无机材料学报 ›› 2020, Vol. 35 ›› Issue (12): 1307-1314.DOI: 10.15541/jim20200105 CSTR: 32189.14.10.15541/jim20200105
所属专题: 功能材料论文精选(2020); 【虚拟专辑】电致变色与热致变色材料
收稿日期:2020-03-02
修回日期:2020-05-07
出版日期:2020-12-20
网络出版日期:2020-06-09
作者简介:王金敏(1975–), 男, 教授. E-mail: wangjinmin@sspu.edu.cn
基金资助:
WANG Jinmin( ),YU Hongyu,MA Dongyun
),YU Hongyu,MA Dongyun
Received:2020-03-02
Revised:2020-05-07
Published:2020-12-20
Online:2020-06-09
About author:WANG Jinmin (1975–), male, professor. E-mail: wangjinmin@sspu.edu.cn
Supported by:摘要:
二氧化锰作为一种重要的过渡金属氧化物, 因其储量丰富、晶型多样、性能优异而备受关注。将二氧化锰纳米化后, 其颗粒尺寸变小、比表面积变大、材料性能优化、应用领域得以拓宽。本文在引言部分从介绍二氧化锰的应用着手, 指出纳米化和晶型多变对二氧化锰的结构和性能有着重要的影响。正文部分主要从纳米二氧化锰的制备方法和纳米二氧化锰的应用两个方面对近年来的研究进展进行了总结和评述。(1)介绍了水热法、溶胶-凝胶法、化学沉淀法、固相合成法等纳米二氧化锰的制备方法, 对各种制备方法的优点与缺点以及所制备纳米二氧化锰的形貌与性能进行了总结。(2)综述了纳米二氧化锰在储能电极、电致变色器件、催化剂、生物传感器等领域的应用研究进展。纳米二氧化锰可作为电池的正极材料和超级电容器的电极材料。通过调控二氧化锰的晶型和复合制备的含锰复合氧化物用于锂离子电池的正极材料, 可提高电池的容量并改善循环稳定性。作为锂离子动力电池的正极材料已有产业化应用, 在新能源汽车领域具有良好的应用前景。由于纯二氧化锰本身的颜色主要是在棕色和黄色之间变化, 光调制幅度较小, 因此作为电致变色器件的电极材料, 通常将其与其它光调制幅度较大的材料进行复合使用。如聚苯胺/二氧化锰杂化电致变色薄膜较纯聚苯胺薄膜在形貌、结构和电致变色性能上有巨大差异, 显示出更高的光调制幅度、着色效率和循环稳定性。纳米二氧化锰在乙苯的催化转化和空气污染物的催化消除方面发挥重要作用。纳米二氧化锰能够增大电流响应、降低检出限, 使检测的灵敏度大大提高, 近年来在生物传感器领域逐渐被大家重视并得到广泛应用, 如二氧化锰纳米片辅助荧光偏振生物传感器可有效检测环境水样中Ag+, PtAu-MnO2二元纳米结构修饰的石墨烯纸在非酶葡萄糖检测中表现出良好的传感性能。在结语部分, 分析了当前纳米二氧化锰的制备和应用方面存在的问题, 指出了纳米二氧化锰在锂离子电池正极材料和电致变色器件中应用的发展方向, 并对其未来的发展前景进行了展望。
中图分类号:
王金敏, 于红玉, 马董云. 纳米二氧化锰的制备及其应用研究进展[J]. 无机材料学报, 2020, 35(12): 1307-1314.
WANG Jinmin, YU Hongyu, MA Dongyun. Progress in the Preparation and Application of Nanostructured Manganese Dioxide[J]. Journal of Inorganic Materials, 2020, 35(12): 1307-1314.
