无机材料学报 ›› 2024, Vol. 39 ›› Issue (11): 1265-1274.DOI: 10.15541/jim20240074 CSTR: 32189.14.10.15541/jim20240074
所属专题: 【能源环境】氢能材料(202506); 【结构材料】高熵陶瓷(202506)
张文宇1,2,3( ), 郭瑞华1,2,3(
), 郭瑞华1,2,3( ), 岳全鑫1,2,3, 黄雅荣1, 张国芳1, 关丽丽1,2
), 岳全鑫1,2,3, 黄雅荣1, 张国芳1, 关丽丽1,2
收稿日期:2024-02-21
修回日期:2024-05-23
出版日期:2024-11-20
网络出版日期:2024-06-24
通讯作者:
郭瑞华, 教授. E-mail: grh7810@163.com作者简介:张文宇(1997-), 男, 硕士研究生. E-mail: zhangwenyu529@qq.com
基金资助:
ZHANG Wenyu1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), YUE Quanxin1,2,3, HUANG Yarong1, ZHANG Guofang1, GUAN Lili1,2
), YUE Quanxin1,2,3, HUANG Yarong1, ZHANG Guofang1, GUAN Lili1,2
Received:2024-02-21
Revised:2024-05-23
Published:2024-11-20
Online:2024-06-24
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:ZHANG Wenyu (1997-), male, Master candidate. E-mail: zhangwenyu529@qq.com
Supported by:摘要:
在电解水制氢过程中, 析氢反应(HER)和析氧反应(OER)的缓慢电催化动力学限制了其能量转换效率。高熵材料具有独特的结构特征和优异的性能, 是一种潜在的电解水催化剂, 有可能取代传统的金属氧化物和贵金属。由于金属与非金属之间的不相容性, 关于高熵化合物特别是高熵金属磷化物合成的报道很少。本研究以柠檬酸为络合剂、磷酸二氢铵为磷源, 采用低温溶胶-凝胶法, 通过添加不同组元金属合成了一系列以碳为基底的高熵合金磷化物纳米颗粒。在1 mol·L-1的KOH介质中, FeCoNiMoCeP/C表现出良好的电解水性能, 在电流密度为10 mA·cm-2条件下, FeCoNiMoCeP/C电极电催化HER和OER所需的过电位分别为119和240 mV。在全解水研究中, FeCoNiMoCeP/C表现出优异的催化活性。在电流密度为10 mA·cm-2条件下, FeCoNiMoCeP/C同时用作阴极和阳极的电解水反应所需过电位仅为1.53 V。这是由于高熵磷化物催化剂原子之间的协同作用可以提供更多的反应位点, 增加反应活性和选择性。本研究可拓展高熵合金在电催化领域的潜在应用范围。
中图分类号:
