无机材料学报 ›› 2023, Vol. 38 ›› Issue (1): 71-78.DOI: 10.15541/jim20220298 CSTR: 32189.14.10.15541/jim20220298
所属专题: 【能源环境】燃料电池(202506)
姚仪帅1,2,3( ), 郭瑞华1,2,3(
), 郭瑞华1,2,3( ), 安胜利1,2,3, 张捷宇4, 周国治4, 张国芳1, 黄雅荣1, 潘高飞1
), 安胜利1,2,3, 张捷宇4, 周国治4, 张国芳1, 黄雅荣1, 潘高飞1
收稿日期:2022-05-27
修回日期:2022-08-01
出版日期:2023-01-20
网络出版日期:2022-08-26
通讯作者:
郭瑞华, 教授. E-mail: grh7810@163.com作者简介:姚仪帅(1996-), 男, 硕士研究生. E-mail: 2563607693@qq.com
基金资助:
YAO Yishuai1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), AN Shengli1,2,3, ZHANG Jieyu4, CHOU Kuochih4, ZHANG Guofang1, HUANG Yarong1, PAN Gaofei1
), AN Shengli1,2,3, ZHANG Jieyu4, CHOU Kuochih4, ZHANG Guofang1, HUANG Yarong1, PAN Gaofei1
Received:2022-05-27
Revised:2022-08-01
Published:2023-01-20
Online:2022-08-26
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:YAO Yishuai(1996-), male, Master candidate. E-mail: 2563607693@qq.com
Supported by:摘要:
直接乙醇燃料电池(DEFC)具有燃料易得、绿色高效的优点, 得到了广泛的研究, 但是DEFC催化剂存在催化效率低、稳定性差的问题, 制约了其快速发展。本研究采用液相水热合成法, 以聚乙烯吡咯烷酮(PVP k-25)为分散剂和还原剂、甘氨酸为表面控制剂和共还原剂, 通过调控Pt-Co金属前驱体的摩尔比, 一步制备了XC-72R炭黑负载的Pt1Cox/C高指数晶面纳米催化剂, 实现了催化剂晶粒在碳载体上的原位生长。Pt1Co1/3/C纳米催化剂暴露的高指数晶面主要包括(410)、(510)和(610)晶面。在晶体生长过程中, Pt1Co1/3/C纳米催化剂晶粒由“类球体”转变立方块, 最终得到具有高指数晶面取向的内凹形貌。Pt1Co1/3/C高指数晶面纳米催化剂的电催化活性最高, 其电化学活性表面积为18.46 m2/g, 对乙醇氧化峰电流密度为48.70 mA/cm2, 稳态电流密度为8.29 mA/cm2, CO氧化峰的电位为0.610 V。这说明具有高指数晶面的催化剂表面存在的台阶、扭结等缺陷原子, 可增加活性位点, 进而显示出优异的电催化性能。本研究可为高指数晶面催化剂材料的开发及工业化应用提供理论依据。
中图分类号:
姚仪帅, 郭瑞华, 安胜利, 张捷宇, 周国治, 张国芳, 黄雅荣, 潘高飞. 原位负载Pt-Co高指数晶面催化剂的制备及其电催化性能[J]. 无机材料学报, 2023, 38(1): 71-78.
YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance[J]. Journal of Inorganic Materials, 2023, 38(1): 71-78.