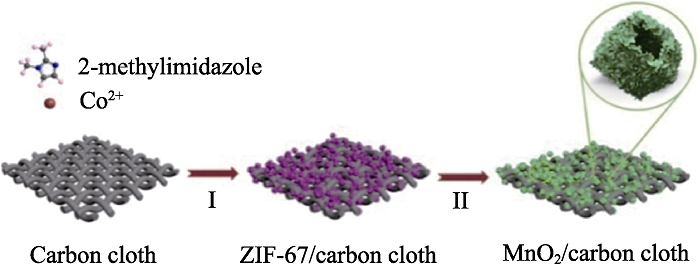
图1 在碳布上两步合成MnO2空心多面体纳米结构的制备过程示意图[13]
Fig. 1 Schematic illustration for the two-step preparation process of MnO2 hollow polyhedrons nanostructures assembled on carbon cloth[13]

图2 采用溶胶-凝胶法与模板法相结合, 以(a) AAO模板A和(b) AAO模板B制备的纳米MnO2的扫描电镜照片[23]
Fig. 2 SEM images of nanostructured MnO2 fabricated by using Sol-Gel and template methods with (a) AAO template A and (b) AAO template B[23]
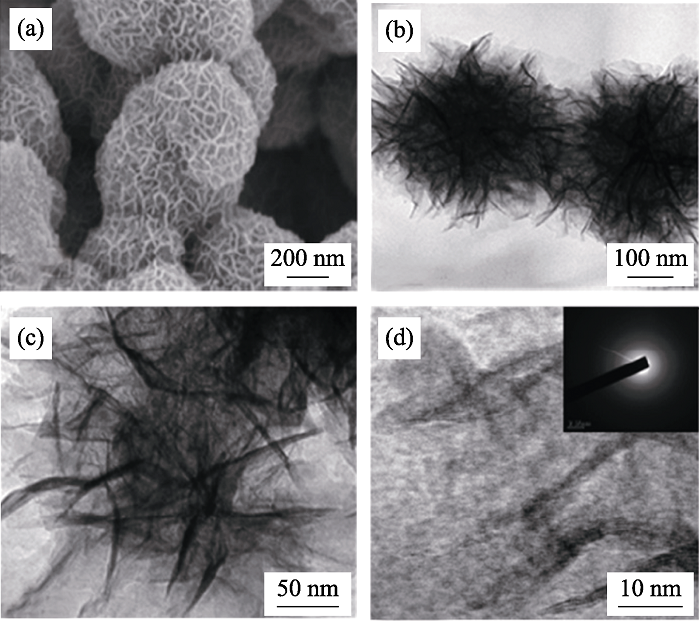
图3 MnO2纳米片组装体的(a) FESEM、(b, c) TEM和(d) HRTEM照片, (d)中插图为SAED花样[28]
Fig. 3 (a) FESEM, (b, c) TEM and (d) HRTEM images of MnO2 assembled nanosheets with inset in (d) showing the corresponding SAED pattern [28]
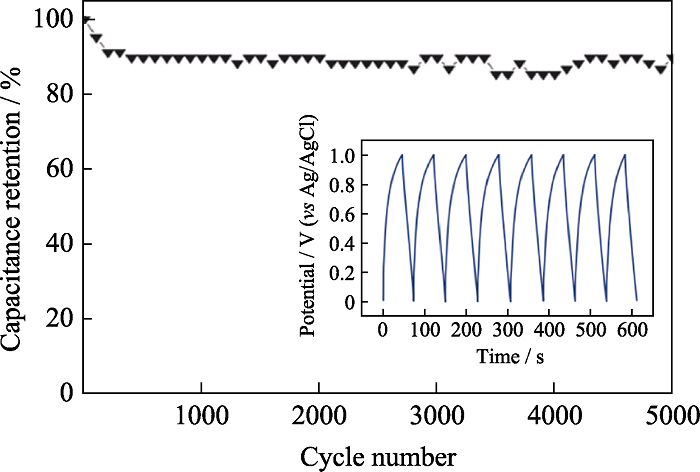
图4 在1 mol/L Na2SO4电解质溶液、2.1 A/g电流密度下δ-MnO2的电容保持率, 插图为相应的δ-MnO2的充放电曲线[16]
Fig. 4 Capacitance retention of δ-MnO2 at current density of 2.1 A/g in 1 mol/L Na2SO4 electrolyte with inset showing the corresponding charge-discharge curves[16]

图5 (a) PANI、(b) MnO2和(c) PANI/MnO2在不同电压下的紫外-可见光透过率光谱, (c)中的插图为PANI/MnO2杂化膜电沉积在ITO/玻璃上的褪色态(上, 浅绿黄色)和着色态(下, 深青绿色)的照片; (d) PANI、MnO2和PANI/MnO2杂化膜在λ680nm (-0.4 V/+0.4 V, 每周期60 s)的响应时间曲线[47]
Fig. 5 UV-Vis transmittance spectra of (a) PANI, (b) MnO2, and (c) PANI/MnO2 at different potentials with insets in (c) showing the photos of PANI/MnO2 hybrid film electrodeposited on ITO/glass at bleached (upper, light greenish yellow) and colored state (lower, dark bluish green), and (d) switching curves comparison between PANI, MnO2, and PANI/MnO2 hybrid films at λ680 nm (-0.4 V/+0.4 V, 60 s/cycle)[47]
