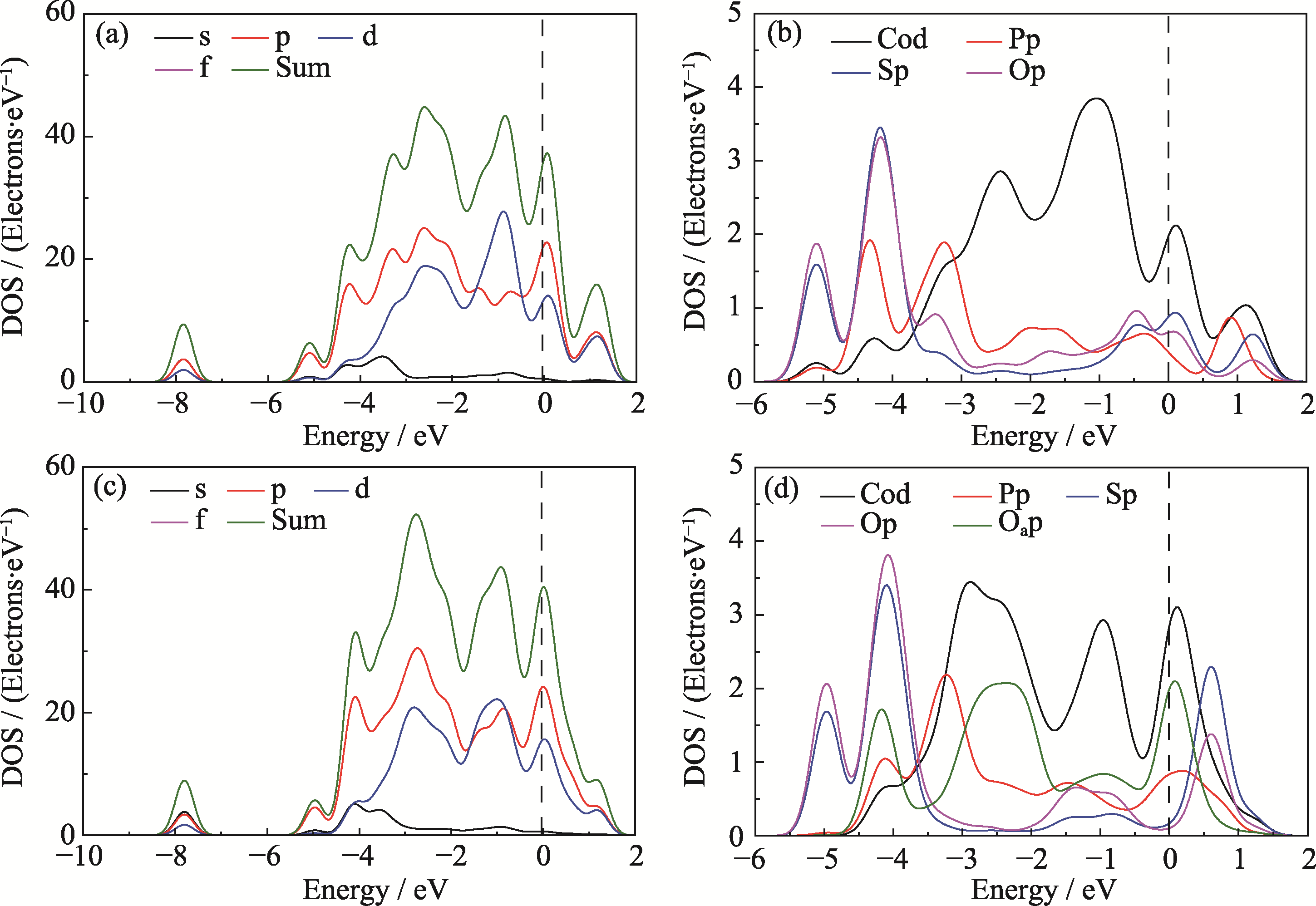
Fig. 6 Comparison of density of states for M4 model before and after adsorption of OOH (a) Total PDOS and (b) atom-resolved PDOS of M4 model; (c) Total PDOS and (d) atom-resolved PDOS of M4 model after adsorbing *OOH. Colorful figures are available on website
| No. | Fractional coordinates | ||
|---|---|---|---|
| X | Y | Z | |
| Co1a | -0.251 | 0.002 | -0.005 |
| Co1b | 0.275 | 0.002 | -0.005 |
| Co2a | -0.128 | 0.266 | 0.015 |
| Co2b | 0.152 | 0.266 | 0.015 |
| Co2c | -0.128 | -0.263 | -0.026 |
| Co2d | 0.152 | -0.263 | -0.026 |
| S1a | -0.239 | 0.167 | -0.175 |
| S1b | 0.263 | 0.167 | -0.175 |
| S1c | -0.238 | -0.164 | 0.165 |
| S1d | 0.262 | -0.164 | 0.165 |
| S2a | -0.315 | 0.187 | 0.137 |
| S2b | 0.339 | 0.187 | 0.137 |
| S2c | -0.316 | -0.184 | -0.147 |
| S2d | 0.340 | -0.184 | -0.147 |
| S3a | 0.012 | 0.374 | 0.185 |
| S3b | 0.012 | -0.369 | -0.197 |
| S4a | 0.012 | 0.394 | -0.130 |
| S4b | 0.012 | -0.392 | 0.119 |
| P1 | 0.012 | 0.070 | 0.084 |
| P2 | 0.012 | -0.066 | -0.094 |
Table S1 Fractional coordinates of each atom in the optimized geometrical structure model of CoPS3-QDs1
| No. | Fractional coordinates | ||
|---|---|---|---|
| X | Y | Z | |
| Co1a | -0.251 | 0.002 | -0.005 |
| Co1b | 0.275 | 0.002 | -0.005 |