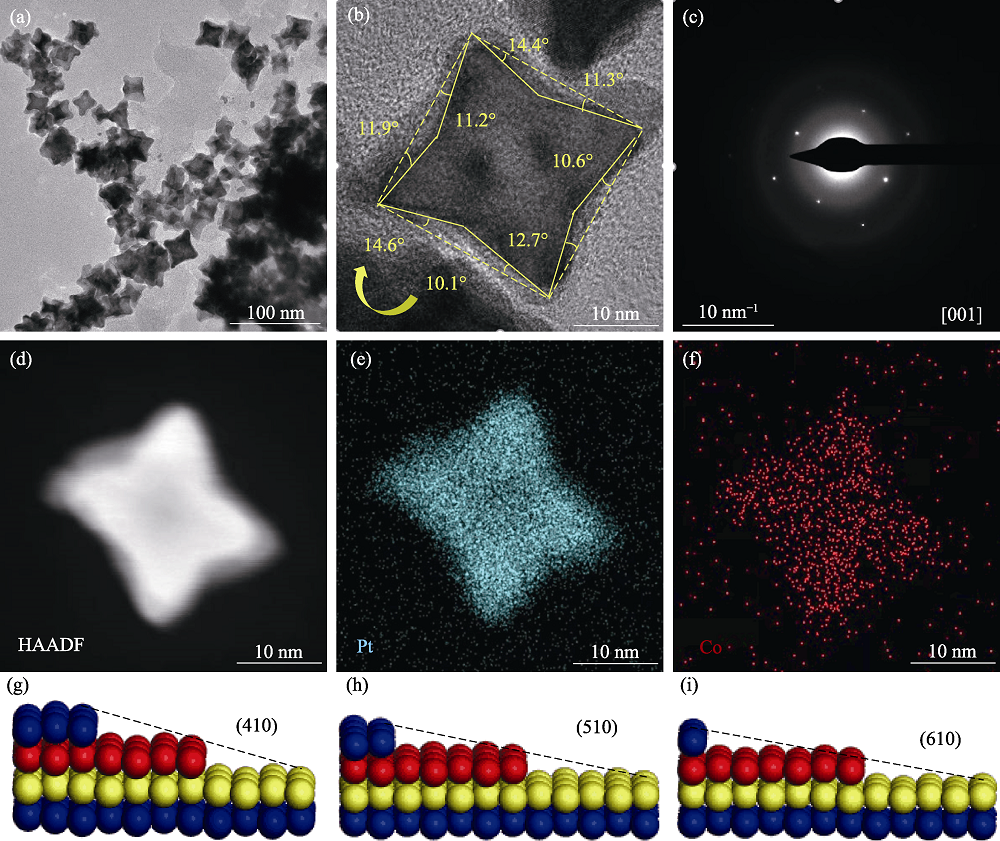
图2 Pt1Co1/3/C高指数晶面纳米催化剂的表面形貌
Fig. 2 Surface morphologies of Pt1Co1/3/C high-index crystalline nanocatalyst (a) TEM and (b) HRTEM images; (c) SAED image; (d-f) EDS surface sweep mapping images; (g-i) High-index crystalline atomic model for Pt1Co1/3/C high-index crystalline nanocatalysts; Colorful spheres in (g-i) represent different layers of atoms
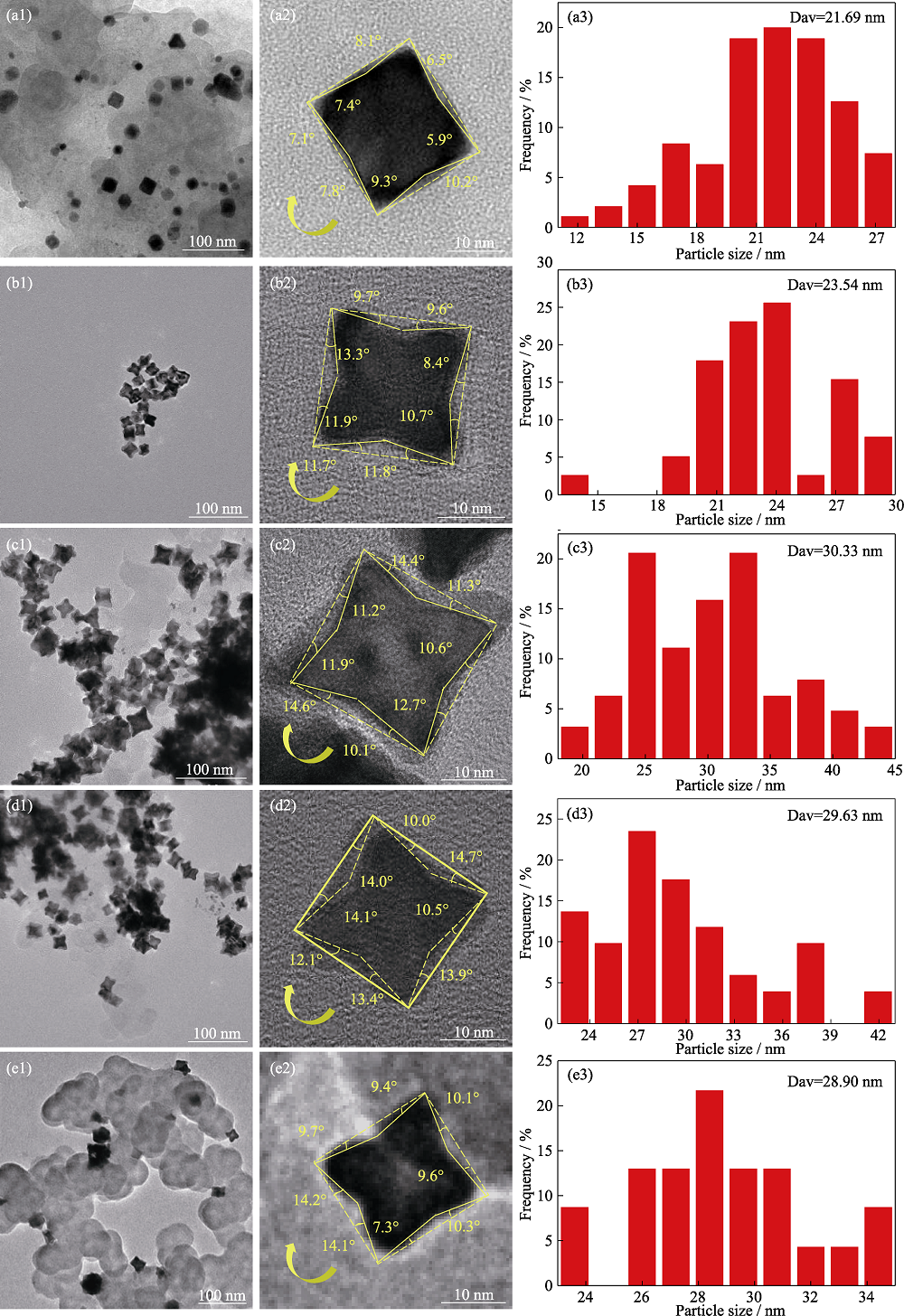
图S1 Pt1Cox/C高指数晶面纳米催化剂TEM、HRTEM照片及粒径分布直方图
Fig. S1 TEM, HRTEM images and histograms of particle size distributions for Pt1Cox/C high index crystalline nanocatalysts (a1-a3) Pt/C; (b1-b3) Pt1Co1/4/C; (c1-c3) Pt1Co1/3/C; (d1-d3) Pt1Co1/2/C; (e1-e3) Pt1Co1/C
| Catalyst | Angle of depression/(°) | Exposure of crystalline surfaces |
|---|---|---|
| Pt/C | 8.1, 6.5, 5.9, 10.2, 9.3, 7.8, 7.1, 7.4 | (610), (710), (810) |