张文宇, 郭瑞华, 岳全鑫, 黄雅荣, 张国芳, 关丽丽. 高熵磷化物双功能催化剂的制备及高效电解水性能[J]. 无机材料学报, 2024, 39(11): 1265-1274.
ZHANG Wenyu, GUO Ruihua, YUE Quanxin, HUANG Yarong, ZHANG Guofang, GUAN Lili. High-entropy Phosphide Bifunctional Catalyst: Preparation and Performance of Efficient Water Splitting[J]. Journal of Inorganic Materials, 2024, 39(11): 1265-1274.
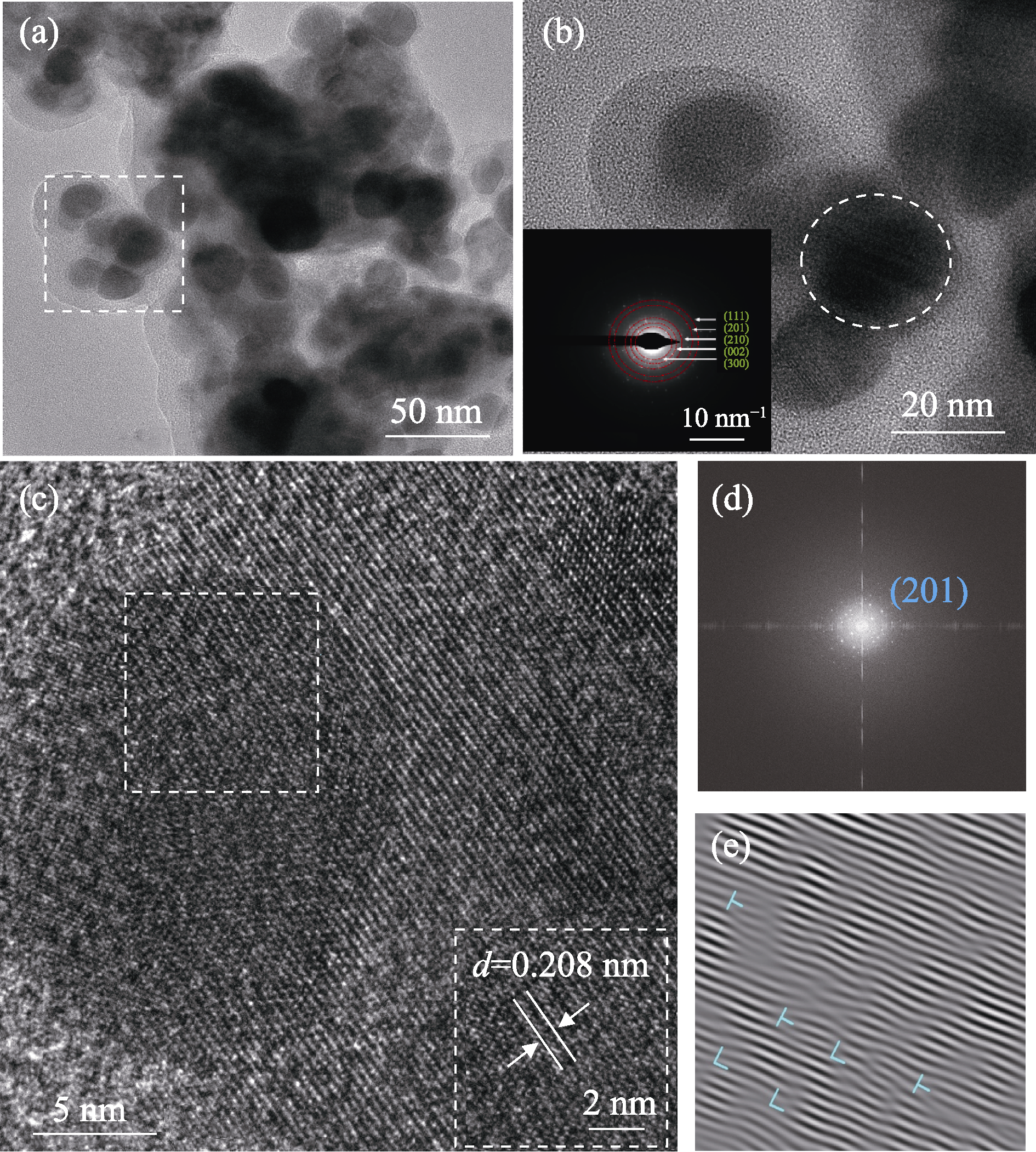
图3 FeCoNiMoCeP/C的TEM和高分辨TEM照片
Fig. 3 TEM and high-resolution TEM (HRTEM) images of FeCoNiMoCeP/C (a) TEM image; (b) HRTEM image and selected-area electron diffraction (SAED) image (inset) of the square region in (a); (c) HRTEM image of the circular region in (b); (d) Fast Fourier transform (FFT) and (e) inverse fast Fourier transform (IFFT) images of the square region in (c)
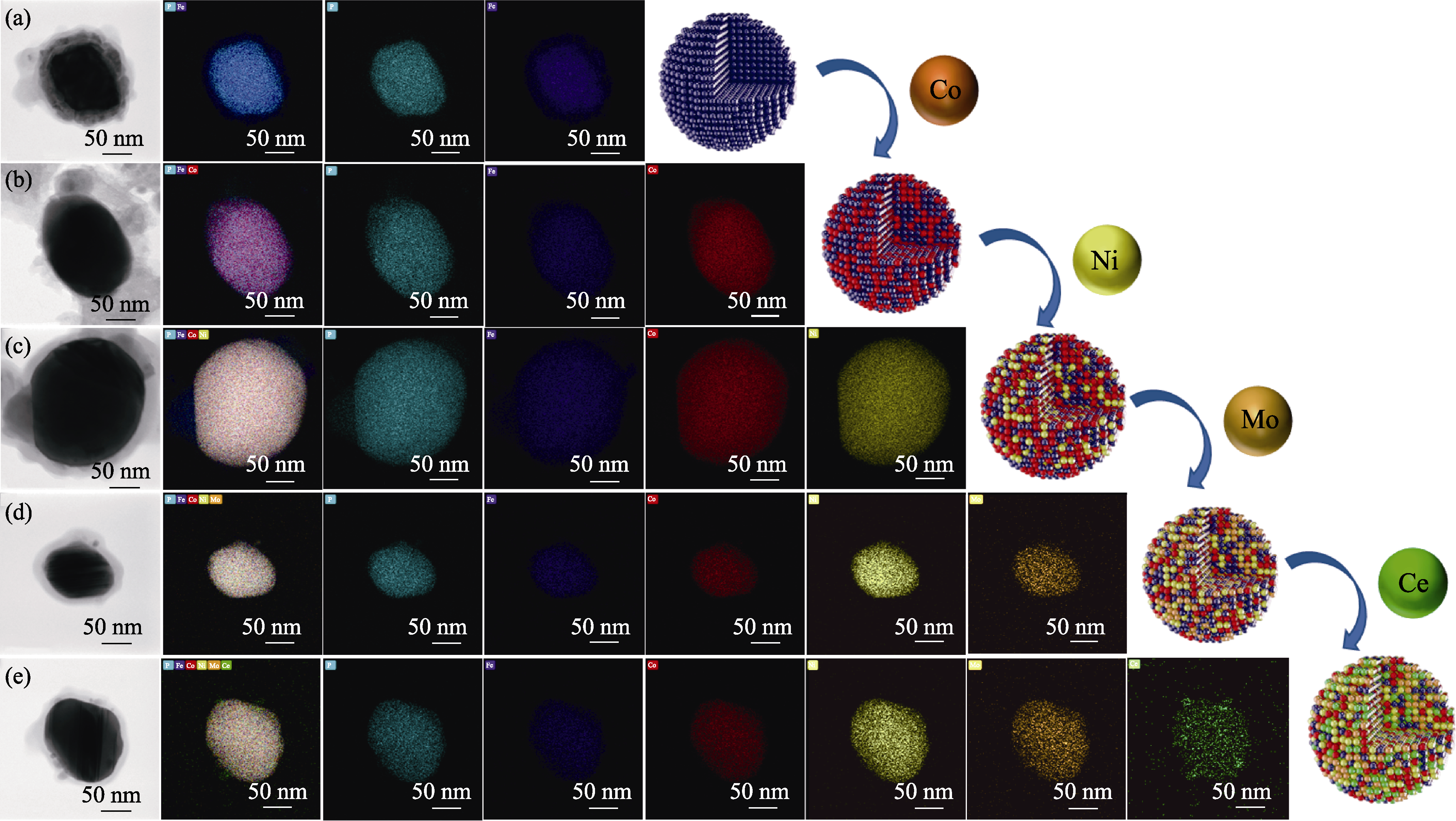
图4 不同样品的高角度环形暗场扫描透射电子显微镜(HAADF-STEM)照片及其相应的EDS元素分布图
Fig. 4 High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images and corresponding EDS elemental mappings of different samples (a) FeP/C; (b) FeCoP/C; (c) FeCoNiP/C; (d) FeCoNiMoP/C; (e) FeCoNiMoCeP/C
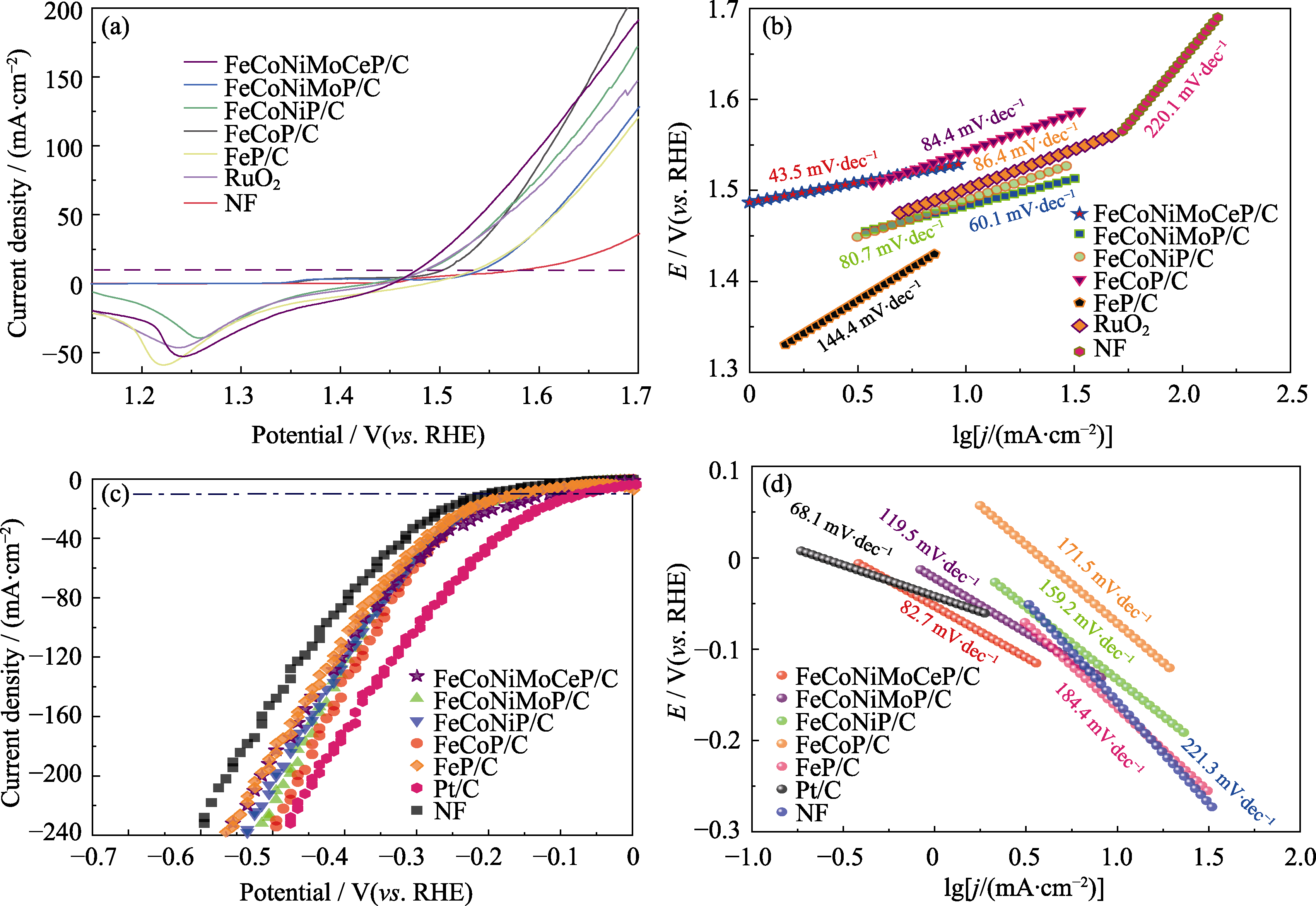
图6 不同催化剂的OER及HER性能
Fig. 6 OER and HER performance of different catalysts (a) OER LSV curves; (b) OER Tafel plots; (c) HER LSV curves; (d) HER Tafel plots. Colorful figures are available on website
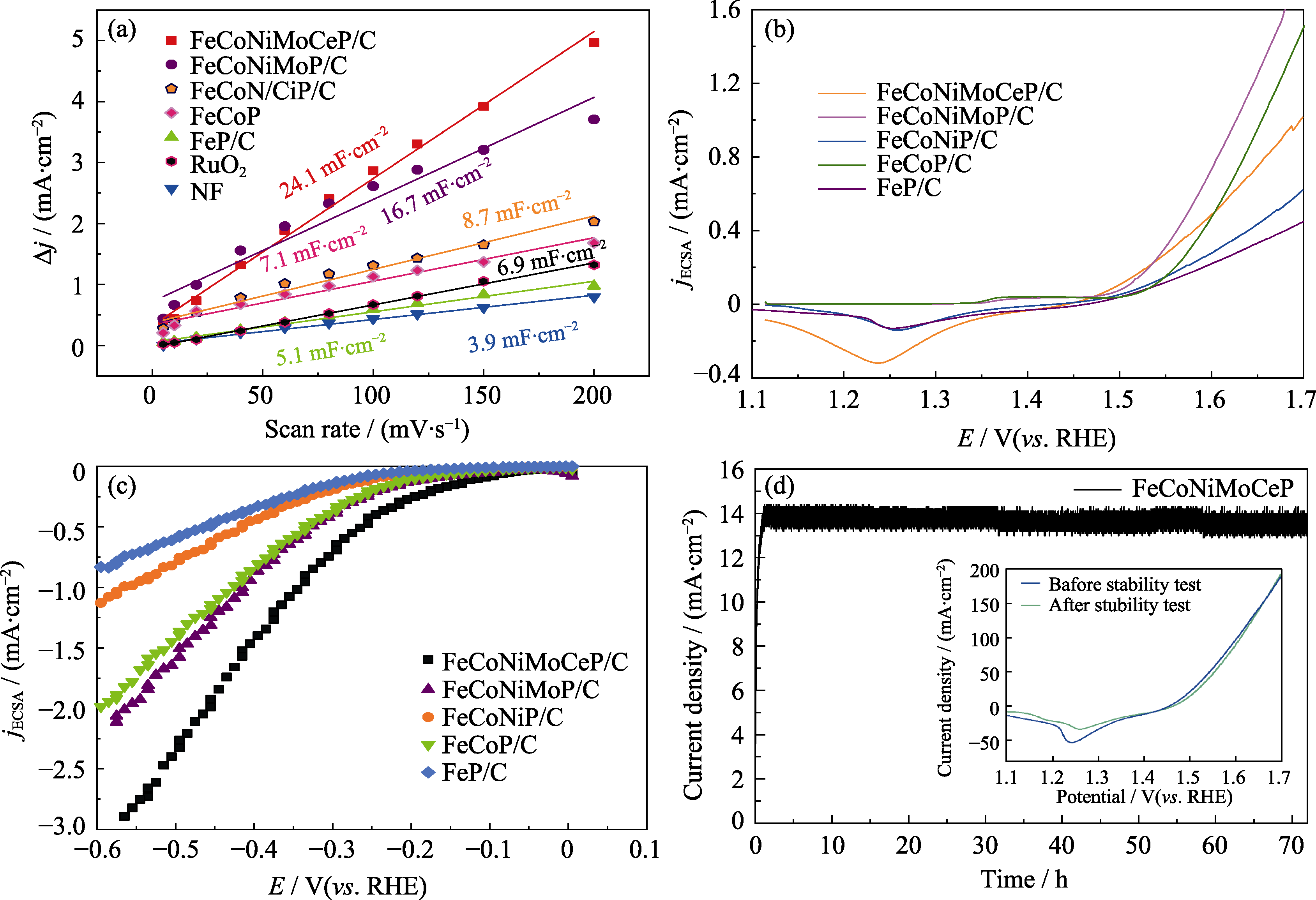
图7 不同催化剂的(a)双层电容(Cdl)曲线、(b) SECSA归一化后的OER LSV曲线、(c) SECSA归一化后的HER LSV曲线; (d) FeCoNiMoCeP/C 催化剂的电流-时间(I-t)曲线