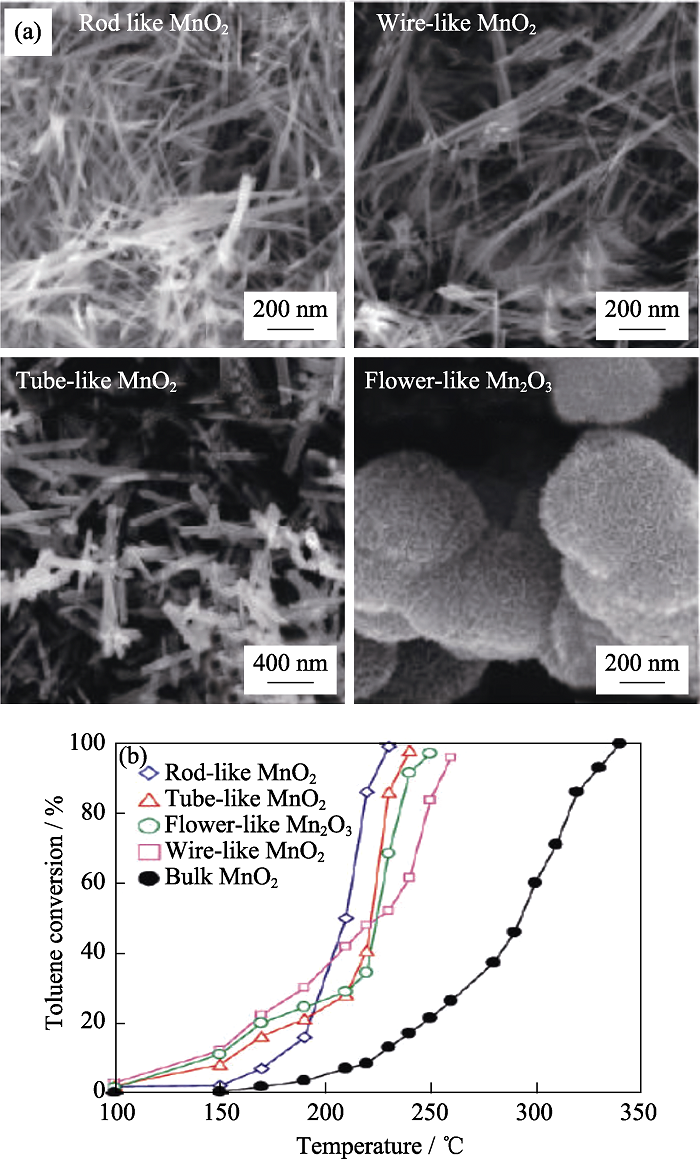
图6 (a)纳米棒状、类丝状、管状α-MnO2和花状球形Mn2O3的SEM照片; (b)在甲苯浓度为10-3、甲苯/O2的摩尔比为1/400和空速为20000 mL/(g?h)的条件下甲苯的转化率随反应温度的变化曲线[51]
Fig. 6 (a) SEM images of rod-like α-MnO2, wire-like α-MnO2, tube-like α-MnO2, and flower-like Mn2O3; (b) Toluene conversion as a function of reaction temperature over the catalysts under the conditions of a toluene concentration of 10-3, toluene/ O2 = 1/400 (mol/mol), and a space velocity of 20000 mL/(g?h)[51]
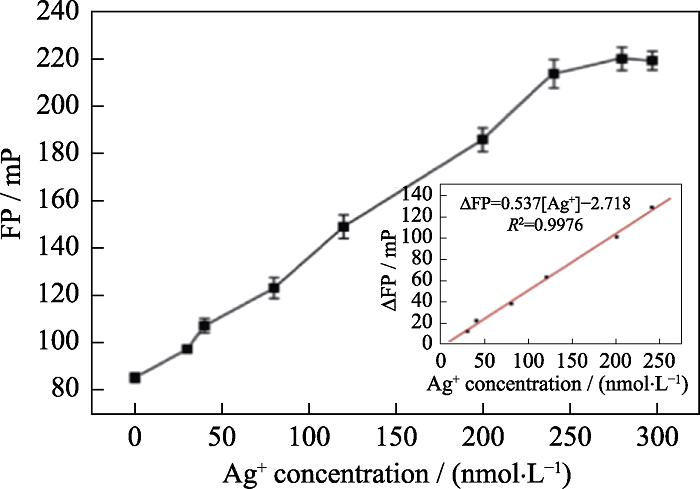
图7 在MnO2纳米片(80 μg/mL)存在下, 加入不同浓度Ag+的荧光偏振值(FP), 插图为ΔFP与Ag+的浓度之间的线性关系[52]
Fig. 7 Measurement of FP following the addition of various concentrations of Ag+ in the presence of MnO2 nanosheets (80 μg/mL) with inset showing the linear relationship between ΔFP and Ag+ concentration[52]
| [1] |
SUN K, LI S Y, WAIGI M G, et al. Nano-MnO2-mediated transformation of triclosan with humic molecules present: kinetics, products, and pathways. Environmental Science & Pollution Research, 2018,25(15):14416-14425.
DOI URL PMID |
| [2] | SEO J K, SHIN J W, CHUNG H, et al. Intercalation and conversion reactions of nanosized β-MnO2 cathode in the secondary Zn/MnO2 alkaline battery. The Journal of Physical Chemistry C, 2018,122(21):11177-11185. |
| [3] | XUE F, WU S, WANG M X, et al. A three-dimensional graphene/ CNT/MnO2 hybrid as supercapacitor electrode. Integrated Ferroelectrics, 2018,190(1):156-163. |