| Pt1Co1/4/C | 9.7, 9.6, 8.4, 10.7, 11.8, 11.7, 11.9, 13.3 | (410), (510), (610), (710) |
| Pt1Co1/3/C | 14.4, 11.3, 10.6, 12.7, 10.1, 14.6, 11.9, 11.2 | (410), (510), (610) |
| Pt1Co1/2/C | 10.0, 14.7, 10.5, 13.9, 13.4, 12.1, 14.1, 14.0 | (410), (510), (610) |
| Pt1Co1/C | 9.4, 10.1, 9.6, 10.3, 7.3, 14.1, 14.2, 9.7 | (410), (610), (810) |
表S1 Pt1Cox/C高指数晶面催化剂的凹陷角度与暴露晶面
Table 1 Pt1Cox/C high index crystalline surface catalyst depression angle vs. exposed crystal surface
| Catalyst | Angle of depression/(°) | Exposure of crystalline surfaces |
|---|---|---|
| Pt/C | 8.1, 6.5, 5.9, 10.2, 9.3, 7.8, 7.1, 7.4 | (610), (710), (810) |
| Pt1Co1/4/C | 9.7, 9.6, 8.4, 10.7, 11.8, 11.7, 11.9, 13.3 | (410), (510), (610), (710) |
| Pt1Co1/3/C | 14.4, 11.3, 10.6, 12.7, 10.1, 14.6, 11.9, 11.2 | (410), (510), (610) |
