
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (5): 549-555.DOI: 10.15541/jim20190190
Special Issue: 2020年能源材料论文精选(三) :太阳能电池、热电材料及其他
Previous Articles Next Articles
ZHENG Kun1,LUO Yongchun1,2( ),DENG Anqiang1,YANG Yang1,ZHANG Haiming1
),DENG Anqiang1,YANG Yang1,ZHANG Haiming1
Received:2019-04-30
Revised:2019-08-02
Published:2020-05-20
Online:2019-09-18
Supported by:CLC Number:
ZHENG Kun, LUO Yongchun, DENG Anqiang, YANG Yang, ZHANG Haiming. Microstructure and Electrochemical Property of A2B7-type La0.3Y0.7Ni3.4-xMnxAl0.1 Hydrogen Storage Alloys[J]. Journal of Inorganic Materials, 2020, 35(5): 549-555.
| Alloy | Element/wt% | Stoichiometric B/A ratio | ||||
|---|---|---|---|---|---|---|
| La | Y | Ni | Mn | Al | ||
| x=0 | 6.73 | 15.33 | 75.75 | 0.00 | 2.19 | 3.53 |
| x=0.15 | 6.82 | 15.56 | 72.38 | 2.90 | 2.34 | 3.47 |
| x=0.2 | 6.86 | 15.47 | 71.57 | 3.86 | 2.24 | 3.48 |
| x=0.3 | 6.91 | 15.71 | 68.61 | 6.41 | 2.37 | 3.42 |
| x=0.5 | 6.94 | 15.77 | 65.02 | 10.11 | 2.17 | 3.40 |
Table 1 Elements content of the annealed alloys by ICP analysis
| Alloy | Element/wt% | Stoichiometric B/A ratio | ||||
|---|---|---|---|---|---|---|
| La | Y | Ni | Mn | Al | ||
| x=0 | 6.73 | 15.33 | 75.75 | 0.00 | 2.19 | 3.53 |
| x=0.15 | 6.82 | 15.56 | 72.38 | 2.90 | 2.34 | 3.47 |
| x=0.2 | 6.86 | 15.47 | 71.57 | 3.86 | 2.24 | 3.48 |
| x=0.3 | 6.91 | 15.71 | 68.61 | 6.41 | 2.37 | 3.42 |
| x=0.5 | 6.94 | 15.77 | 65.02 | 10.11 | 2.17 | 3.40 |
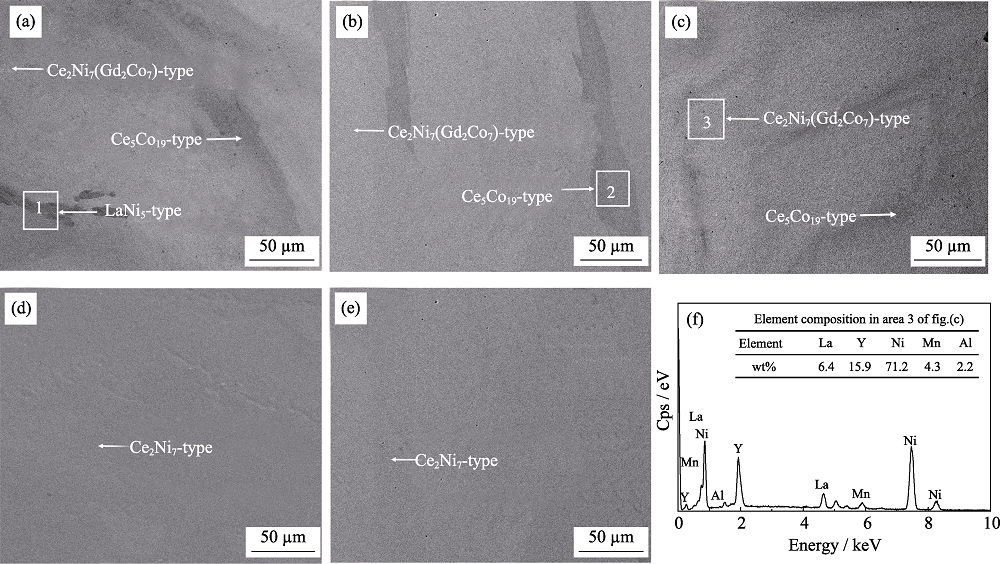
Fig. 1 SEM images of the annealed alloys (a-e) and EDS pattern of A2B7-type phase in area 3 (a)x=0; (b)x=0.15; (c)x=0.2; (d)x=0.3; (e)x=0.5; (f) EDS pattern of A2B7-type phase in area 3 of fig.(c)
| [1] | YASUOKA S, MAGARI Y, MURTA T , et al. Development of high- capacity nickel-metal hydride batteries using superlattice hydrogen- absorbing alloys. Journal of Power Sources, 2006,156(2):662-666. |
| [2] | YING YAN-JUN, CHEN LI-FANG, ZENG XIAO-QING , et al. Theoretical and experimental investigations of the effect of Co addition on the structural and properties of AB3.5-type hydrogen storage alloys. Journal of Inorganic Materials, 2012,27(9):568-574. |
| [3] | LIU J, LI Y, HAN D , et al. Electrochemical performance and capacity degradation mechanism of single-phase La-Mg-Ni-based hydrogen storage alloys. Journal of Power Sources, 2015,300:77-86. |
| [4] | BADDOUR-HADJEAN R, MEYER L, PEREIRA-RAMOS J P , et al. An electrochemical study of new La1-xCexY2Ni9( 0≤x≤1) hydrogen storage alloys. Electrochimica Acta, 2001,46(15):2385-2393. |
| [5] | BEREZOVETS’ V V, DENYS R V, RYABOV O B , et al. Hydrides of substituted derivatives based on the YNi3 compound. Materials Science, 2007,43(4):499-507. |
| [6] | CHARBONNIER V, ZHANG J, MONNIER J , et al. Structural and hydrogen storage properties of Y2Ni7 deuterides studied by neutron powder diffraction. Journal of Physical Chemistry C, 2015,119(22):12218-12225. |