| Co2a | -0.128 | 0.266 | 0.015 |
| Co2b | 0.152 | 0.266 | 0.015 |
| Co2c | -0.128 | -0.263 | -0.026 |
| Co2d | 0.152 | -0.263 | -0.026 |
| S1a | -0.239 | 0.167 | -0.175 |
| S1b | 0.263 | 0.167 | -0.175 |
| S1c | -0.238 | -0.164 | 0.165 |
| S1d | 0.262 | -0.164 | 0.165 |
| S2a | -0.315 | 0.187 | 0.137 |
| S2b | 0.339 | 0.187 | 0.137 |
| S2c | -0.316 | -0.184 | -0.147 |
| S2d | 0.340 | -0.184 | -0.147 |
| S3a | 0.012 | 0.374 | 0.185 |
| S3b | 0.012 | -0.369 | -0.197 |
| S4a | 0.012 | 0.394 | -0.130 |
| S4b | 0.012 | -0.392 | 0.119 |
| P1 | 0.012 | 0.070 | 0.084 |
| P2 | 0.012 | -0.066 | -0.094 |

Fig. S2 Schematic structure models of six active sites (S1-S4, Co1 and Co2) of CoPS3-QDs1 adsorbing three oxygen intermediates (*OOH, *OH, and *O), illustrated using site S1 and Co2 as example
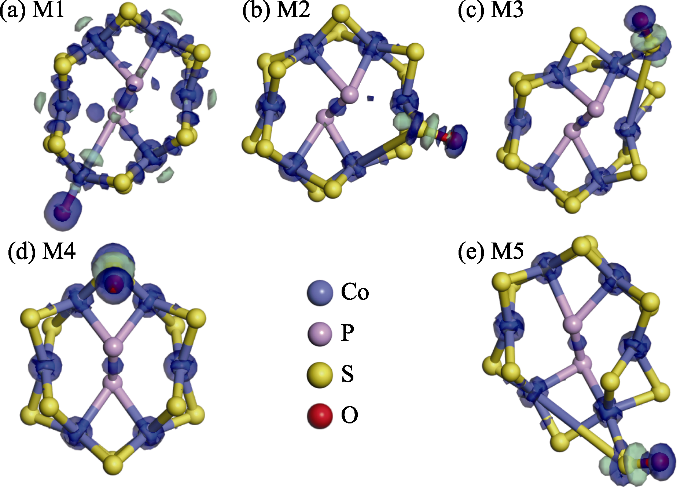
Fig. S3 Optimized structural models and charge density difference maps of O modification at the Co2 site of CoPS3-QDs1 and its surrounding four inequivalent S atoms, where blue regions indicate electron accumulation and green regions indicate electron depletion, with isovalue=0.2 e/Å3
| [1] |
TEE S Y, WIN K Y, TEO W S, et al. Recent progress in energy- driven water splitting. Advanced Science, 2017, 4(5): 1600337.