| Pt1Co1/2/C | 10.0, 14.7, 10.5, 13.9, 13.4, 12.1, 14.1, 14.0 | (410), (510), (610) |
| Pt1Co1/C | 9.4, 10.1, 9.6, 10.3, 7.3, 14.1, 14.2, 9.7 | (410), (610), (810) |
| Catalyst | Pt(0)/eV | Relative ratio/% | Pt(II)/eV | Relative ratio/% |
|---|---|---|---|---|
| Pt/C | 70.30,73.60 | 52.67 | 70.95,74.45 | 47.33 |
| Pt1Co1/3/C | 70.15,73.55 | 53.04 | 70.85,74.35 | 46.96 |
表1 Pt/C与Pt1Co1/3/C催化剂的XPS拟合结果
Table 1 XPS fitting results for Pt/C and Pt1Co1/3/C catalysts
| Catalyst | Pt(0)/eV | Relative ratio/% | Pt(II)/eV | Relative ratio/% |
|---|---|---|---|---|
| Pt/C | 70.30,73.60 | 52.67 | 70.95,74.45 | 47.33 |
| Pt1Co1/3/C | 70.15,73.55 | 53.04 | 70.85,74.35 | 46.96 |
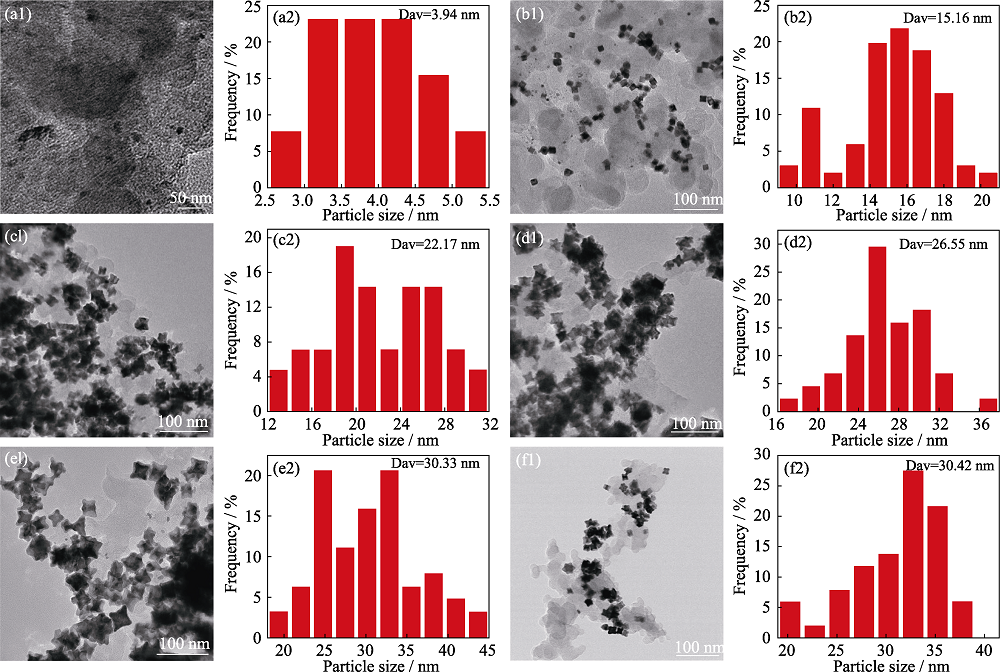
图S2 Pt1Co1/3/C高指数晶面催化剂在不同保温时间的TEM照片及粒径分布直方图
Fig. S2 TEM images and histograms of particle size distributions of Pt1Co1/3/C high index crystalline catalysts at different holding time (a1, a2) 1 h; (b1, b2) 3 h; (c1, c2) 5h; (d1, d2) 7 h; (e1, e2) 9 h; (f1, f2) 10 h