| [4] | GU X, YUE J, LI L J, et al. General synthesis of MnOx (MnO2, Mn2O3, Mn3O4, MnO) hierarchical microspheres as lithium-ion battery anodes. Electrochimica Acta, 2015,184:250-256. |
| [5] | FENG Q, KANOH H, OOI K. Manganese oxide porous crystals. Journal of Materials Chemistry, 1999,9(2):319-333. |
| [6] | POST J E. Manganese oxide minerals: crystal structures and economic and environmental significance. Proceedings of the National Academy of Sciences, 1999,96(7):3447-3454. |
| [7] | JIA Z J, WANG J, WANG Y, et al. Interfacial synthesis of δ-MnO2 nano-sheets with a large surface area and their application in electrochemical capacitors. Journal of Materials Science & Technology, 2016,32(2):147-152. |
| [8] | HUANG Y J, LI W S. Preparation of manganese dioxide for oxygen reduction in zinc air battery by hydro thermal method. Journal of Inorganic Materials, 2013,28(3):341-346. |
| [9] | WEN J G, RUAN X Y, ZHOU Z T. Characterization of MnO2 aerogels prepared via supercritical drying technique. Journal of Inorganic Materials, 2009,24(3):521-524. |
| [10] | XIAO X Z, YI Q F. Synthesis and electochemical capacity of MnO2/SMWCNT/PANI ternarycomposites. Journal of Inorganic Materials, 2013,28(8):825-830. |
| [11] |
DARR J A, ZHANG J Y, MAKWANA N M, et al. Continuous hydrothermal synthesis of inorganic nanoparticles: applications and future directions. Chemical Reviews, 2017,117(17):11125-11238.