Fig. 7 (a) Double-layer capacitance (Cdl) curves, (b) SECSA-normalized OER LSV curves, and (c) SECSA-normalized HER LSV curves for different catalysts; (d) Current-time (I-t) curve of FeCoNiMoCeP/C catalyst Inset in (d): LSV curves of FeCoNiMoCeP/C before and after 72 h stability test at 1.5 V. Colorful figures are available on website
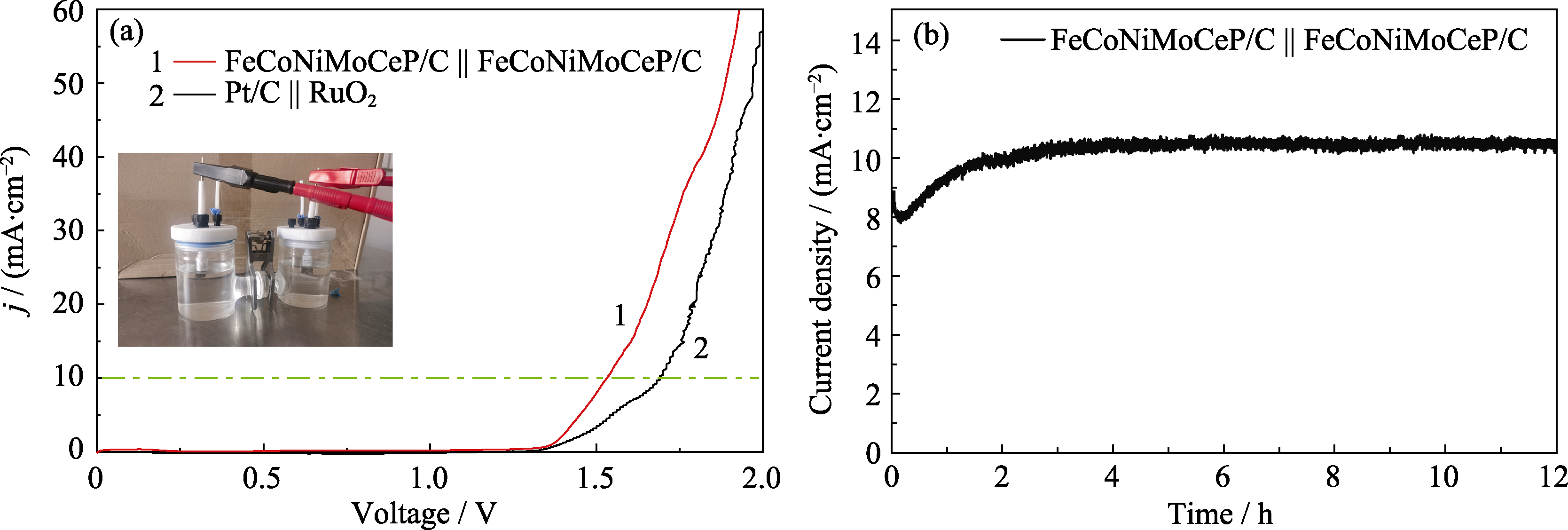
图8 FeCoNiMoCeP/C || FeCoNiMoCeP/C全解水性能分析
Fig. 8 Performance analysis of FeCoNiMoCeP/C || FeCoNiMoCeP/C for overall water splitting (a) LSV curves of FeCoNiMoCeP/C || FeCoNiMoCeP/C and Pt/C || RuO2 with inset showing photograph of overall water splitting equipment with two electrodes; (b) I-t curve of FeCoNiMoCeP/C || FeCoNiMoCeP/C
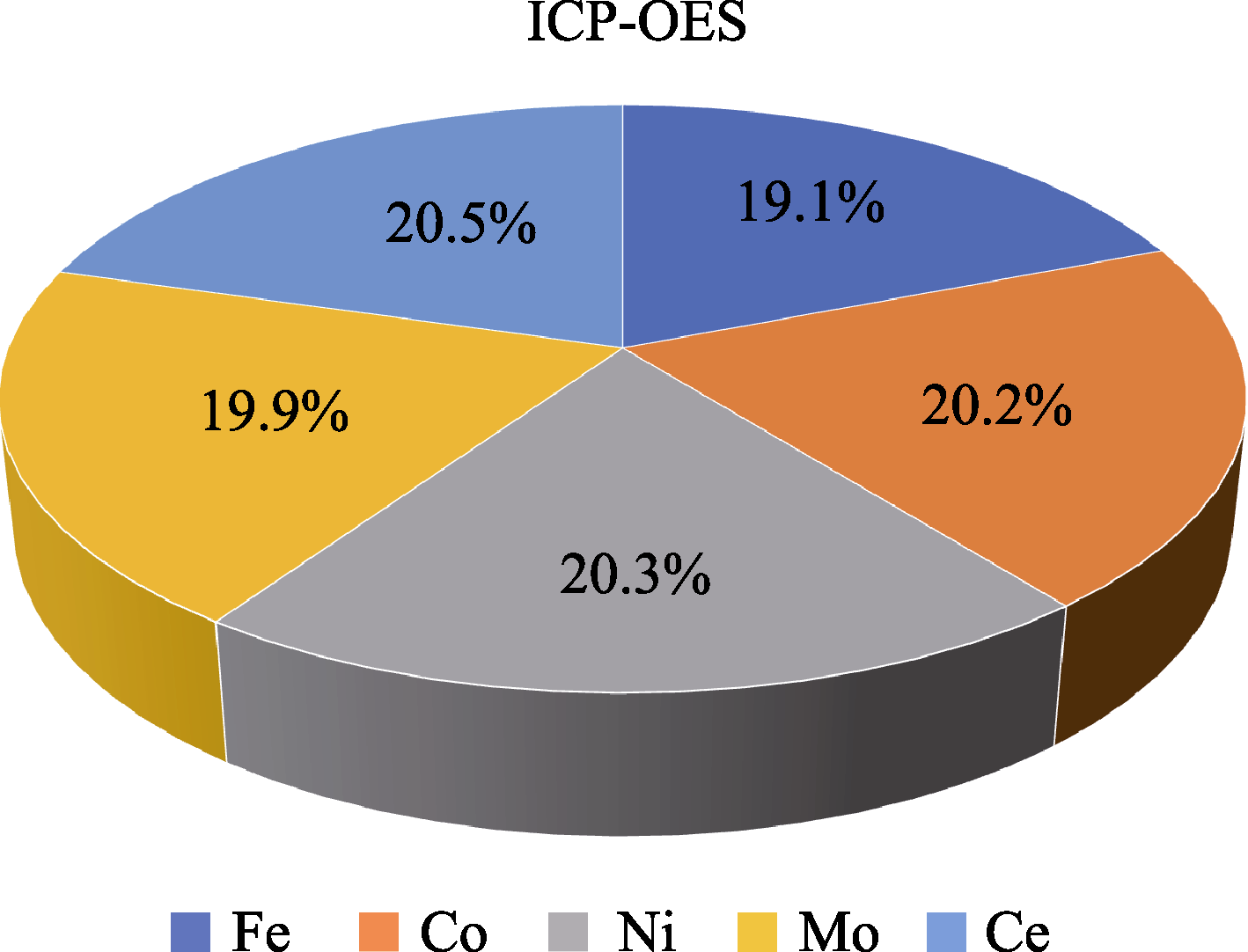
图S1 通过 ICP-OES 测量FeCoNiMoCeP/C 催化剂中金属元素Fe、Co、Ni、Mo和Ce的原子分数
Fig. S1 Atomic percentages of metal elements Fe, Co, Ni, Mo, and Ce in FeCoNiMoCeP/C catalyst measured by ICP-OES
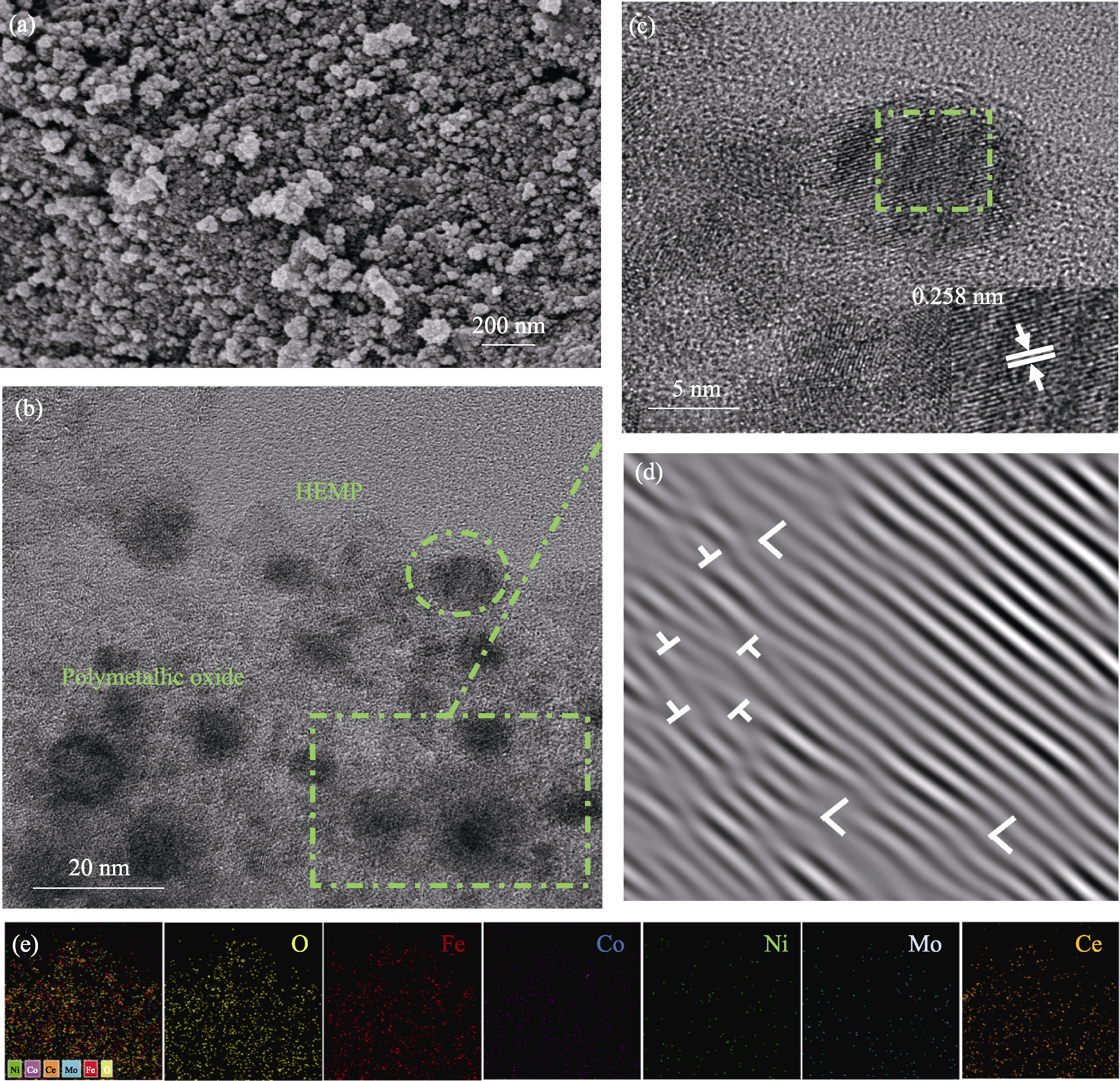
图S4 FeCoNiMoCeP/C在72 h稳定性测试后的结构形貌以及EDS元素分布图
Fig. S4 Structure morphology and EDS elemental mappings of FeCoNiMoCeP/C after stability test for 72 h (a) SEM image; (b) HRTEM image; (c) Crystallographic planes and interplanar spacing marked by dashed boxes in (b); (d) IFFT pattern corresponding to the selected area marked by the dashed array in (c); (e) Elemental mappings
