无机材料学报 ›› 2024, Vol. 39 ›› Issue (11): 1254-1264.DOI: 10.15541/jim20240098 CSTR: 32189.14.10.15541/jim20240098
所属专题: 【能源环境】氢能材料(202506)
岳全鑫1,2,3( ), 郭瑞华1,2,3(
), 郭瑞华1,2,3( ), 王瑞芬1,2,3, 安胜利1,2,3, 张国芳1, 关丽丽1,2
), 王瑞芬1,2,3, 安胜利1,2,3, 张国芳1, 关丽丽1,2
收稿日期:2024-03-04
修回日期:2024-05-15
出版日期:2024-11-20
网络出版日期:2024-05-31
通讯作者:
郭瑞华, 教授. E-mail: grh7810@163.com作者简介:岳全鑫(1998-), 男, 硕士研究生. E-mail: 2438079303@qq.com
基金资助:
YUE Quanxin1,2,3( ), GUO Ruihua1,2,3(
), GUO Ruihua1,2,3( ), WANG Ruifen1,2,3, AN Shengli1,2,3, ZHANG Guofang1, GUAN Lili1,2
), WANG Ruifen1,2,3, AN Shengli1,2,3, ZHANG Guofang1, GUAN Lili1,2
Received:2024-03-04
Revised:2024-05-15
Published:2024-11-20
Online:2024-05-31
Contact:
GUO Ruihua, professor. E-mail: grh7810@163.comAbout author:YUE Quanxin (1998-), male, Master candidate. E-mail: 2438079303@qq.com
Supported by:摘要:
电解水制氢因具有绿色环保、制氢纯度高等优点而得到了科学界的广泛关注。然而, 电催化水分解过程中缓慢的阳极析氧反应(OER)极大地阻碍了电解水制氢的发展进程, 使其在实际应用中面临着许多挑战。本研究采用水热和电沉积相结合的策略, 成功在导电泡沫镍(NF)基底上制得了一种以晶体NiMoO4纳米棒为“核”、非晶态CoFe-LDH纳米片为“壳”的新型三维(3D)核壳异质结构催化剂。这种特殊的3D核壳结构充分激发了NiMoO4和CoFe-LDH的电催化潜力, 极大地提升了电化学水分解反应的效率。通过NiMoO4和非晶态CoFe-LDH的协同作用, NiMoO4@CoFe-LDH/NF纳米催化剂产生了更多的活性位点, 表现出了高效的电子转移能力和优异的OER电催化活性。电化学测试表明, 当电沉积时间为60 s时, NiMoO4@CoFe-LDH/NF具有最优异的电化学性能, 在10和100 mA·cm−2下的过电位η10和η100只有168和216 mV, 且具有极小的Tafel斜率和出色的长期稳定性。同时, NiMoO4@CoFe-LDH||NiMoO4全解水系统也表现出了较低的驱动电压, 在1.57 V电压下即可产生10 mA·cm−2 的电流密度。这项工作为设计开发高效的电解水催化材料提供了新的思路。
中图分类号:
岳全鑫, 郭瑞华, 王瑞芬, 安胜利, 张国芳, 关丽丽. 3D核壳结构NiMoO4@CoFe-LDH纳米棒的高效析氧及全解水性能研究[J]. 无机材料学报, 2024, 39(11): 1254-1264.
YUE Quanxin, GUO Ruihua, WANG Ruifen, AN Shengli, ZHANG Guofang, GUAN Lili. 3D Core-shell Structured NiMoO4@CoFe-LDH Nanorods: Performance of Efficient Oxygen Evolution Reaction and Overall Water Splitting[J]. Journal of Inorganic Materials, 2024, 39(11): 1254-1264.