| [7] | CHARBONNIER V, ZHANG J, MONNIER J , et al. Relationship between H2, sorption properties and aqueous corrosion mechanisms in A2Ni7, hydride forming alloys (A=Y, Gd or Sm). Journal of Power Sources, 2016,326:146-155. |
| [8] | XIONG W, YAN H Z, WANG L , et al. Characteristics of A2B7-type La-Y-Ni-based hydrogen storage alloys modified by partially substituting Ni with Mn. International Journal of Hydrogen Energy, 2017,42(15):10131-10141. |
| [9] | ZHAO LEI, LUO YONG-CHUN, DENG AN-QIANG , et al. Hydrogen storage and electrochemical properties of the Mg-free A2B7-type La1-xYxNi3.25Mn0.15Al0.1 alloys with superlattice structure. Chemical Journal of Chinese Universities, 2018,39(9):1993-2002. |
| [10] | LIU J, HAN S, LI Y , et al. An investigation on phase transformation and electrochemical properties of as-cast and annealed La0.75Mg0.25Nix,( x=3.0, 3.3, 3.5, 3.8) alloys. Journal of Alloys & Compounds, 2013,552(3):119-126. |
| [11] | YOUN R A, The Rietveld Method. London: Oxford University Press, 1995: 1-75. |
| [12] | ZHENG G, POPOV B N, WHITE R E . Electrochemical determination of the diffusion coefficient of hydrogen through an LaNi4.25Al0.25 electrode in alkaline aqueous solution. Journal of The Electrochemical Society, 1995,142(8):2695-2698. |
| [13] | NOTTEN P H L, HOKKEL P . Double phase hydride forming compounds: a new class of highly electrocatalytic materials. Journal of The Electrochemical Society, 1991,138(7):1877-1885. |
| [14] |
ZHANG J, ZHOU G, CHEN G , et al. Relevance of hydrogen storage properties of ANi3 intermetallics (A=La, Ce, Y) to the ANi2 subunits in their crystal structures. Acta Materilia, 2008,56(19):5388-5394.
DOI URL PMID |
| [15] | GUZIK M N, HAUBACK B C, YVON K , et al. Hydrogen atom distribution and hydrogen induced site depopulation for the La2-xMgxNi7H system. Journal of Solid State Chemistry, 2012,186(2):9-16. |
| [16] | YAN H, XIONG W, WANG L , et al. Investigations on AB3, A2B7 and A5B19-type La-Y-Ni system hydrogen storage alloys. International Journal of Hydrogen Energy, 2017,42(4):2257-2264. |
| [17] | DENYS R V, RIABOV B, YARTYS V A , et al. Hydrogen storage properties and structure of La1-xMgx( Ni1-yMny)3 intermetallics and their hydrides. Journal of Alloys and Compounds, 2006,137:446-447. |
| [18] |
FANG F, CHEN Z L, WU D Y , et al. Subunit volume control mechanism for dehydrogenation performance of AB3-type superlattice intermetallics. Journal of Power Sources, 2019,427:145-153.
DOI URL |
| [19] | LIU J J, HAN S M, LI Y , et al. Effect of Pr on phase structure and cycling stability of La-Mg-Ni-based alloy with A2B7 and A5B19-type superlattice structures. Electrochimica Acta, 2015,184:257-263. |
| [20] |
WANG HAO, LUO YONG-CHUN, DENG AN-QIANG , et al. Annealing temperature on structural and electrochemical property of Mg-free La-Y-Ni based A2B7-type hydrogen storage alloys. Journal of Inorganic Material, 2018,33(4):434-440.