| Sample | Electrolyte solvent | η10 OER/ mV | η10 HER/ mV | OER Tafel slope/ (mV·dec-1) | HER Tafel slope/ (mV·dec-1) | η10 overall water splitting/V | Stability/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeCoNiMoCeP/C | 1.0 mol·L-1 KOH | 240 | 119 | 63.4 | 82.7 | 1.53 | 72 | This work |
| NiCoFeMnCrP | 1.0 mol·L-1 KOH | 270 | 220 | 52.5 | 94.5 | 1.55 | 24 | [ |
| FeNiCoMnCu@CNT | 1.0 mol·L-1 KOH+Sea water | 380 | 290 | 130.0 | 171.0 | 1.60 | 40 | [ |
| CoNiCuMnAl@C 2 h | 1.0 mol·L-1 KOH+Sea water | 290 | - | 66.8 | - | 1.54 | 30 | [ |
| CoCrFeNiMo | 1.0 mol·L-1 KOH | 270 | 157 | 62.5 | 46.7 | 1.86 | 22 | [ |
| CoZnCdCuMnS@CF | 1.0 mol·L-1 KOH | 220 | 173 | 69.8 | 98.5 | 1.63 | 70 | [ |
| La-HEO@NiFeOOH(1 : 3) | 1.0 mol·L-1 KOH | 262 | - | 38.0 | - | 1.57 | 30 | [ |
表S1 FeCoNiMoCeP/C与文献报道催化剂在10 mA·cm-2条件下的OER、HER和全解水性能比较
Table S1 OER, HER, and overall water splitting performance of the FeCoNiMoCeP/C and catalysts in literature at 10 mA·cm-2
| Sample | Electrolyte solvent | η10 OER/ mV | η10 HER/ mV | OER Tafel slope/ (mV·dec-1) | HER Tafel slope/ (mV·dec-1) | η10 overall water splitting/V | Stability/h | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeCoNiMoCeP/C | 1.0 mol·L-1 KOH | 240 | 119 | 63.4 | 82.7 | 1.53 | 72 | This work |
| NiCoFeMnCrP | 1.0 mol·L-1 KOH | 270 | 220 | 52.5 | 94.5 | 1.55 | 24 | [ |