DOI URL |
| [2] |
YUE M, LAMBERT H, PAHON E, et al. Hydrogen energy systems: a critical review of technologies, applications, trends and challenges. Renewable and Sustainable Energy Reviews, 2021, 146: 111180.
DOI URL |
| [3] |
SUN H, XU X, KIM H, et al. Electrochemical water splitting: bridging the gaps between fundamental research and industrial applications. Energy & Environmental Materials, 2023, 6(5): e12441.
DOI URL |
| [4] |
LI J, HOU C, CHEN C, et al. Collaborative interface optimization strategy guided ultrafine RuCo and MXene heterostructure electrocatalysts for efficient overall water splitting. ACS Nano, 2023, 17(11): 10947.
DOI URL |
| [5] |
CHEN R, YANG Y, WU W, et al. Reconstructed β-NiOOH enabling highly efficient and ultrastable oxygen evolution at large current density. Chemical Engineering Journal, 2024, 480: 148100.
DOI URL |
| [6] |
LI Y, LIU J, LI S, et al. Codecoration of phosphate and iron for improving oxygen evolution reaction of layered Ni (OH)2/NiOOH. ACS Catalysis, 2024, 14(7): 4807.
DOI URL |
| [7] |
YU M, BUDIYANTO E, TUYSUZ H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angewandte Chemie International Edition, 2022, 61(1): e202103824.
DOI URL |
| [8] |
ZHOU B, GAO R, ZOU J, et al. Surface design strategy of catalysts for water electrolysis. Small, 2022, 18(27): 2202336.
DOI URL |
| [9] |
HUANG H, KIM H, LEE A, et al. Structure engineering defective and mass transfer enhanced RuO2 nanosheets for proton exchange membrane water electrolyzer. Nano Energy, 2021, 88: 106276.
DOI URL |
| [10] |
ZHANG L, LU C, YE F, et al. Selenic acid etching assisted vacancy engineering for designing highly active electrocatalysts toward the oxygen evolution reaction. Advanced Materials, 2021, 33(14): 2007523.
DOI URL |
| [11] |
ZHAO S, CHEN Y, LIU Y, et al. Shining light on layered metal phosphosulphide catalysts for efficient water electrolysis: preparation, promotion strategies, and perspectives. Green Chemistry, 2023, 25(16): 6170.
DOI URL |
| [12] |
XUE S, CHEN L, LIU Z, et al. NiPS3 nanosheet-graphene composites as highly efficient electrocatalysts for oxygen evolution reaction. ACS Nano, 2018, 12(6): 5297.
DOI URL |
| [13] | OLIVEIRA F, PASTIK J, MAZÁNEK V, et al. Cobalt phosphorous trisulfide as a high-performance electrocatalyst for the oxygen evolution reaction. ACS Applied Materials & Interfaces, 2021, 13(20): 23638. |
| [14] |
KONKENA B, MASA J, BOTZ A, et al. Metallic NiPS3@NiOOH core-shell heterostructures as highly efficient and stable electrocatalyst for the oxygen evolution reaction. ACS Catalysis, 2017, 7(1): 229.
DOI URL |
| [15] |
LI W, LI C, DONG H, et al. Expediting oxygen evolution by optimizing cation and anion complexity in electrocatalysts based on metal phosphorous trichalcogenides. Angewandte Chemie International Edition, 2023, 62(9): e202214570.
DOI URL |
| [16] |
OLIVEIRA F, PAŠTIKA J, AYAZ I, et al. Alkaline water electrolysis performance of mixed cation metal phosphorous trichalcogenides. Materials Today Energy, 2024, 39: 101468.
DOI URL |
| [17] |
SONG B, LI K, YIN Y, et al. Tuning mixed nickel iron phosphosulfide nanosheet electrocatalysts for enhanced hydrogen and oxygen evolution. ACS Catalysis, 2017, 7: 8549.
DOI URL |
| [18] |
LIU P, PU Y. Construction of two-dimensional CoPS3@defective N-doped carbon composites for enhanced oxygen evolution reaction. International Journal of Hydrogen Energy, 2022, 47(1): 197.
DOI URL |
| [19] |
HUANG C, LIN H, CHIANG C, et al. Manipulating spin exchange interactions and spin-selected electron transfers of 2D metal phosphorus trisulfide crystals for efficient oxygen evolution reaction. Advanced Functional Materials, 2023, 33(43): 2305792.
DOI URL |
| [20] |
YU J, SONG H, LI X, et al. Computational studies on carbon dots electrocatalysis: a review. Advanced Functional Materials, 2021, 31(49): 2107196.