DOI URL |
| [21] | YANG C C, WANG C C, LI M M , et al. A start of the renaissance for nickel metal hydride batteries: a hydrogen storage alloy series with an ultra-long cycle life. Journal of Materials Chemistry A, 2017,5:1145-1152. |
| [1] | ZHANG Yaping,LEI Yuxuan,DING Wenming,YU Lianqing,ZHU Shuaifei. Preparation and Photoelectrochemical Property of the Dual-ferroelectric Composited Material [J]. Journal of Inorganic Materials, 2020, 35(9): 987-992. |
| [2] | ZHANG Ya-Ping, DING Wen-Ming, ZHU Hai-Feng, HUANG Cheng-Xing, YU Lian-Qing, WANG Yong-Qiang, LI Zhe, XU Fei. Photoelectrochemical Properties of MoSe2 Modified TiO2 Nanotube Arrays [J]. Journal of Inorganic Materials, 2019, 34(8): 797-802. |
| [3] | WANG Hao, LUO Yong-Chun, DENG An-Qiang, ZHAO Lei, JIANG Wan-Ting. Annealing Temperature on Structural and Electrochemical Property of Mg-free La-Y-Ni Based A2B7-type Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2018, 33(4): 434-440. |
| [4] | TONG Yan-Wei, ZHANG Xue-Feng, FANG Min-Xian. Structures and Electrochemical Properties of V2Ti0.5Cr0.5Ni1-xMox(x=0.02-0.08) Ni/MH Battery Anode Materials [J]. Journal of Inorganic Materials, 2016, 31(2): 148-152. |
| [5] |
YANG Xue -Mei, LIU Zhong -Ping, LI Xiao -Dong, Zhang Ze -Ming, JI Lan-Xiang, DENG Jian-Guo.
Ultra-long VO2(B) Nanobelts : One-step Hydrothermal Synthesis and Electrochemical Properties [J]. Journal of Inorganic Materials, 2015, 30(4): 443-448. |
| [6] | MEI Xing-Zhi, LUO Yong-Chun, ZHANG Guo-Qing, KANG Long. Structural and Electrochemical Properties of A2B7-type La1-xScxNi2.6Co0.3Mn0.5Al0.1 (x = 0~0.5) Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2015, 30(10): 1049-1055. |
| [7] | CHEN Ming-Zhe, GUO Xiao-Dong, ZHONG Ben-He, YAN Hui-Min, ZHANG Ji-Bin, LIU Ju. High Energy Density Spinel LiCr0.2Ni0.4Mn1.4O4 Cathode Material Prepared by Spray Pyrolysis Method [J]. Journal of Inorganic Materials, 2014, 29(9): 917-923. |
| [8] | LI Jian, YAO Shu-Heng, ZHOU Hong-Ming, GENG Wen-Jun. Preparation of LiMn0.4Fe0.6PO4/C Composite by A New Route Combining Solid-state Reaction with Hydrothermal Synthesis [J]. Journal of Inorganic Materials, 2014, 29(4): 443-448. |
| [9] | ZHANG Guo-Fang, ZHANG Yang-Huan, LIU Zhuo-Cheng, XU Jian-Yi, ZHANG Yin. Effect of CeO2-based Solid Solutions on Hydrogen Storage Kinetic Properties of Mg2Ni Alloy [J]. Journal of Inorganic Materials, 2014, 29(12): 1241-1245. |
| [10] | ZHANG Qian, LIU Wei-Wei, FANG Guo-Qing, XIA Bing-Bo, SUN Hong-Dan, KANEKO Shingo, YANG Yu-Sheng, ZHENG Jun-Wei, LI De-Cheng. Structural and Electrochemical Performances of Li1+2xMn0.3+xNi0.3-3xCr0.4O2 Synthesized by Spray-dry Method [J]. Journal of Inorganic Materials, 2013, 28(06): 616-622. |
| [11] | YING Yan-Jun, CHENG Li-Fang, ZENG Xiao-Qin, ZOU Jian-Xin, DING Wen-Jiang. Theoretical and Experimental Investigations of the Effect of Co Addition on the Structural and Properties of AB3.5-type Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2012, 27(6): 568-574. |
| [12] | WANG Yan-Zhi, ZHAO Min-Shou. Structure and Electrochemical Characteristics of Ti-V-based Solid Solution/AB5-type La-Mg-based Alloy Composite Hydrogen Storage Material [J]. Journal of Inorganic Materials, 2012, 27(5): 463-468. |
| [13] | TIAN Xiao, Tegus O, HAI Shan, YAO Zhan-Quan. Effects of Rapid Quenching on Electrochemical Properties of MlNi3.55Co0.75Mn0.4Al0.3/5wt% Mg2Ni Composite Hydrogen Storage Alloy [J]. Journal of Inorganic Materials, 2012, 27(11): 1179-1184. |
| [14] | DING Hui-Ling1,2, HAO Jian-Sheng1,3, ZHU Xi-Lin2,3, LI Yuan2, HAN Shu-Min1,2, ZHANG Jing-Wu1. Electrochemical Performance Studies on Nickel-cobalt Electroplated La-Mg-Ni-based Hydrogen Storage Alloys [J]. Journal of Inorganic Materials, 2010, 25(6): 647-652. |
| [15] | LIU Yi-Xin,YANG Shu-Quan,ZHANG Dan-Dan,LI Guang-Xu,WEI Wen-Lou,GUO Jin. Effects of B and C on Hydrogen Storage Properties of Li-N-H Complex Hydrides [J]. Journal of Inorganic Materials, 2009, 24(4): 813-816. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||