无机材料学报 ›› 2024, Vol. 39 ›› Issue (5): 467-476.DOI: 10.15541/jim20230491 CSTR: 32189.14.10.15541/jim20230491
所属专题: 【能源环境】氢能材料(202506)
收稿日期:2023-10-23
修回日期:2024-01-03
出版日期:2024-05-20
网络出版日期:2024-01-31
通讯作者:
陈必清, 教授. E-mail: chenbq2332@163.com作者简介:景欣欣(1998-), 女, 硕士研究生. E-mail: 470375822@qq.com
基金资助:
JING Xinxin( ), CHEN Biqing(
), CHEN Biqing( ), ZHAI Jiaxin, YUAN Meiling
), ZHAI Jiaxin, YUAN Meiling
Received:2023-10-23
Revised:2024-01-03
Published:2024-05-20
Online:2024-01-31
Contact:
CHEN Biqing, professor. E-mail: chenbq2332@163.comAbout author:JING Xinxin (1998-), female, Master candidate. E-mail: 470375822@qq.com
Supported by:摘要:
开发低成本、高活性的非贵金属电催化剂对电解水的实际应用具有重要意义, 稀土(Rare Earth, RE)元素因其独特的电子结构成为金属催化剂改性的研究热点, 但在泡沫镍(Nickel Foam, NF)基体上制备稀土复合催化剂的方法存在成本高、工艺复杂和耗时长等问题。本研究采用一步化学沉积法, 在NF基体上制备了三种稀土复合电极Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF, 对催化电极的结构和形貌进行了表征, 并研究其在1 mol·L-1 KOH溶液中的析氢性能。结果表明, 添加Sm、Dy和Tb可使电极的电子结构发生改变, 改善催化剂材料的本征性质, 进而提高析氢反应(Hydrogen Evolution Reaction, HER)的催化性能。其中, Ni-Co-B-Tb/NF表现出最佳的析氢性能, 达到10 mA·cm-2的电流密度仅需58 mV的过电位, Tafel斜率为65 mV·dec-1, 析氢反应由Volmer-Heyrovsky步骤控制。进一步研究发现稀土浓度对电催化性能影响较大, 当Tb浓度为3 g·L-1时, Ni-Co-B-Tb/NF表面颗粒尺寸较小且分布均匀, 暴露出更多的活性位点, 有利于HER的电荷转移, 析氢性能最佳。此外, 经过100 h长期稳定性测试和2000次循环伏安(Cyclic Voltammetry, CV)测试后, 该催化剂表现出良好的电化学稳定性。
中图分类号:
景欣欣, 陈必清, 翟佳鑫, 袁美玲. Ni-Co-B-RE(Sm、Dy、Tb)复合电极: 化学沉积法制备及电催化析氢性能研究[J]. 无机材料学报, 2024, 39(5): 467-476.
JING Xinxin, CHEN Biqing, ZHAI Jiaxin, YUAN Meiling. Ni-Co-B-RE (Sm, Dy, Tb) Composite Electrodes: Preparation by Chemical Deposition Method and Electrocatalytic Hydrogen Evolution Performance[J]. Journal of Inorganic Materials, 2024, 39(5): 467-476.

图1 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF的(a~d)低倍率和(e~h)高倍率SEM照片
Fig. 1 (a-d) Low-magnification and (e-h) high-magnification SEM images of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF, and Ni-Co-B-Dy/NF

图2 (a) Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF样品粉末的XRD谱图; (b) Ni-Co-B-Tb/NF的EDS谱图
Fig. 2 (a) XRD patterns of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF powders, and (b) EDS mappings of Ni-Co-B-Tb/NF
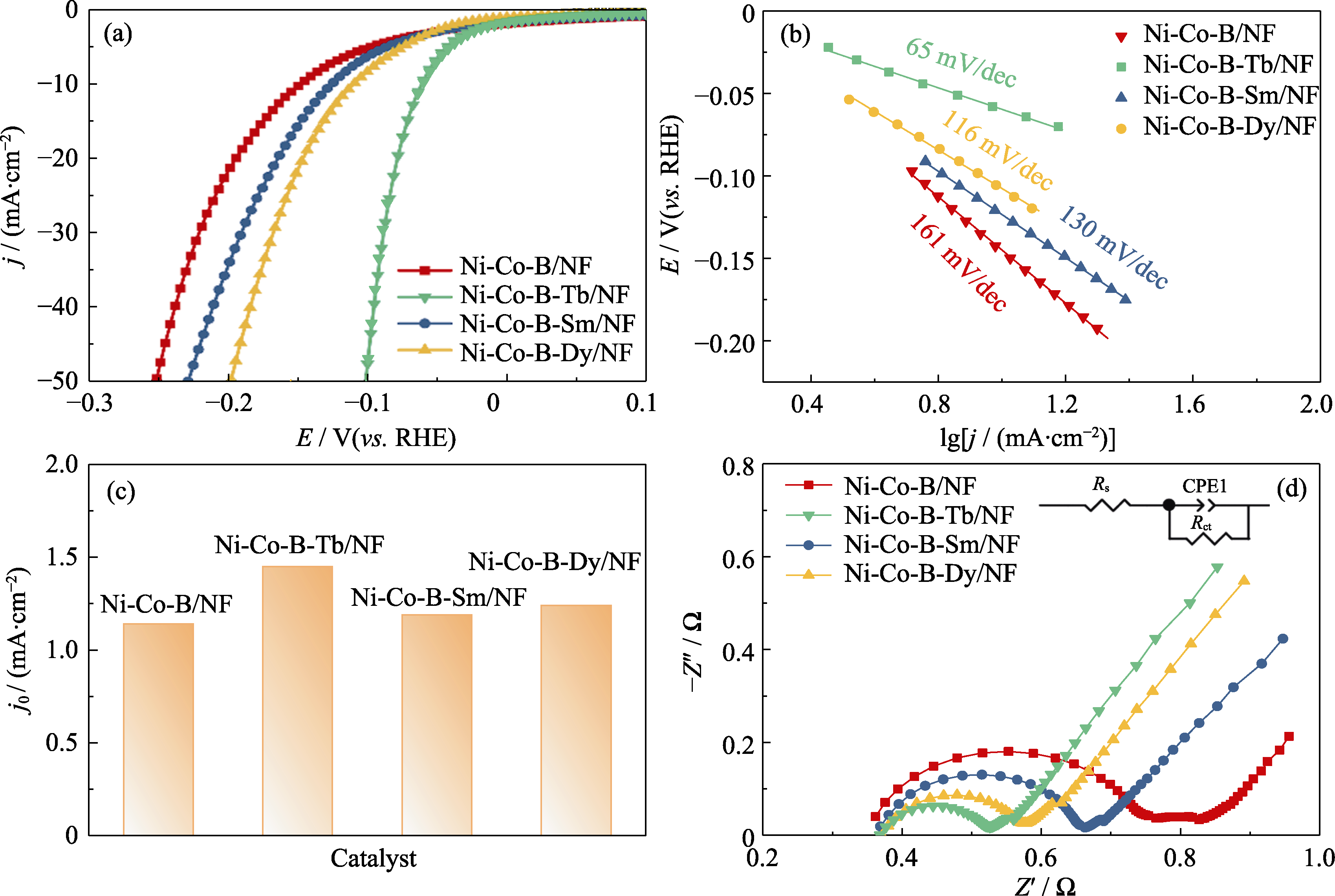
图5 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF电极的(a)LSV曲线, (b) Tafel曲线, (c) Tafel曲线外推得到的交换电流密度(j0)和(d)EIS图谱(插图为等效电路图)