DOI URL |
| [21] |
SU H, WANG W, SHI R, et al. Recent advances in quantum dot catalysts for hydrogen evolution: synthesis, characterization, and photocatalytic application. Carbon Energy, 2023, 5(9): e280.
DOI URL |
| [22] |
MOHANTY B, MITRA A, JENA B, et al. MoS2 quantum dots as efficient electrocatalyst for hydrogen evolution reaction over a wide pH range. Energy & Fuels, 2020, 34(8): 10268.
DOI URL |
| [23] |
YANG M, LIAN Z, SI C, et al. Revealing the intrinsic relation between heteroatom dopants and graphene quantum dots as a bi- functional ORR/OER catalyst. Molecular Catalysis, 2022, 518: 112109.
DOI URL |
| [24] |
XIA C, FENG J, MA C, et al. Exploring the underlying oxygen reduction reaction electrocatalytic activities of pyridinic-N and pyrrolic-N doped graphene quantum dots. Molecular Catalysis, 2023, 535: 112880.
DOI URL |
| [25] |
MOHAMMADI N, ESRAFILI M, SARDROODI J. Defect stabilized Fe atom on porous BN sheet as a potential electrocatalyst for oxygen reduction reaction: a first-principles investigation. Applied Surface Science, 2022, 580: 152271.
DOI URL |
| [26] |
PERDEW J, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Physical Review Letters, 1996, 77: 3865.
DOI PMID |
| [27] |
DELLEY B. Hardness conserving semilocal pseudopotentials. Physical Review B, 2002, 66: 155125.
DOI URL |
| [28] |
HEHRE W. Ab initio molecular orbital theory. Accounts of Chemical Research, 1976, 9: 399.
DOI URL |
| [29] |
NØRSKOV J K, ROSSMEISL J, LOGADOTTIR A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry B, 2004, 108(46): 17886.
DOI URL |
| [30] |
NØRSKOV J K, BLIGAARD T, LOGADOTTIR A, et al. Trends in the exchange current for hydrogen evolution. Journal of The Electrochemical Society, 2005, 152(3): J23.
DOI URL |
| [31] |
MAN I C, SU H, CALLE-VALLEJO F, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem, 2011, 3(7): 1159.
DOI URL |
| [32] |
TAN G, ZHAO X, ZHANG Z, et al. First-principles study of the oxygen evolution reaction on Ni3Fe-layered double hydroxides surfaces with varying sulfur coverage. Molecular Catalysis, 2022, 519: 112116.
DOI URL |
| [33] | 刘婧, 冷艳丽, 慕红梅, 等. 双金属团簇Cu12Fe吸附CO和H2的理论研究. 原子与分子物理学报, 2023, 40(2): 92. |
| [34] |
JIN Y, JIN Y, LI K, et al. Mixed insulating state for van der Waals CoPS3. The Journal of Physical Chemistry Letters, 2022, 13(45): 10486.
DOI URL |
| [35] |
LI K, LI N, YAN N, et al. Adsorption of small hydrocarbons on pristine, N-doped and vacancy graphene by DFT study. Applied Surface Science, 2020, 515: 146028.
DOI URL |
| [36] |
LI J. Oxygen evolution reaction in energy conversion and storage: design strategies under and beyond the energy scaling relationship. Nano-Micro Letters, 2022, 14(1): 112.
DOI PMID |
| [37] |
ZHOU G, LI M, LI Y, et al. Regulating the electronic structure of CoP nanosheets by O incorporation for high-efficiency electrochemical overall water splitting. Advanced Functional Materials, 2020, 30(7): 1905252.
DOI URL |
| [38] |
LU S, ZHANG Y, LOU F, et al. Non-precious metal activated MoSi2N4 monolayers for high-performance OER and ORR electrocatalysts: a first-principles study. Applied Surface Science, 2022, 579: 152234.