| FeNiCoMnCu@CNT | 1.0 mol·L-1 KOH+Sea water | 380 | 290 | 130.0 | 171.0 | 1.60 | 40 | [ |
| CoNiCuMnAl@C 2 h | 1.0 mol·L-1 KOH+Sea water | 290 | - | 66.8 | - | 1.54 | 30 | [ |
| CoCrFeNiMo | 1.0 mol·L-1 KOH | 270 | 157 | 62.5 | 46.7 | 1.86 | 22 | [ |
| CoZnCdCuMnS@CF | 1.0 mol·L-1 KOH | 220 | 173 | 69.8 | 98.5 | 1.63 | 70 | [ |
| La-HEO@NiFeOOH(1 : 3) | 1.0 mol·L-1 KOH | 262 | - | 38.0 | - | 1.57 | 30 | [ |
| [1] | STAFFELL I, SCAMMAN D, ABAD V A, et al. The role of hydrogen and fuel cells in the global energy system. Energy & Environmental Science, 2019, 12(2): 463. |
| [2] |
KIBSGAARD J, CHORKENDORFF I. Considerations for the scaling-up of water splitting catalysts. Nature Energy, 2019, 4(6): 430.
DOI |
| [3] | ANWAR S, KHAN F, ZHANG Y, et al. Recent development in electrocatalysts for hydrogen production through water electrolysis. International Journal of Hydrogen Energy, 2021, 46(63): 32284. |
| [4] |
BATCHELOR T A A, PEDERSEN J K, WINTHER S H, et al. High-entropy alloys as a discovery platform for electrocatalysis. Joule, 2019, 3(3): 834.
DOI |
| [5] | CHEN P C, LIU M, DU J S, et al. Interface and heterostructure design in polyelemental nanoparticles. Science, 2019, 363(6430): 959. |
| [6] |
YAO Y, HUANG Z, XIE P, et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science, 2018, 359(6383): 1489.
DOI PMID |