Fig. 5 (a) LSV curves, (b) Tafel curves, (c) exchange current densities (j0) extrapolated from Tafel plots and (d) EIS spectra with inset showing equivalent circuit of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF, and Ni-Co-B-Dy/NF electrodes Colorful figures are available on website

图6 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF(a~d)在不同温度下的LSV曲线(插图为相应的Tafel曲线)和(e)阿伦尼乌斯曲线
Fig. 6 (a-d) LSV curves at different temperatures with insets showing corresponding Tafel curves and (e) Arrhenius plots of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF Colorful figures are available on website

图7 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF电极(a~d)在不同扫描速率下的CV曲线, (e)充电双电层库仑曲线和(f) ECSA归一化后的LSV曲线
Fig. 7 (a-d) CV curves at different scan rates, (e) charge double-layer voltammetry, and (f) ECSA-normalized LSV curves of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF, and Ni-Co-B-Dy/NF electrodes Colorful figures are available on website

图8 x-TNCB/NF(x=1, 2, 3, 4, 5)电极的(a)LSV曲线, (b) Tafel曲线, (c) Tafel曲线外推得到的交换电流密度(j0)和(d)EIS图谱(插图为等效电路图)
Fig. 8 (a) LSV curves, (b) Tafel curves, (c) exchange current densities (j0) extrapolated from Tafel plots, and (d) EIS spectra with inset showing equivalent circuit of x-TNCB/NF (x=1, 2, 3, 4, 5) electrodes Colorful figures are available on website

图9 3-TNCB/NF电极(a)在2000圈CV扫描前后的LSV曲线和(b)在100 mV电位下电解100 h的电流-时间曲线
Fig. 9 (a) LSV curves before and after 2000 CV sweeps, and (b) I-t curve under 100 mV static overpotential for 100 h electrolysis of 3-TNCB/NF electrode
| Composition of plating solution | Concentration/(g·L-1) |
|---|---|
| Anhydrous nickel chloride (NiCl2) | 10.0 |
| Anhydrous cobalt chloride (CoCl2) | 5.0 |
| Borane dimethylamine | 1.0 |
| Samarium nitrate [Sm(NO3)3] | 3.0 |
| Terbium nitrate [Tb(NO3)3] | 1.0-5.0 |
| Dysprosium nitrate [Dy(NO3)3] | 3.0 |
| Succinic acid | 1.5 |
| Citric acid | 1.5 |
| Malic acid | 1.5 |
表S1 化学沉积镀液配方
Table S1 Chemical deposition plating solution formula
| Composition of plating solution | Concentration/(g·L-1) |
|---|---|
| Anhydrous nickel chloride (NiCl2) | 10.0 |
| Anhydrous cobalt chloride (CoCl2) | 5.0 |
| Borane dimethylamine | 1.0 |
| Samarium nitrate [Sm(NO3)3] | 3.0 |
| Terbium nitrate [Tb(NO3)3] | 1.0-5.0 |
| Dysprosium nitrate [Dy(NO3)3] | 3.0 |
| Succinic acid | 1.5 |
| Citric acid | 1.5 |
| Malic acid | 1.5 |
| Catalyst | Element | Content/%(in mass) |