DOI URL PMID |
| [12] | ZHAO P, YAO M Q, REN H B, et al. Nanocomposites of hierarchical ultrathin MnO2 nanosheets/hollow carbon nanofibers for high-performance asymmetric supercapacitors. Applied Surface Science, 2019,463:931-938. |
| [13] |
WU F F, GAO X B, XU X L, et al. Boosted Zn storage performance of MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth via a facile wet-chemical synthesis. ChemSusChem, 2020,13(6):1537-1545.
DOI URL PMID |
| [14] | LU C J, ZHU F Q, YIN J G, et al. Synthesis of α-MnO2 nanowires via facile hydrothermal method and their application in Li-O2 battery. Journal of Inorganic Materials, 2018,33(9):1029-1034. |
| [15] | ZHU K, WANG C, CAMARGO P H C, et al. Investigating the effect of MnO2 band gap in hybrid MnO2-Au materials over the SPR-mediated activities under visible light. Journal of Materials Chemistry A, 2019,7(3):925-931. |
| [16] | WANG L, MA W L, LI Y H, et al. Synthesis of δ-MnO2 with nanoflower-like architecture by a microwave-assisted hydrothermal method. Journal of Sol-Gel Science and Technology, 2017,82:85-91. |
| [17] | MA Z C, WEI X Y, XING S T, et al. Hydrothermal synthesis and characterization of surface-modified δ-MnO2 with high Fenton-like catalytic activity. Catalysis Communications, 2015,67:68-71. |
| [18] | LIU D Y, DONG L M, SHAN L W, et al. Preparation of Fe-MnO2/RGO electrode and electrochemical properties. Ferroelectrics, 2019,546(1):41-47. |
| [19] | XIE Y M, WANG L J, GUO Q Y, et al. Preparation of MnO2/porous carbon material with core-shell structure and its application in supercapacitor. Journal of Materials Science Materials in Electronics, 2018,29(10):1-8. |
| [20] | MATHUR A, HALDER A. One step synthesis of bifunctional iron-doped manganese oxide nanorods for rechargeable zinc-air batteries. Catalysis Science & Technology, 2019,9(5):1245-1254. |
| [21] |
JITTIARPORN P, BADILESCU S, Al SAWAFTA M N, et al. Electrochromic properties of Sol-Gel prepared hybrid transition metal oxides - a short review. Journal of Science: Advanced Materials and Devices, 2017,2(3):286-300.
DOI URL |
| [22] |
MOHAMED M A, SALLEH W N W, JAAFAR J, et al. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: bio-template assisted Sol-Gel synthesis and photocatalytic activity. Applied Surface Science, 2017,393:46-59.
DOI URL |
| [23] | WANG X Y, WANG X Y, HUANG W G, et al. Sol-Gel template synthesis of highly ordered MnO2 nanowire arrays. Journal of Power Sources, 2005,140(1):211-215. |
| [24] | 赵娜英, 卞洁鹏, 杨雪健, 等. 溶胶-凝胶法制备掺镧改性纳米MnO2. 化工新型材料, 2019,47(5):164-171. |
| [25] | 李哲, 汤化伟, 王百年. 纳米MnO2负载硅藻土对苯酚废水的吸附性能研究. 合肥工业大学学报(自然科学版), 2016,39(5):695-700. |
| [26] | THEISS F L, AYOKO G A, FROST R L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods-a review. Applied Surface Science, 2016,383:200-213. |
| [27] | LI X L, ZHU J F, JIAO Y H, et al. Manganese dioxide morphology on electrochemical performance of Ti3C2Tx@MnO2 composites. Journal of Inorganic Materials, 2020,35(1):119-125. |
| [28] | MAHAMALLIK P, SAHA S, PAL A. Tetracycline degradation in aquatic environment by highly porous MnO2 nanosheet assembly. Chemical Engineering Journal, 2015,276:155-165. |
| [29] | 吴昊天, 张振忠, 赵芳霞, 等. 低温固相法制备的纳米α-MnO2的性能. 电池, 2015,45(3):157-159. |
| [30] | 龚良玉. 固相合成MnO2纳米棒的电容性能及其PbO改性研究. 青岛农业大学学报(自然科学版), 2011,28(2):157-161. |
| [31] | 李娟, 夏熙. 纳米MnO2的固相合成及其电化学性能的研究(Ι): 纳米γ-MnO2的合成及表征. 高等学校化学学报, 1999,20(9):1434-1437. |
| [32] | SHIN J, SEO J K, YAYLIAN R, et al. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. International Materials Reviews, 2019: 1-32. |
| [33] | SHAFI P M, BOSE A C. Structural evolution of tetragonal MnO2 and its electrochemical behavior. AIP Conference Proceedings, 2016,1731(1):050038. |
| [34] | HAN S D, KIM S, LI D G, et al. Mechanism of Zn insertion into nanostructured δ-MnO2: a nonaqueous rechargeable Zn metal battery. Chemistry of Materials, 2017,29(11):4874-4884. |
| [35] |
ZERAATI A S, ARJMAND M, SUNDARARAJ U. Silver nanowire/MnO2 nanowire hybrid polymer nanocomposites: materials with high dielectric permittivity and low dielectric loss. ACS Applied Materials & Interfaces, 2017,9(16):14328-14336.