| [7] | YAN L, CAO L, DAI P, et al. Metal-organic frameworks derived nanotube of nickel-cobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Advanced Functional Materials, 2017, 27(40): 1703455. |
| [8] | ZHAO Y, JIA N, WU X R, et al. Rhodium phosphide ultrathin nanosheets for hydrazine oxidation boosted electrochemical water splitting. Applied Catalysis B: Environmental, 2020, 270: 118880. |
| [9] | YANG X, GUO R, CAI R, et al. Engineering high-entropy materials for electrocatalytic water splitting. International Journal of Hydrogen Energy, 2022, 47(28): 13561. |
| [10] | SUN M, LIU H, QU J, et al. Earth-rich transition metal phosphide for energy conversion and storage. Advanced Energy Materials, 2016, 6(13): 1600087. |
| [11] | WU R, XIAO B, GAO Q, et al. A Janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting. Angewandte Chemie International Edition, 2018, 57(47): 15445. |
| [12] | TIAN L, YAN X, CHEN X. Electrochemical activity of iron phosphide nanoparticles in hydrogen evolution reaction. ACS Catalysis, 2016, 6(8): 5441. |
| [13] | LAI D, KANG Q, GAO F, et al. High-entropy effect of a metal phosphide on enhanced overall water splitting performance. Journal of Materials Chemistry A, 2021, 9(33): 17913. |
| [14] | WANG X, DONG Q, QIAO H, et al. Continuous synthesis of hollow high-entropy nanoparticles for energy and catalysis applications. Advanced Materials, 2020, 32(46): 2002853. |