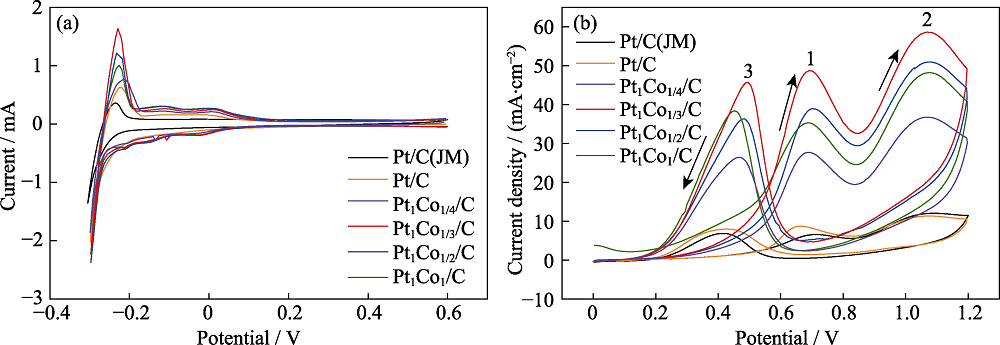
图4 催化剂的电催化性能
Fig. 4 Electrocatalytic performance of catalysts (a) H adsorption-desorption curves of the catalysts in 0.5 mol/L H2SO4 saturated with N2; (b) Cyclic voltammetric curves of the catalysts in 0.5 mol/L H2SO4+1 mol/L CH3CH2OH Colorful figures are available on website
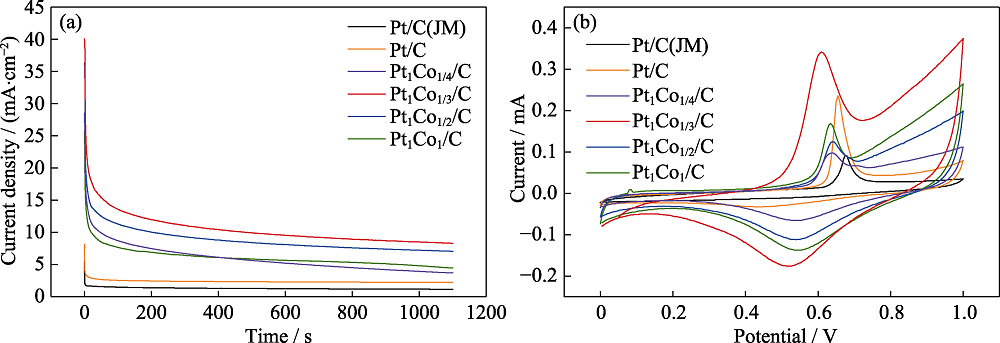
图5 催化剂的稳定性和抗中毒性能
Fig. 5 Stability and anti-poisoning performance of catalysts (a) Timing current curves of the catalysts in 0.5 mol/L H2SO4+1 mol/L CH3CH2OH; (b) CO dissolution curves of catalysts in 0.5 mol/L H2SO4 Colorful figures are available on website
| [1] |
LUO S P, SHEN P K. Concave platinum-copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis. ACS Nano, 2017, 11(12): 11946.