DOI URL PMID |
| [36] | REHMAN S, TANG T Y, ALI Z, et al. Integrated design of MnO2@carbon hollow nanoboxes to synergistically encapsulate polysulfides for empowering lithium sulfur batteries. Small, 2017,13(20):1700087. |
| [37] | LUO P F, HUANG Z. Fabrication of scandium-doped lithium manganese oxide as a high-rate capability cathode material for lithium energy storage. Solid State Ionics, 2019,338:20-24. |
| [38] | WANG Y M, WANG F, FENG X J. Porous nest-like LiMnPO4 microstructures assembled by nanosheets for lithium ion battery cathodes. Journal of Materials Science: Materials in Electronics, 2018,29(2):1426-1434. |
| [39] | 李俊豪, 冯斯桐, 张圣洁, 等. 高性能磷酸锰锂正极材料的研究进展. 材料导报, 2019,33(9):2854-2861. |
| [40] | ZHAO J X, WANG G H, ZHANG Q, et al. An underlying intercalation ion for fast-switching and stable electrochromism. Journal of Materials Science Materials in Electronics, 2019,30(13):12753-12756. |
| [41] | LIU Y R, RYOTA S, CHEUK L H, et al. Electrochromic triphenylamine-based cobalt (II) complex nanosheets. Journal of Materials Chemistry C, 2019,7(30):9159-9166. |
| [42] | CHEN C W, BRIGEMAN A N, HO T J, et al. Normally transparent smart window based on electrically induced instability in dielectrically negative cholesteric liquid crystal. Optical Materials Express, 2018,8(3):691. |
| [43] |
TONG Z Q, LIU S K, LI X G, et al. Achieving rapid Li-ion insertion kinetics in TiO2 mesoporous nanotube arrays for bifunctional high-rate energy storage smart windows. Nanoscale, 2018,10:3254-3261.
DOI URL PMID |
| [44] | CANNAVALE A, AYR U, FIORITO F, et al. Smart electrochromic windows to enhance building energy efficiency and visual comfort. Energies, 2020,13(6):1449. |
| [45] | BECHINGER C, FERRERE S, ZABAN A, et al. Photoelectrochromic windows and displays. Nature, 1996,383(6601):608-610. |
| [46] | CHO S I, KWON W J, CHOI S J, et al. Nanotube-based ultrafast electrochromic display. Advanced Materials, 2005,17(2):171-175. |
| [47] | ZHOU D, CHE B Y, LU X H. Rapid one-pot electrodeposition of polyaniline/manganese dioxide hybrids: a facile approach to stable high-performance anodic electrochromic materials. Journal of Materials Chemistry C, 2017(5):1758-1766. |
| [48] | SAKAI N, EBINA Y, TAKADA K, et al. Electrochromic films composed of MnO2 nanosheets with controlled optical density and high coloration efficiency. Journal of the Electrochemical Society, 2005,152(12):E384-E389. |
| [49] | FALAHATGAR S S, GHODSI F E, TEPEHAN F Z, et al. Electrochromic performance of Sol-Gel derived amorphous MnO2-ZnO nanogranular composite thin films. Journal of Non-Crystalline Solids, 2015,427:1-9. |
| [50] | LYU W M, YANG L, FAN B B, et al. Silylated MgAl LDHs intercalated with MnO2 nanowires: highly efficient catalysts for the solvent-free aerobic oxidation of ethylbenzene. Chemical Engineering Journal, 2015,263:309-316. |
| [51] |
WANG F, DAI H X, DENG J G, et al. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: highly effective catalysts for the removal of toluene. Environmental Science & Technology, 2012,46(7):4034-4041.