|---|---|---|
| Ni-Co-B-Tb/NF | Ni | 49.08 |
| Co | 22.20 | |
| B | 2.71 | |
| Tb | 1.57 | |
| Ni-Co-B-Sm/NF | Ni | 39.48 |
| Co | 9.41 | |
| B | 2.16 | |
| Sm | 1.35 | |
| Ni-Co-B-Dy/NF | Ni | 53.89 |
| Co | 21.66 | |
| B | 2.49 | |
| Dy | 1.94 |
表S2 Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF电极的ICP-MS数据
Table S2 ICP-MS data of Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF electrodes
| Catalyst | Element | Content/%(in mass) |
|---|---|---|
| Ni-Co-B-Tb/NF | Ni | 49.08 |
| Co | 22.20 | |
| B | 2.71 | |
| Tb | 1.57 | |
| Ni-Co-B-Sm/NF | Ni | 39.48 |
| Co | 9.41 | |
| B | 2.16 | |
| Sm | 1.35 | |
| Ni-Co-B-Dy/NF | Ni | 53.89 |
| Co | 21.66 | |
| B | 2.49 | |
| Dy | 1.94 |
| Catalyst | Rs/(Ω·cm-2) | Rct/(Ω·cm-2) |
|---|---|---|
| Ni-Co-B/NF | 0.379 | 0.171 |
| Ni-Co-B-Tb/NF | 0.376 | 0.144 |
| Ni-Co-B-Sm/NF | 0.390 | 0.251 |
| Ni-Co-B-Dy/NF | 0.393 | 0.357 |
表S3 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF电极的EIS参数
Table S3 EIS parameters of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF electrodes
| Catalyst | Rs/(Ω·cm-2) | Rct/(Ω·cm-2) |
|---|---|---|
| Ni-Co-B/NF | 0.379 | 0.171 |
| Ni-Co-B-Tb/NF | 0.376 | 0.144 |
| Ni-Co-B-Sm/NF | 0.390 | 0.251 |
| Ni-Co-B-Dy/NF | 0.393 | 0.357 |
| Catalyst | T/K | Tafel slope/(mV·dec-1) | j0/(mA·cm-2) | Ea/(kJ·mol-1) |
|---|---|---|---|---|
| Ni-Co-B/NF | 298 | 115 | 1.86 | 32.2 |
| 313 | 88 | 4.36 | ||
| 323 | 104 | 8.12 | ||
| 333 | 99 | 18.6 | ||
| Ni-Co-B-Tb/NF | 298 | 123 | 2.69 | 11.5 |
| 313 | 125 | 3.71 | ||
| 323 | 125 | 4.89 | ||
| 333 | 114 | 6.02 | ||
| Ni-Co-B-Sm/NF | 298 | 152 | 1.65 | 19.8 |
| 313 | 140 | 2.57 | ||
| 323 | 150 | 3.46 | ||
| 333 | 219 | 6.30 | ||
| Ni-Co-B-Dy/NF | 298 | 164 | 1.99 | 17.2 |
| 313 | 168 | 3.01 | ||
| 323 | 165 | 4.36 | ||
| 333 | 187 | 6.30 |
表S4 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF的动力学参数
Table S4 Kinetic parameters of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF
| Catalyst | T/K | Tafel slope/(mV·dec-1) | j0/(mA·cm-2) | Ea/(kJ·mol-1) |
|---|---|---|---|---|
| Ni-Co-B/NF | 298 | 115 | 1.86 | 32.2 |
| 313 | 88 | 4.36 | ||
| 323 | 104 | 8.12 | ||
| 333 | 99 | 18.6 | ||
| Ni-Co-B-Tb/NF | 298 | 123 | 2.69 | 11.5 |
| 313 | 125 | 3.71 | ||
| 323 | 125 | 4.89 | ||
| 333 | 114 | 6.02 | ||
| Ni-Co-B-Sm/NF | 298 | 152 | 1.65 | 19.8 |