| [15] | JING X X, CHEN B Q, ZHAI J X. Ni-Co-B-RE (Sm, Dy, Tb) Composite electrodes: preparation by chemical deposition method and their electrocatalytic hydrogen evolution performance. Journal of Inorganic Materials, 2024, 39(5): 467. |
| [16] | SIVANANTHAM A, LEE H, HWANG S W, et al. Preparation, electrical and electrochemical characterizations of CuCoNiFeMn high-entropy-alloy for overall water splitting at neutral-pH. Journal of Materials Chemistry A, 2021, 9(31): 16841. |
| [17] | LI M, MEI S, ZHENG Y, et al. High-entropy oxides as photocatalysts for organic conversion. Chemical Communications, 2023, 59(90): 13478. |
| [18] | HUANG K, ZHANG B, WU J, et al. Exploring the impact of atomic lattice deformation on oxygen evolution reactions based on a sub-5 nm pure face-centred cubic high-entropy alloy electrocatalyst. Journal of Materials Chemistry A, 2020, 8(24): 11938. |
| [19] | HUANG K, PENG D, YAO Z, et al. Cathodic plasma driven self- assembly of HEAs dendrites by pure single FCC FeCoNiMnCu nanoparticles as high efficient electrocatalysts for OER. Chemical Engineering Journal, 2021, 425: 131533. |
| [20] |
ROMERO J, VARELA M, ASSEBBAN M, et al. Insights into the formation of metal carbon nanocomposites for energy storage using hybrid NiFe layered double hydroxides as precursors. Chemical Science, 2020, 11(29): 7626.