DOI PMID |
| [2] |
XUE S F, DENG W T, YANG F, et al. Hexapod Pt RuCu nanocrystalline alloy for highly efficient and stablemethanol oxidation. ACS Catalysis, 2018, 8(8): 7578.
DOI URL |
| [3] |
TANG M, LUO S P, WANG K, et al. Simultaneous formation of trimetallic Pt-Ni-Cu excavated rhombicdodecahedrons with enhanced catalytic performance for the methanol oxidation reaction. Nano Research, 2018, 11(9): 4786.
DOI URL |
| [4] | MA Y X, YIN L S, YANG T, et al. One-pot synthesis of concave platinum-cobalt nanocrystals and their superior catalytic performances for methanol electrochemical oxidation and oxygen electrochemical reduction. ACS Applied Materials & Interfaces, 2017, 9(41): 36164. |
| [5] |
LUO M C, SUN Y J, QIN Y N, et al. Surface and near-surface engineering of PtCo nanowires at atomic scale for enhanced electrochemical sensing and catalysis. Chemistry of Materials, 2018, 30(19): 6660.
DOI URL |
| [6] |
ZHANG T, BAI Y, SUN Y Q, et al. Laser-irradiation induced synthesis of spongy AuAgPt alloy nanospheres with high-index facets, rich grain boundaries and subtle lattice distortion for enhanced electrocatalytic activity. Journal of Materials Chemistry A, 2018, 6(28): 13735.
DOI URL |
| [7] |
YANG P P, YUAN X L, HU H C, et al. Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation. Advanced Functional Materials, 2018, 28(1): 1704774.
DOI URL |
| [8] |
ZHU Y, GU J, YU T, et al. Synthesis and property of platinum- cobalt alloy nano catalyst. Journal of Inorganic Materials, 2021, 36(3): 299.
DOI URL |
| [9] | ZHANG Z C, LIU G G, CUI X Y, et al. Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution. Advanced Materials, 2018, 30(30): e1801741. |
| [10] | LUO S P, TANG M, SHEN P K, et al. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts. Advanced Materials, 2017, 29(8): 01687. |
| [11] |
LUO M C, SUN Y J, ZHANG X, et al. Stable high-index faceted Pt skin on zigzag-like Pt Fe nanowires enhances oxygen reduction catalysis. Advanced Materials, 2018, 30(10): 1705515.
DOI URL |
| [12] |
XIE S F, CHOI S, LU N, et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Letters, 2014, 14(6): 3570.
DOI PMID |
| [13] |
LUO Y, FENG J Z, FENG J, et al. Research progress on advanced carbon materials as Pt support for proton exchange membrane fuel cells. Journal of Inorganic Materials, 2020, 35(4): 407.
DOI |
| [14] |
ZHANG J, LANGILLE M, PERSONICK M L, et al. Concave cubic gold nanocrystals with high-index facets. Journal of the American Chemical Society, 2010, 132(40): 14012.
DOI PMID |
| [15] |
QIN Y C, ZHANG X, DAI X P, et al. Graphene oxide-assisted synthesis of Pt-Co alloy nanocrystals with high-index facets and enhanced electrocatalytic properties. Small, 2016, 12(4): 524.
DOI PMID |
| [16] |
TIAN N, XIAO J, ZHOU Z Y, et al. Pt-group bimetallic nanocrystals with high-index facets as high performance electrocatalysts. Faraday Discussions, 2013, 162: 77.
PMID |
| [17] | ZHU C L, YIN Z X, LAI W H, et al. Fe-Ni-Mo nitride porous nanotubes for full water splitting and Zn-air batteries. Advanced Energy Materials, 2018, 8(36): 1802327. |
| [18] |
OUYANG Y R, CAO H J, WU H J, et al. Tuning Pt-skinned PtAg nanotubes in nanoscales to efficiently modify electronic structure for boosting performance of methanol electrooxidation. Applied Catalysis B: Environmental, 2020, 265(C): 118606.