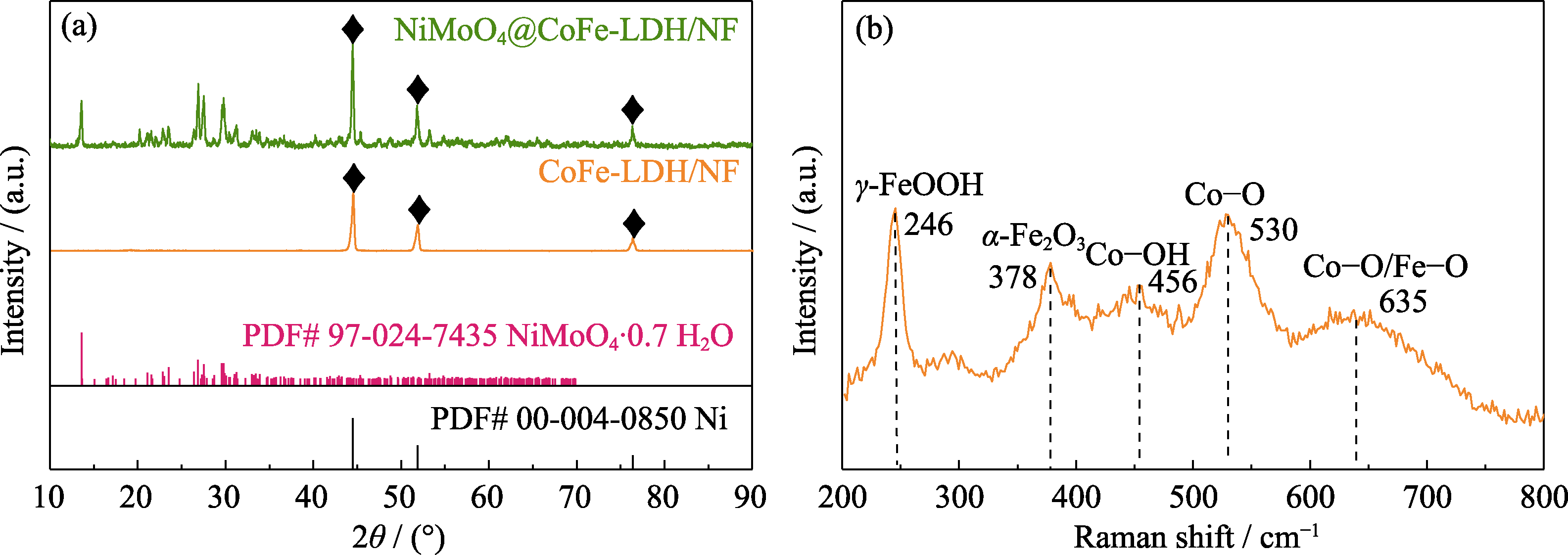
图2 (a) NiMoO4@CoFe-LDH/NF、CoFe-LDH/NF的XRD图谱和(b) CoFe-LDH的拉曼光谱图
Fig. 2 (a) XRD patterns of NiMoO4@CoFe-LDH/NF and CoFe-LDH/NF, and (b) Raman spectrum of CoFe-LDH

图3 催化剂样品的SEM照片
Fig. 3 SEM images of catalyst samples (a) NF; (b, c) CoFe-LDH/NF; (d) NiMoO4/NF; (e, f) NiMoO4@CoFe-LDH-30s/NF; (g, h) NiMoO4@CoFe-LDH-60s/NF; (i, j) NiMoO4@CoFe-LDH-90s/NF; (k, l) NiMoO4@CoFe-LDH-120s/NF
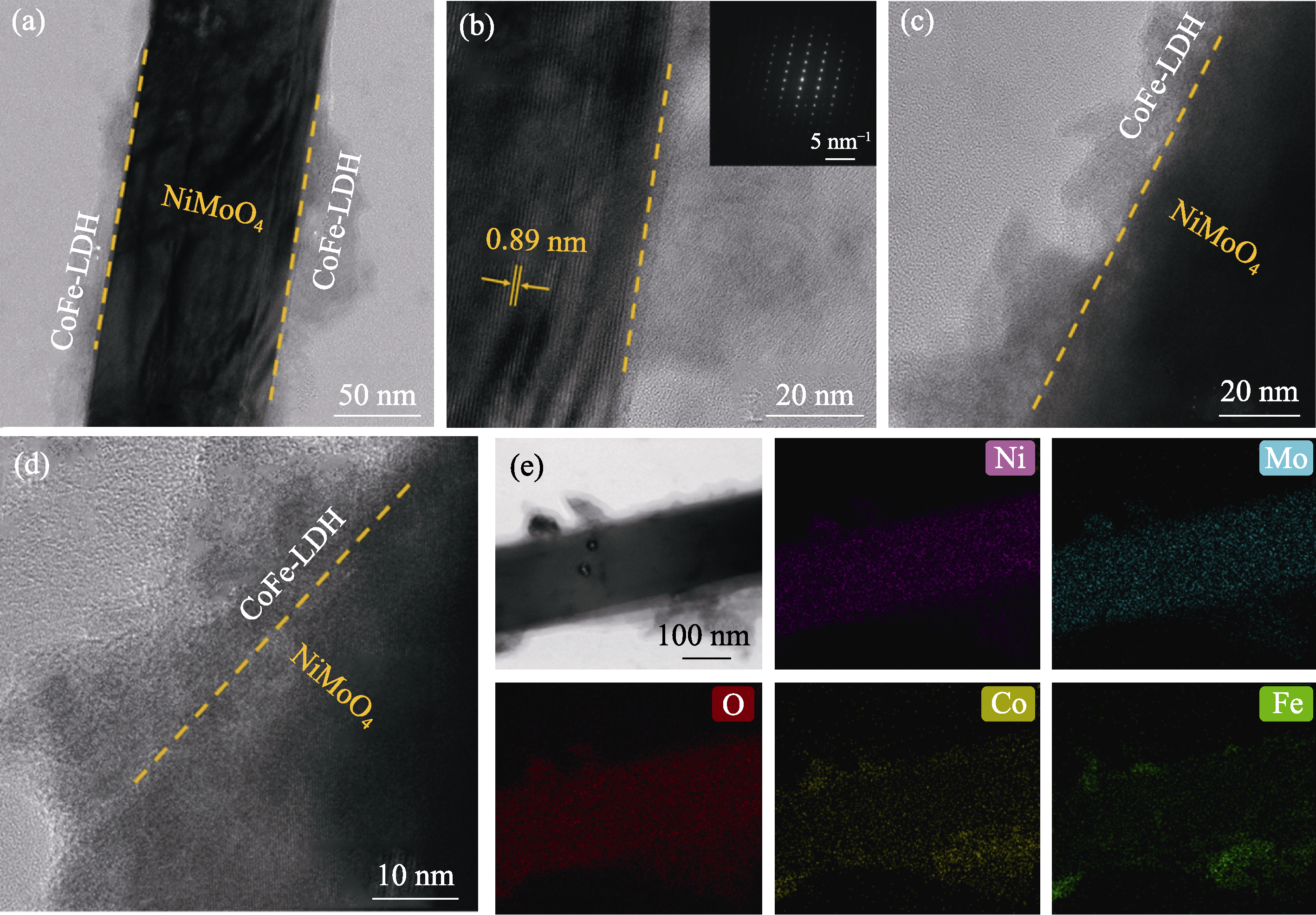
图4 NiMoO4@CoFe-LDH/NF的TEM、HRTEM和EDS面扫照片
Fig. 4 TEM, HRTEM and EDS mapping images of NiMoO4@CoFe-LDH/NF (a) TEM; (b-d) HRTEM images; (e) EDS elemental mappings
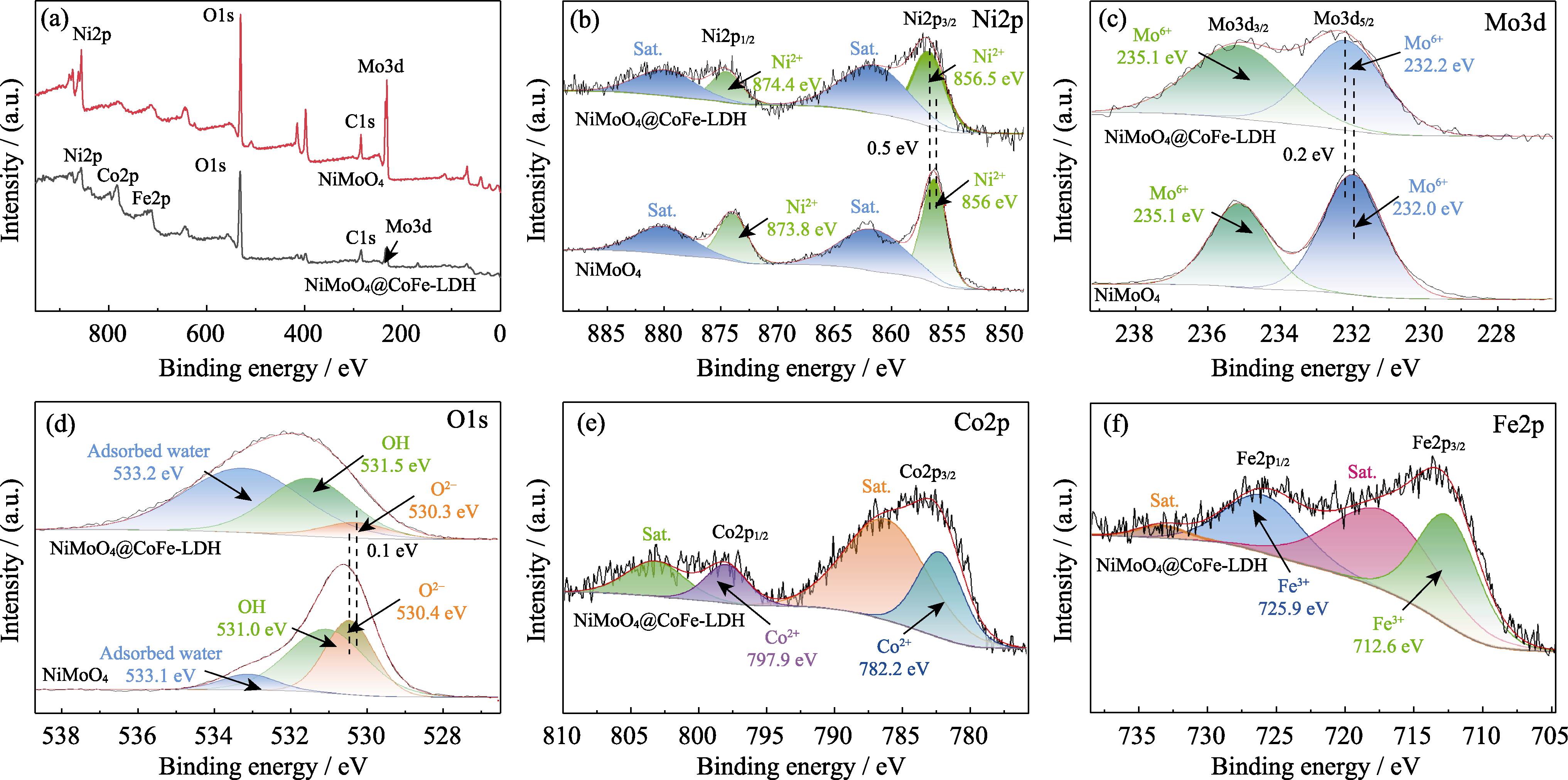