| 313 | 140 | 2.57 | ||
| 323 | 150 | 3.46 | ||
| 333 | 219 | 6.30 | ||
| Ni-Co-B-Dy/NF | 298 | 164 | 1.99 | 17.2 |
| 313 | 168 | 3.01 | ||
| 323 | 165 | 4.36 | ||
| 333 | 187 | 6.30 |
| Catalyst | ECSA/(cm2·g-1) |
|---|---|
| Ni-Co-B/NF | 677.5 |
| Ni-Co-B-Tb/NF | 1517.5 |
| Ni-Co-B-Sm/NF | 980 |
| Ni-Co-B-Dy/NF | 1262.5 |
表S5 Ni-Co-B/NF、Ni-Co-B-Tb/NF、Ni-Co-B-Sm/NF和Ni-Co-B-Dy/NF催化剂的ECSA数据
Table S5 ECSA of Ni-Co-B/NF, Ni-Co-B-Tb/NF, Ni-Co-B-Sm/NF and Ni-Co-B-Dy/NF catalysts
| Catalyst | ECSA/(cm2·g-1) |
|---|---|
| Ni-Co-B/NF | 677.5 |
| Ni-Co-B-Tb/NF | 1517.5 |
| Ni-Co-B-Sm/NF | 980 |
| Ni-Co-B-Dy/NF | 1262.5 |
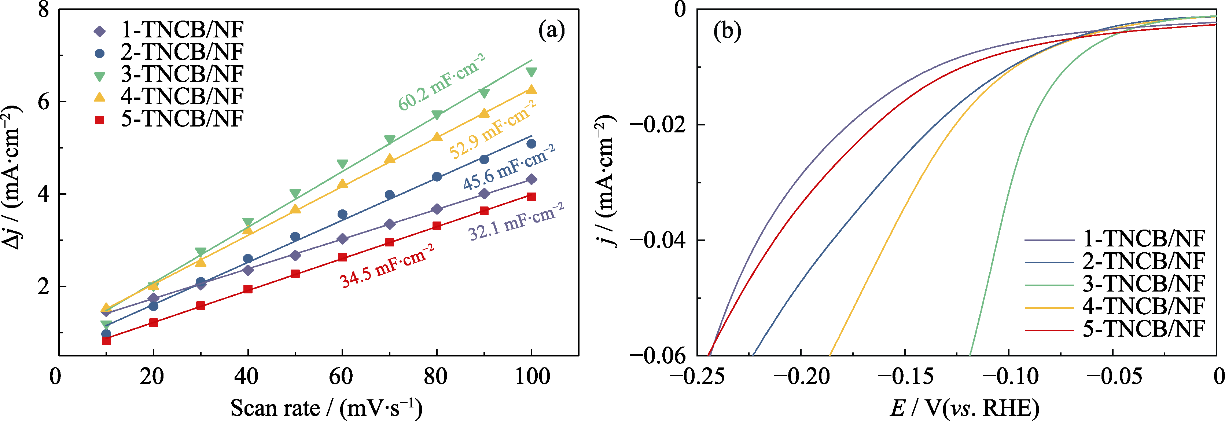
图S4 x-TNCB/NF (x=1, 2, 3, 4, 5)电极的(a)充电双电层库仑曲线和(b)ECSA归一化后LSV曲线
Fig. S4 (a) Charge double-layer voltammetry and (b) LSV curves with normalized ECSA of x-TNCB/NF (x=1, 2, 3, 4, 5) electrodes
| Catalyst | ECSA/(cm2·g-1) |
|---|---|
| 1-TNCB/NF | 802.5 |
| 2-TNCB/NF | 1140.0 |
| 3-TNCB/NF | 1505.0 |
| 4-TNCB/NF | 1322.5 |
| 5-TNCB/NF | 862.5 |
表S6 x-TNCB/NF (x=1, 2, 3, 4, 5)催化剂的ECSA数据
Table S6 ECSA of x-TNCB/NF (x=1, 2, 3, 4, 5) catalysts
| Catalyst | ECSA/(cm2·g-1) |
|---|---|
| 1-TNCB/NF | 802.5 |
| 2-TNCB/NF | 1140.0 |
| 3-TNCB/NF | 1505.0 |
| 4-TNCB/NF | 1322.5 |
| 5-TNCB/NF | 862.5 |
| Catalyst | Rs/(Ω·cm-2) | Rct/(Ω·cm-2) |
|---|---|---|
| 1-TNCB/NF | 0.307 | 0.376 |
| 2-TNCB/NF | 0.299 | 0.275 |
| 3-TNCB/NF | 0.320 | 0.222 |
| 4-TNCB/NF | 0.306 | 0.258 |
| 5-TNCB/NF | 0.315 | 0.353 |
表S7 x-TNCB/NF (x=1, 2, 3, 4, 5)电极的EIS参数
Table S7 EIS parameters of x-TNCB/NF (x=1, 2, 3, 4, 5) electrodes
| Catalyst | Rs/(Ω·cm-2) | Rct/(Ω·cm-2) |
|---|---|---|
| 1-TNCB/NF | 0.307 | 0.376 |
| 2-TNCB/NF | 0.299 | 0.275 |
| 3-TNCB/NF | 0.320 | 0.222 |
| 4-TNCB/NF | 0.306 | 0.258 |
| 5-TNCB/NF | 0.315 | 0.353 |

图S5 x-TNCB/NF(x=1, 2, 3, 4, 5)电极(a~e)在不同温度下LSV曲线(插图为不同温度下Tafel曲线)和(f)阿伦尼乌斯曲线
Fig. S5 (a-e) LSV curves at different temperatures and (f) Arrhenius plots with inset showing corresponding Tafel curves of x-TNCB/NF (x=1, 2, 3, 4, 5)
| Catalyst | T/K | Tafel slope/(mV·dec-1) | j0/(mA·cm-2) | Ea/(kJ·mol-1) |
|---|---|---|---|---|
| 1-TNCB/NF | 298 | 138 | 1.94 | 24.8 |
| 313 | 152 | 3.23 | ||
| 323 | 141 | 6.02 | ||
| 333 | 130 | 10.71 | ||
| 2-TNCB/NF | 298 | 177 | 1.73 | 19.7 |
| 313 | 154 | 2.75 | ||
| 323 | 137 | 4.46 | ||
| 333 | 130 | 7.24 | ||