DOI URL |
| [19] |
QIN C L, FAN A X, ZHANG X, et al. The in situ etching assisted synthesis of Pt-Fe-Mn ternary alloys with high-index facets as efficient catalysts for electro-oxidation reactions. Nanoscale, 2019, 11(18): 9061.
DOI URL |
| [20] |
JOHANEK V, LAURINA M, GRANT A W, et al. Fluctuations and bistabilities on catalyst nanoparticles. Science, 2004, 304(5677): 1639.
PMID |
| [21] |
HONKALA K, HELLMAN A, REMEDIAKIS I N, et al. Ammonia synthesis from first-principles calculations. Science, 2005, 307(5709): 555.
DOI PMID |
| [22] |
SHAO M H, CHANG Q W, DODELET J P, et al. Recent advances in electrocatalysts for oxygen reduction reaction. Chemical Reviews, 2016, 116(6): 3594.
DOI PMID |
| [23] | ZHANG Z C, HUI J F, LIU Y S, et al. Glycine-mediated syntheses of Pt concave nanocubes with high-index {hk0} facets and their enhanced electrocatalytic activities. Langmuir, 2012, 28(42): 148458. |
| [24] |
GUO S J, DONG S J, WANG E K. Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano, 2010, 4(1): 547.
DOI PMID |
| [25] |
PARK K W, CHOI J H, SUNG Y E, et al. Structural, chemical, and electronic properties of Pt/Ni thin film electrodes for methanol electrooxidation. The Journal of Physical Chemistry B, 2003, 107(24): 5851.
DOI URL |
| [26] |
YU X F, WANG D S, PENG Q, et al. High performance electrocatalyst: Pt-Cu hollow nanocrystals. Chemical Communications, 2011, 47(28): 8094.
DOI URL |
| [27] |
WANG Y, ZHUO H Y, ZHANG X, et al. Synergistic effect between undercoordinated platinum atoms and defective nickel hydroxide on enhanced hydrogen evolution reaction in alkaline solution. Nano Energy, 2018, 48: 590.
DOI URL |
| [28] |
GUO R H, QIAN F, AN S L, et al. Effect of acid treatment on electrocatalytic performance of PtNi catalyst. Chemical Research in Chinese Universities, 2020, 37(3): 686.
DOI URL |
| [29] |
ZHU T W, HENSEN E J M, SANTEN R A, et al. Roughening of Pt nanoparticles induced by surface-oxide formation. Physical Chemistry Chemical Physics, 2013, 15(7): 2268.
DOI PMID |
| [30] | YU T, KIM D Y, ZHANG H, et al. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angewandte Chemie International Edition, 2011, 123(12): 2825. |
| [31] |
HUANG M H, JIANG Y Y, JIN C H, et al. Pt-Cu alloy with high density of surface Pt defects for efficient catalysis of breaking C-C bond in ethanol. Electrochimica Acta, 2014, 125: 29.
DOI URL |
| [32] |
SHI Y, MA Z R, XIAO Y Y, et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nature Communications, 2021, 12(1): 3021.
DOI PMID |
| [33] |
WEI Z Z, YAO Z H, ZHOU Q, et al. Optimizing alkyne hydrogenation performance of Pd on carbon in situ decorated with oxygen-deficient TiO2 by integrating the reaction and diffusion. ACS Catalysis, 2019, 9(12): 10656.
DOI URL |
| [34] |
CHEN W L, GAO W P, TU P, et al. Neighboring Pt atom sites in ultrathin FePt nanosheet for efficient and highly CO-tolerant oxygen reduction reaction. Nano Letters, 2018, 18(9): 5905.