DOI URL PMID |
| [52] |
QI L, YAN Z, HUO Y, et al. MnO2 nanosheet-assisted ligand-DNA interaction-based fluorescence polarization biosensor for the detection of Ag+ ions. Biosensors and Bioelectronics, 2017,87:566-571.
DOI URL PMID |
| [53] |
XIAO F, LI Y Q, GAO H C, et al. Growth of coral-like PtAu-MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosensors & Bioelectronics, 2013,41:417-423.
DOI URL PMID |
| [1] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [2] | 甄明硕, 刘晓然, 范向前, 张文平, 严东东, 刘磊, 李晨. 电致变色型智能可视化湿度系统[J]. 无机材料学报, 2024, 39(4): 432-440. |
| [3] | 冯星哲, 马董云, 王金敏. 多孔NiMn-LDH纳米片薄膜的溶剂热生长及其电致变色性能[J]. 无机材料学报, 2024, 39(12): 1391-1396. |
| [4] | 李跃军, 曹铁平, 孙大伟. S型异质结Bi4O5Br2/CeO2的制备及其光催化CO2还原性能[J]. 无机材料学报, 2023, 38(8): 963-970. |
| [5] | 牛海滨, 黄佳慧, 李倩文, 马董云, 王金敏. 多孔NiMoO4纳米片薄膜的直接水热生长及其电致变色性能[J]. 无机材料学报, 2023, 38(12): 1427-1433. |
| [6] | 孙佳伟, 宛心怡, 杨婷, 马董云, 王金敏. Ti2Nb10O29薄膜的制备及其电致变色性能[J]. 无机材料学报, 2023, 38(12): 1434-1440. |
| [7] | 陈长, 赵若伊, 韩少杰, 王焕燃, 杨群, 高彦峰. 纳米晶液相镀膜制备WO3电致变色薄膜研究和性能优化[J]. 无机材料学报, 2023, 38(11): 1355-1363. |
| [8] | 姚仪帅, 郭瑞华, 安胜利, 张捷宇, 周国治, 张国芳, 黄雅荣, 潘高飞. 原位负载Pt-Co高指数晶面催化剂的制备及其电催化性能[J]. 无机材料学报, 2023, 38(1): 71-78. |
| [9] | 张家强, 邹馨蕾, 王能泽, 贾春阳. 两步电沉积法制备Zn-Fe PBA薄膜及其在电致变色器件中的性能研究[J]. 无机材料学报, 2022, 37(9): 961-968. |
| [10] | 张笑宇, 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿, 王宏志. 基于Cu3(HHTP)2薄膜的离子液体电致变色电极[J]. 无机材料学报, 2022, 37(8): 883-890. |
| [11] | 黄郅航, 滕官宏伟, 铁鹏, 范德松. 钙钛矿陶瓷薄膜的电致变色特性[J]. 无机材料学报, 2022, 37(6): 611-616. |
| [12] | 张弦, 张策, 姜文君, 冯德强, 姚伟. 四元BiMnVO5的合成、电子结构与可见光催化性能研究[J]. 无机材料学报, 2022, 37(1): 58-64. |
| [13] | 王金敏, 后丽君, 马董云. 氧化钼电致变色材料与器件[J]. 无机材料学报, 2021, 36(5): 461-470. |
| [14] | 张翔, 李文杰, 王乐滨, 陈曦, 赵九蓬, 李垚. 无机电致变色材料反射特性研究进展[J]. 无机材料学报, 2021, 36(5): 451-460. |
| [15] | 武琦, 丛杉, 赵志刚. 多彩氧化钨薄膜的红外电致变色性能研究[J]. 无机材料学报, 2021, 36(5): 485-491. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||