| 3-TNCB/NF | 298 | 141 | 3.01 | 11.5 |
| 313 | 130 | 3.98 | ||
| 323 | 163 | 5.24 | ||
| 333 | 162 | 6.76 | ||
| 4-TNCB/NF | 298 | 148 | 2.13 | 16.2 |
| 313 | 128 | 3.31 | ||
| 323 | 161 | 4.89 | ||
| 333 | 195 | 6.60 | ||
| 5-TNCB/NF | 298 | 169 | 1.69 | 19.8 |
| 313 | 167 | 2.57 | ||
| 323 | 163 | 4.57 | ||
| 333 | 205 | 6.91 |
表S8 x-TNCB/NF (x=1, 2, 3, 4, 5)的动力学参数
Table S8 Kinetic parameters of x-TNCB/NF (x=1, 2, 3, 4, 5)
| Catalyst | T/K | Tafel slope/(mV·dec-1) | j0/(mA·cm-2) | Ea/(kJ·mol-1) |
|---|---|---|---|---|
| 1-TNCB/NF | 298 | 138 | 1.94 | 24.8 |
| 313 | 152 | 3.23 | ||
| 323 | 141 | 6.02 | ||
| 333 | 130 | 10.71 | ||
| 2-TNCB/NF | 298 | 177 | 1.73 | 19.7 |
| 313 | 154 | 2.75 | ||
| 323 | 137 | 4.46 | ||
| 333 | 130 | 7.24 | ||
| 3-TNCB/NF | 298 | 141 | 3.01 | 11.5 |
| 313 | 130 | 3.98 | ||
| 323 | 163 | 5.24 | ||
| 333 | 162 | 6.76 | ||
| 4-TNCB/NF | 298 | 148 | 2.13 | 16.2 |
| 313 | 128 | 3.31 | ||
| 323 | 161 | 4.89 | ||
| 333 | 195 | 6.60 | ||
| 5-TNCB/NF | 298 | 169 | 1.69 | 19.8 |
| 313 | 167 | 2.57 | ||
| 323 | 163 | 4.57 | ||
| 333 | 205 | 6.91 |
| [1] | MIDILLI A, KUCUK H, TOPAL M E, et al. A comprehensive review on hydrogen production from coal gasification: challenges and opportunities. International Journal of Hydrogen Energy, 2021, 46(50): 25385. |
| [2] | SHI P D, ZHANG Y, ZHANG G L, et al. A crystalline/amorphous CoP@CoB hierarchical core-shell nanorod array for enhanced hydrogen evolution. Journal of Materials Chemistry A, 2021, 9(35): 19719. |
| [3] | HOU Z M, XIONG Y, LUO J S, et al. International experience of carbon neutrality and prospects of key technologies: lessons for China. Petroleum Science, 2023, 20(2): 893. |
| [4] | LI H, GUO J, LI Z, et al. Research progress of hydrogen production technology and related catalysts by electrolysis of water. Molecules, 2023, 28(13): 5010. |
| [5] | YANG J S, LI J, WANG Y, et al. Tailoring the pore structure of porous Ni-Sn alloys for boosting hydrogen evolution reaction in alkali solution. Metals, 2022, 12(12): 2123. |
| [6] | GAO Y C, JIANG J G, MENG Y, et al. A review of recent developments in hydrogen production via biogas dry reforming. Energy Conversion and Management, 2018, 171: 133. |
| [7] | YAN S, PAN W, CHUN W, et al. Electrodeposition of amorphous Ni-Fe-Mo composite as a binder-free and high-performance electrocatalyst for hydrogen generation from alkaline water electrolysis. International Journal of Hydrogen Energy, 2023, 48(85): 33130. |