DOI PMID |
| [21] | SONG H, WU M, TANG Z, et al. Single atom ruthenium-doped CoP/CDs nanosheets via splicing of carbon-dots for robust hydrogen production. Angewandte Chemie International Edition, 2021, 60(13): 7234. |
| [22] |
LI D, GUO Z, ZHAO R, et al. An efficient cerium dioxide incorporated nickel cobalt phosphide complex as electrocatalyst for all-pH hydrogen evolution reaction and overall water splitting. Journal of Colloid and Interface Science, 2024, 653: 1725.
DOI PMID |
| [23] | WANG X, ZHANG J, WANG P, et al. Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction. Energy & Environmental Science, 2023, 16(11): 5500. |
| [24] | WANG Y, TAO S, LIN H, et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy, 2021, 81: 105606. |
| [25] | JIN L, WANG Q, WANG K, et al. Engineering NiMoO4/NiFe LDH/rGO multicomponent nanosheets toward enhanced electrocatalytic oxygen evolution reaction. Dalton Transactions, 2022, 51(16): 6448. |
| [26] | LV Q, YAO B, ZHANG W, et al. Controlled direct electrodeposition of crystalline NiFe/amorphous NiFe-(oxy)hydroxide on NiMo alloy as a highly efficient bifunctional electrocatalyst for overall water splitting. Chemical Engineering Journal, 2022, 446: 137420. |
| [27] | LUO M, ZHAO Z, ZHANG Y, et al. PdMo bimetallene for oxygen reduction catalysis. Nature, 2019, 574(7776): 81. |
| [28] | XU H, WANG B, SHAN C, et al. Ce-doped NiFe-layered double hydroxide ultrathin nanosheets/nanocarbon hierarchical nanocomposite as an efficient oxygen evolution catalyst. ACS Applied Materials & Interfaces, 2018, 10(7): 6336. |
| [29] | HA D H, HAN B, RISCH M, et al. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy, 2016, 29: 37. |
| [30] | TAO H B, XU Y, HUANG X, et al. A general method to probe oxygen evolution intermediates at operating conditions. Joule, 2019, 3(6): 1498. |
| [31] | ZHANG Y, ZHU X, ZHANG G, et al. Rational catalyst design for oxygen evolution under acidic conditions: strategies toward enhanced electrocatalytic performance. Journal of Materials Chemistry A, 2021, 9(10): 5890. |
| [32] | BIAN H, QI P, XIE G, et al. HEA-NiFeCuCoCe/NF through ultra-fast electrochemical self-reconstruction with high catalytic activity and corrosion resistance for seawater electrolysis. Chemical Engineering Journal, 2023, 477: 147286. |
| [33] | CONG Y, CHEN X, MEI Y, et al. CeO2 decorated bimetallic phosphide nanowire arrays for enhanced oxygen evolution reaction electrocatalysis via interface engineering. Dalton Transactions, 2022, 51(7): 2923. |
| [34] | WANG A J, CHEN J, ZHANG P F, et al. Relation between NiMo(O) phase structures and hydrogen evolution activities of water electrolysis. Acta Physico-Chimica Sinica, 2023, 39(4): 2301023. |
| [35] |
ZHANG Q, LIAN K, LIU Q, et al. High entropy alloy nanoparticles as efficient catalysts for alkaline overall seawater splitting and Zn-air batteries. Journal of Colloid and Interface Science, 2023, 646: 844.
DOI PMID |
| [36] | WANG S, HUO W, FANG F, et al. High entropy alloy/C nanoparticles derived from polymetallic MOF as promising electrocatalysts for alkaline oxygen evolution reaction. Chemical Engineering Journal, 2022, 429: 132410. |
| [37] | HUO X, ZUO X, WANG X, et al. High entropy alloy CoCrFeNiMo reinforced electrocatalytic performance for high-efficient electrocatalytic water splitting. Chemistry-An Asian Journal, 2023, 18(15): e202300456. |
| [38] | LEI Y, ZHANG L, XU W, et al. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Research, 2022, 15(7): 6054. |
| [39] | WANG Z, HAN S, ZHANG Y, et al. Decorated NiFeOOH on high entropy perovskite oxide by interface engineering for efficient oxygen evolution and overall water splitting. Fuel, 2024, 357: 129946. |
| [1] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [2] | 岳全鑫, 郭瑞华, 王瑞芬, 安胜利, 张国芳, 关丽丽. 3D核壳结构NiMoO4@CoFe-LDH纳米棒的高效析氧及全解水性能研究[J]. 无机材料学报, 2024, 39(11): 1254-1264. |
| [3] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [4] | 马龙涛, 支春义. Fe, N掺杂二维多孔碳双功能催化剂及锌-空气电池中的应用[J]. 无机材料学报, 2019, 34(1): 103-108. |
| [5] | 赵晓婵, 房 艳, 房春晖, 周永全, 戈海文, 朱发岩. 石墨烯包覆分子筛复合电极材料的制备及其性能研究[J]. 无机材料学报, 2017, 32(4): 386-392. |
| [6] | 王艳芝, 赵敏寿. Ti-V基固溶体/AB5型镧镁基合金复合储氢材料的结构与电化学性能[J]. 无机材料学报, 2012, 27(5): 463-468. |
| [7] | 周 艺,黄可龙1,朱志平,杨 波,夏畅斌,肖汉宁. 酸催化溶胶-凝胶法Eu2+、Gd3+共掺杂TiO2的制备及光催化活性[J]. 无机材料学报, 2008, 23(5): 1085-1088. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||