DOI URL |
| [1] | CHEN Zi, ZHANG Aidi, GONG Ke, LIU Haihua, YU Gang, SHAN Qingsong, LIU Yong, ZENG Haibo. High-brightness and Monodisperse Quaternary CuInZnS@ZnS Quantum Dots with Tunable and Long-lived Emission [J]. Journal of Inorganic Materials, 2025, 40(4): 433-339. |
| [2] | LÜ Xinyi, XIANG Hengyang, ZENG Haibo. Long-range Ordered Films Boost Efficient Perovskite Quantum Dot Light-emitting Devices [J]. Journal of Inorganic Materials, 2025, 40(1): 111-112. |
| [3] | LI Jiaqi, LI Xiaosong, LI Xuanhe, ZHU Xiaobing, ZHU Aimin. Transition Metal-doped Manganese Oxide: Synthesis by Warm Plasma and Electrocatalytic Performance for Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2024, 39(7): 835-844. |
| [4] | YUE Zihao, YANG Xiaotu, ZHANG Zhengliang, DENG Ruixiang, ZHANG Tao, SONG Lixin. Effect of Pb2+ on the Luminescent Performance of Borosilicate Glass Coated CsPbBr3 Perovskite Quantum Dots [J]. Journal of Inorganic Materials, 2024, 39(4): 449-456. |
| [5] | YUE Quanxin, GUO Ruihua, WANG Ruifen, AN Shengli, ZHANG Guofang, GUAN Lili. 3D Core-shell Structured NiMoO4@CoFe-LDH Nanorods: Performance of Efficient Oxygen Evolution Reaction and Overall Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1254-1264. |
| [6] | WANG Peng, JIN Zunlong, CHEN Ningguang, LIU Yonghao. Theoretical Investigation of Mo Doped α-MnO2 Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(5): 541-546. |
| [7] | FU Yongsheng, BI Min, LI Chun, SUN Jingwen, WANG Xin, ZHU Junwu. Research Progress on Non-noble Metal/Nitrogen-doped Carbon Composite Materials in Electrocatalytic Oxygen Evolution Reaction [J]. Journal of Inorganic Materials, 2022, 37(2): 163-172. |
| [8] | ZHANG Fengjuan, HAN Boning, ZENG Haibo. Perovskite Quantum Dot Photovoltaic and Luminescent Concentrator Cells: Current Status and Challenges [J]. Journal of Inorganic Materials, 2022, 37(2): 117-128. |
| [9] | TIAN Jianjian, MA Xia, WANG Min, YAO Heliang, HUA Zile, ZHANG Lingxia. Sn Quantum Dots for Electrocatalytic Reduction of CO2 to HCOOH [J]. Journal of Inorganic Materials, 2021, 36(12): 1337-1342. |
| [10] | SHU Mengyang, LU Jialin, ZHANG Zhijie, SHEN Tao, XU Jiayue. CsPbBr3 Perovskite Quantum Dots/Ultrathin C3N4 Nanosheet 0D/2D Composite: Enhanced Stability and Photocatalytic Activity [J]. Journal of Inorganic Materials, 2021, 36(11): 1217-1222. |
| [11] | CHEN Ting, XU Yanqiao, JIANG Weihui, XIE Zhixiang, WANG Lianjun, JIANG Wan. Ionic Liquid Assisted Microwave Synthesis of Cu-In-Zn-S/ZnS Quantum Dots and Their Application in White LED [J]. Journal of Inorganic Materials, 2020, 35(4): 439-446. |
| [12] | LI Sheng-Song, ZHENG Yong-Chao, MENG Shu-Lin, WU Li-Zhu, ZHONG Jin- Yi, ZHAO Chong-Lin. Core/Shell Quantum Dots and Au Nanoparticles Assembly for Effective Detection of Nerve Agent Mimic [J]. Journal of Inorganic Materials, 2019, 34(8): 893-898. |
| [13] | Yang LIU, Shan YU, Kai-Wen ZHENG, Wei-Wei CHEN, Xing-An DONG, Fan DONG, Ying ZHOU. NO Photo-oxidation and In-situ DRIFTS Studies on N-doped Bi2O2CO3/CdSe Quantum Dot Composite [J]. Journal of Inorganic Materials, 2019, 34(4): 425-432. |
| [14] | YANG Ying, PAN De-Qun, ZHANG Zheng, CHEN Tian, HAN Xiao-Min, ZHANG Li-Song, GUO Xue-Yi. Photovoltaic Performance of Ag2Se Quantum Dots Co-sensitized Solid-state Dye-sensitized Solar Cells [J]. Journal of Inorganic Materials, 2019, 34(2): 137-144. |
| [15] | GAO Dong, ZHANG Yu-Liang, SUN Jing, FAN Hong-Jun. One-step Synthesis of Specific pH-responsive Carbon Quantum Dots and Their Luminescence Mechanism [J]. Journal of Inorganic Materials, 2019, 34(12): 1309-1315. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||