| [8] | LI H, LU X L, DING H H, et al. Copper-doped NiC2S4 nanosheets grown on Ni foam efficient hydrogen evolution catalyst in alkaline solution. ECS Journal of Solid State Science and Technology, 2023, 12(6): 063004. |
| [9] |
RAFAEL C, CARMEN N. Chemicals from alkynes with palladium catalysts. Chemical Reviews, 2014, 114(3): 1783.
DOI PMID |
| [10] | XU Y, FENG T, WANG Y, et al. Constructing bifunctional Fe7S8/CoS heterostructures for efficient water electrolysis. International Journal of Hydrogen Energy, 2023, 48(1): 113. |
| [11] | KADREKAR R, PATEL N, ARYA A. Understanding the role of boron and stoichiometric ratio in the catalytic performance of amorphous Co-B catalyst. Applied Surface Science, 2020, 518: 146199. |
| [12] | LONG H Y, GAO D D, WANG P, et al. Amorphization-induced reverse electron transfer in NiB cocatalyst for boosting photocatalytic H2 production. Applied Catalysis B-Environmental, 2024, 340: 123270. |
| [13] | WANG Y, REN J, MA J, et al. Co-Fe-B@g-C3N4/Cu sheet for promoting hydrolytic dehydrogenation from the hydrolysis of NaBH4 solution. Materials Letters, 2023, 351: 135099. |
| [14] | LIU H X, LI X Y, CHEN L L, et al. Monolithic Ni-Mo-B bifunctional electrode for large current water splitting. Advanced Functional Materials, 2022, 32(4): 21107308. |
| [15] | CHEN Z J, DUAN X G, WEI W, et al. Boride-based electrocatalysts: emerging candidates for water splitting. Nano Research, 2020, 13(2): 293. |
| [16] | DOMINIC J, KARTHIKEYAN M, KUMAR K K S. Polyaniline- rare earth metal chloride composites as an adsorbent cum electrode material for supercapacitor performance investigation. Journal of Energy Storage, 2022, 48: 103971. |
| [17] | CHEN H, HUANG H B, LI H H, et al. Self-supporting Co/CeO2 heterostructures for ampere-level current density alkaline water electrolysis. Inorganic Chemistry, 2023, 62(7): 3297. |
| [18] |
ZHU Y N, CHEN B Q, CHENG T S, et al. Amorphous Nd-Ni-B/NF rare earth composites: preparation and her electrocatalytic performance. Journal of Inorganic Materials, 2021, 36(6): 637.
DOI |
| [19] |
LI Y F, YUAN X L, WANG P, et al. Rare earth alloy nanomaterials in electrocatalysis. Journal of Energy Chemistry, 2023, 83: 574.