DOI URL |
| [35] |
LIAN X, GUO W L, LIU F L, et al. DFT studies on Pt3M (M=Pt, Ni, Mo, Ru, Pd, Rh) clusters for CO oxidation. Computational Materials Science, 2015, 96: 237.
DOI URL |
| [36] |
ZHU Y M, BU L Z, SHAO Q, et al. Structurally ordered Pt3Sn nanofibers with highlighted antipoisoning property as efficient ethanol oxidation electrocatalysts. ACS Catalysis, 2020, 10(5): 3455.
DOI URL |
| [1] | 信震宇, 郭瑞华, 乌仁托亚, 王艳, 安胜利, 张国芳, 关丽丽. Pt-Fe/GO纳米催化剂的制备及其电催化乙醇氧化性能研究[J]. 无机材料学报, 2025, 40(4): 379-387. |
| [2] | 刘磊, 郭瑞华, 王丽, 王艳, 张国芳, 关丽丽. Pt3Co高指数晶面氧还原过程的密度泛函理论研究[J]. 无机材料学报, 2025, 40(1): 39-46. |
| [3] | 刘锁兰, 栾福园, 吴子华, 寿春晖, 谢华清, 杨松旺. 原位生长钙钛矿太阳能电池共形氧化锡薄膜[J]. 无机材料学报, 2024, 39(12): 1397-1403. |
| [4] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [5] | 牛海滨, 黄佳慧, 李倩文, 马董云, 王金敏. 多孔NiMoO4纳米片薄膜的直接水热生长及其电致变色性能[J]. 无机材料学报, 2023, 38(12): 1427-1433. |
| [6] | 张弦, 张策, 姜文君, 冯德强, 姚伟. 四元BiMnVO5的合成、电子结构与可见光催化性能研究[J]. 无机材料学报, 2022, 37(1): 58-64. |
| [7] | 肖昱旻, 李彬, 覃礼钊, 林华, 李庆, 廖斌. 用CuCl2为铜源高效制备形貌可控CuGeO3的研究[J]. 无机材料学报, 2021, 36(1): 69-74. |
| [8] | 王举汉,文雄,刘成超,张煜华,赵燕熹,李金林. 多级孔Co/Al-SiO2催化剂制备及其费-托合成催化性能[J]. 无机材料学报, 2020, 35(9): 999-1004. |
| [9] | 王金敏, 于红玉, 马董云. 纳米二氧化锰的制备及其应用研究进展[J]. 无机材料学报, 2020, 35(12): 1307-1314. |
| [10] | 李孟夏, 陆越, 王利斌, 胡先罗. Mn3O4@ZnO核壳结构纳米片阵列的可控合成及其在水系锌离子电池中的应用[J]. 无机材料学报, 2020, 35(1): 86-92. |
| [11] | 许云青,王海增. EDTA辅助水热法制备不同形貌的氟化镁钠[J]. 无机材料学报, 2019, 34(9): 933-937. |
| [12] | 苟生莲, 乃学瑛, 肖剑飞, 叶俊伟, 董亚萍, 李武. 碱式氯化镁晶须制备纳米氧化镁热分解动力学研究[J]. 无机材料学报, 2019, 34(7): 781-785. |
| [13] | 刘伟, 郑凯, 王东红, 雷忆三, 范怀林. Co3O4纳米线阵列@活性炭纤维复合材料的水热合成及电化学应用[J]. 无机材料学报, 2019, 34(5): 487-492. |
| [14] | 王伟, 罗世阶, 鲜聪, 肖群, 杨洋, 欧云, 刘运牙, 谢淑红. 水热合成BiCl3/Bi2S3复合材料的热电性能[J]. 无机材料学报, 2019, 34(3): 328-334. |
| [15] | 刘小元, 刘宝丹, 姜亚南, 王柯, 周洋, 杨兵, 张兴来, 姜辛. 形貌可控及光学吸收性能可调的钙钛矿型SrTiO3纳米结构的原位生长[J]. 无机材料学报, 2019, 34(1): 65-71. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||