DOI |
| [20] | CARDOSO D S P, AMARAL L, SANTOS D M F, et al. Enhancement of hydrogen evolution in alkaline water electrolysis by using nickel-rare earth alloys. International Journal of Hydrogen Energy, 2015, 40(12): 4295. |
| [21] | WANG X, TANG Y W, LEE J M, et al. Recent advances in rare-earth-based materials for electrocatalysis. Chem Catalysis, 2022, 2(5): 967. |
| [22] | XIA B Y, WU H B, WANG X, et al. One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. Journal of the American Chemical Society, 2012, 134(34): 13934. |
| [23] | RYOO R, KIM J, JO C, et al. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature, 2020, 585(7824): 221. |
| [24] | ZHANG G D, DAI J Q, LIANG X L. Enhanced ferroelectric properties in La-doped BiFeO3 films by the sol-gel method. Journal of Sol-Gel Science and Technology, 2023, 105(2): 489. |
| [25] |
LI L, WANG X R, GUO Y, et al. Synthesis of an ultrafine CoP nanocrystal/graphene sandwiched structure for efficient overall water splitting. Langmuir, 2020, 36(8): 1916.
DOI PMID |
| [26] | ANASTASIADOU D, LIGT B, HE Y Y, et al. Carbon dioxide and nitrate co-electroreduction to urea on CuOxZnOy. Communications Chemistry, 2023, 6(1): 199. |
| [27] | LEWANDOWSKI M, BARTOSZEWICZ M, JAROSZEWSKA K, et al. Transition metal borides of Ni-B (Co-B) as alternative non-precious catalytic materials: advances, potentials, and challenges. Short review. Journal of Industrial and Engineering Chemistry, 2022, 116: 75. |
| [28] |
ZHANG S M, CHEN B Q, GAO L X, et al. Exploration of electrocatalytic preparation of sodium borohydride with sodium metaborate based on Eu-Ni-B rare earth-composite electrode. Journal of Functional Materials, 2020, 51(4): 4207.
DOI |
| [29] | LIU W K, CHEN R F, LIU L J, et al. Amorphous NiB/carbon nanohybrids: synthesis and catalytic enhancement induced by electron transfer. RSC Advances, 2016, 6(97): 94451. |
| [30] |
LI D X, GUO Z M, ZHAO R H, et al. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for all-pH hydrogen evolution reaction and overall water splitting. Journal of Colloid and Interface Science, 2024, 653: 1725.
DOI PMID |
| [31] | WANG X, ZHANG J, WANG P, et al. Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction. Energy & Environmental Science, 2023, 16(11): 5500. |
| [32] | JOKAR A, TOGHRAEI A, MALEKI M, et al. Facile electrochemical synthesis of Ni-Co-B film on Cu sheet for dual-electrocatalysis of hydrogen and oxygen evolution reactions. Electrochimica Acta, 2021, 389: 10. |
| [33] | YU S, YANG S, CAI D, et al. Regulating f orbital of Tb electronic reservoir to activate stepwise and dual-directional sulfur conversion reaction. InfoMat, 2023, 5(1): e12381. |
| [34] | CHEN R J, WU C L, PAN J X, et al. Microstructure of electroless Ni-B alloy deposits. Heat Treatment of Metals, 2011, 36(7): 88. |
| [35] | LI X S, ZHOU J, SHEN L Q, et al. Exceptionally high saturation magnetic flux density and ultralow coercivity via an amorphous- nanocrystalline transitional microstructure in an FeCo-based alloy. Advanced Materials, 2022, 35(50): 2205863. |
| [36] | GAO W, WEN D, HO J C, et al. Incorporation of rare earth elements with transition metal-based materials for electrocatalysis: a review for recent progress. Materials Today Chemistry, 2019, 12: 266. |
| [37] | ASGARI M, DARBAND G B, MONIRVAGHEFI M. Electroless deposition of Ni-W-Mo-Co-P films as a binder-free, efficient, and durable electrode for electrochemical hydrogen evolution. Electrochimica Acta, 2023, 446: 142001. |
| [38] | XU Y F, YANG H W, CHANG X X, et al. Introduction to electrocatalytic kinetics. Acta Physico-Chimica Sinica, 2023, 39(4): 2210025. |
| [39] | LIU B, HE J B, CHEN Y J, et al. Phytic acid-coated titanium as electrocatalyst of hydrogen evolution reaction in alkaline electrolyte. International Journal of Hydrogen Energy, 2013, 38(8): 3130. |
| [40] | LIN T W, LIU C J, DAI C S. Ni3S2/carbon nanotube nanocomposite as electrode material for hydrogen evolution reaction in alkaline electrolyte and enzyme-free glucose detection. Applied Catalysis B-Environmental, 2014, 154: 213. |
| [41] | PANG Y, ZHU S L, CUI Z D, et al. Self-supported amorphous nanoporous nickel-cobalt phosphide catalyst for hydrogen evolution reaction. Progress in Natural Science-Materials International, 2021, 31(2): 201. |
| [42] | WANG A Q, CHEN J, ZHANG P F, et al. Relation between NiMo(O) phase structures and hydrogen evolution activities of water electrolysis. Acta Physico-Chimica Sinica, 2023, 39(4): 2301023. |
| [43] | WANG H W, GU X K, ZHENG X S, et al. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Science Advances, 2019, 5(1): eaat6413. |
| [44] | GUAN Q Q, ZHU C W, LIN Y, et al. Bimetallic monolayer catalyst breaks the activity-selectivity trade-off on metal particle size for efficient chemoselective hydrogenations. Nature Catalysis, 2021, 4(10): 840. |
| [45] | WANG J, SHAO H T, REN S R, et al. Fabrication of porous Ni-Co catalytic electrode with high performance in hydrogen evolution reaction. Applied Surface Science, 2021, 539: 148045. |
| [1] | 潘泽晟, 游雅萍, 郑雅, 陈海杰, 王连军, 江莞. 面向紫光激发白光LED用荧光材料的耐候性[J]. 无机材料学报, 2025, 40(3): 314-322. |
| [2] | 陈甲, 范依然, 闫文馨, 韩颖超. 聚丙烯酸-钙(铈)纳米团簇荧光探针用于无机磷定量检测研究[J]. 无机材料学报, 2024, 39(9): 1053-1062. |
| [3] | 李刘媛, 黄开明, 赵秀艺, 刘会超, 王超. RE-Si-Al-O玻璃相对高熵稀土双硅酸盐微结构及耐CMAS腐蚀性能的影响[J]. 无机材料学报, 2024, 39(7): 793-802. |
| [4] | 李众少, 李明, 曹逊. 基于含氧氢化钆薄膜的宽波段调制光致变色智能窗[J]. 无机材料学报, 2024, 39(4): 441-448. |
| [5] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [6] | 范栋, 钟鑫, 王亚文, 张振忠, 牛亚然, 李其连, 张乐, 郑学斌. 富铝CMAS对稀土硅酸盐环境障涂层的腐蚀行为与机制研究[J]. 无机材料学报, 2023, 38(5): 544-552. |
| [7] | 王志强, 吴济安, 陈昆峰, 薛冬峰. 大尺寸Er,Yb:YAG单晶的生长及其性能[J]. 无机材料学报, 2023, 38(3): 329-334. |
| [8] | 杨艳国, 任海深, 何代华, 林慧兴. 阳离子场强对BaO-SiO2-Ln2O3微晶玻璃结构及高温性能影响[J]. 无机材料学报, 2023, 38(10): 1207-1215. |
| [9] | 陆晨辉, 葛万银, 宋盼盼, 张盼锋, 徐美美, 张伟. 用于白光LED稀土Eu掺杂SiAlON基荧光粉的发光性能[J]. 无机材料学报, 2023, 38(1): 97-104. |
| [10] | 蔚海浪, 曹学强, 邓龙辉, 蒋佳宁. LaMeAl11O19/YSZ热障涂层热力学性能和热循环寿命[J]. 无机材料学报, 2022, 37(12): 1259-1266. |
| [11] | 马玲玲, 常江. Nd掺杂硅酸钙及其复合电纺丝膜的制备及性能研究[J]. 无机材料学报, 2021, 36(9): 974-980. |
| [12] | 胡靖三, 顾建飞, 章维益. 晶场劈裂序参量导致稀土-硼十二化合物中磁性和比热反常的理论研究[J]. 无机材料学报, 2021, 36(8): 865-870. |
| [13] | 朱云娜, 陈必清, 程天舒, 杜婵, 张士民, 赵静. 非晶Nd-Ni-B/NF稀土复合电极材料的制备及其析氢性能[J]. 无机材料学报, 2021, 36(6): 637-644. |
| [14] | 朱嘉桐, 楼志豪, 张萍, 赵佳, 孟轩宇, 许杰, 高峰. 稀土钽酸盐(RETaO4)高熵陶瓷的制备与热学性能研究[J]. 无机材料学报, 2021, 36(4): 411-417. |
| [15] | 孙鲁超, 任孝旻, 杜铁锋, 罗颐秀, 张洁, 王京阳. 高熵化设计: 稀土硅酸盐材料关键性能优化新策略[J]. 无机材料学报, 2021, 36(4): 339-346. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||