图5 NiMoO4@CoFe-LDH和NiMoO4的XPS谱图
Fig. 5 XPS spectra of NiMoO4@CoFe-LDH and NiMoO4 (a) Total, (b) Ni2p, (c) Mo3d, (d) O1s XPS spectra of NiMoO4@CoFe-LDH and NiMoO4; (e) Co2p and (f) Fe2p XPS spectra of NiMoO4@CoFe-LDH
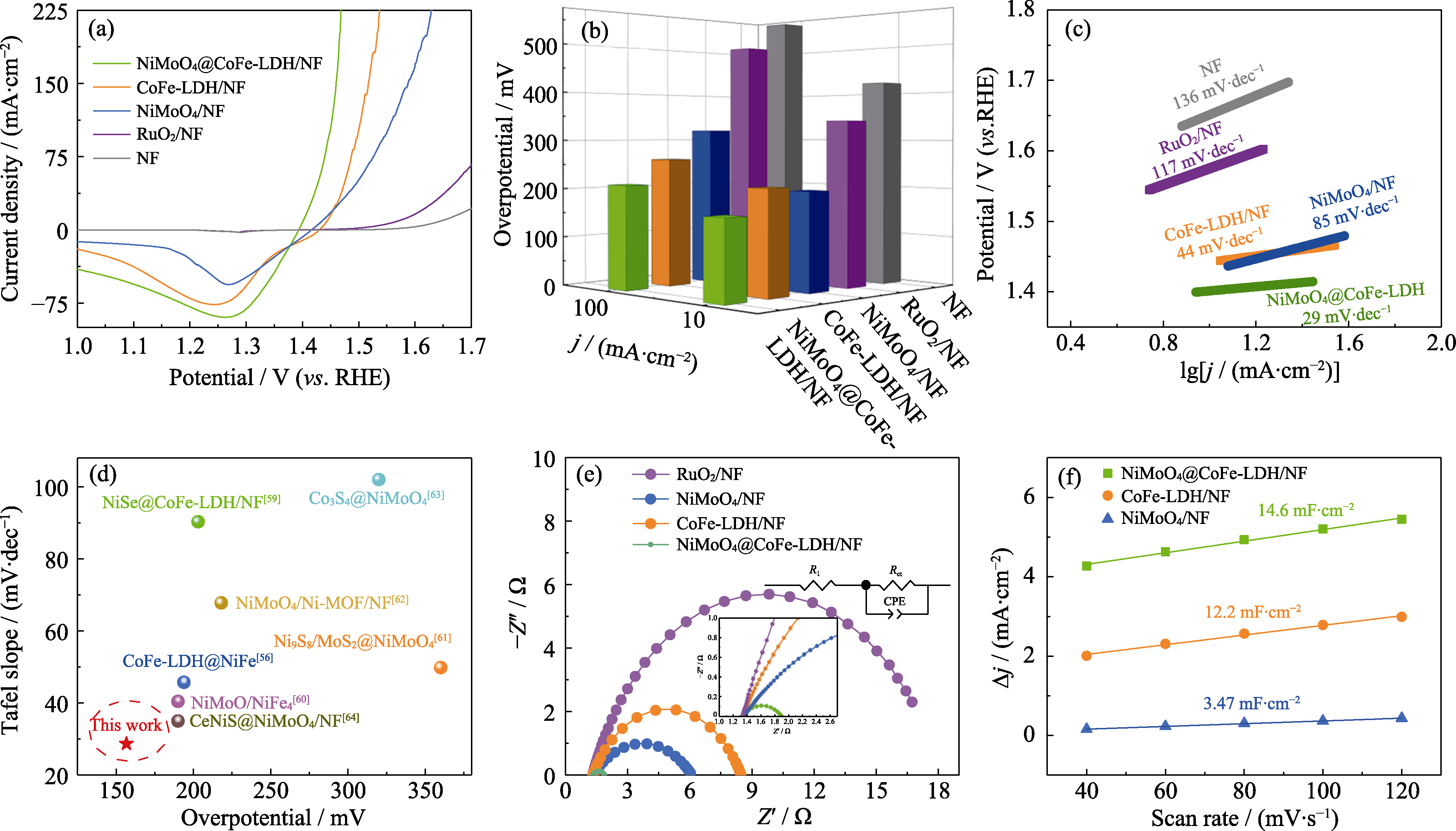
图6 不同催化剂样品的电化学性能
Fig. 6 Electrochemical performance of different catalytic samples (a) OER-LSV curves; (b) OER overpotentials at 10 and 100 mA·cm−2; (c) Tafel plots; (d) Comparison of overpotentials and Tafel slopes at 10 mA·cm-2 for currently reported OER electrocatalysts[56,59⇓⇓⇓⇓ -64]; (e) Nyquist plots; (f) Double-layer capacitance plots. Colorful figures are available on website
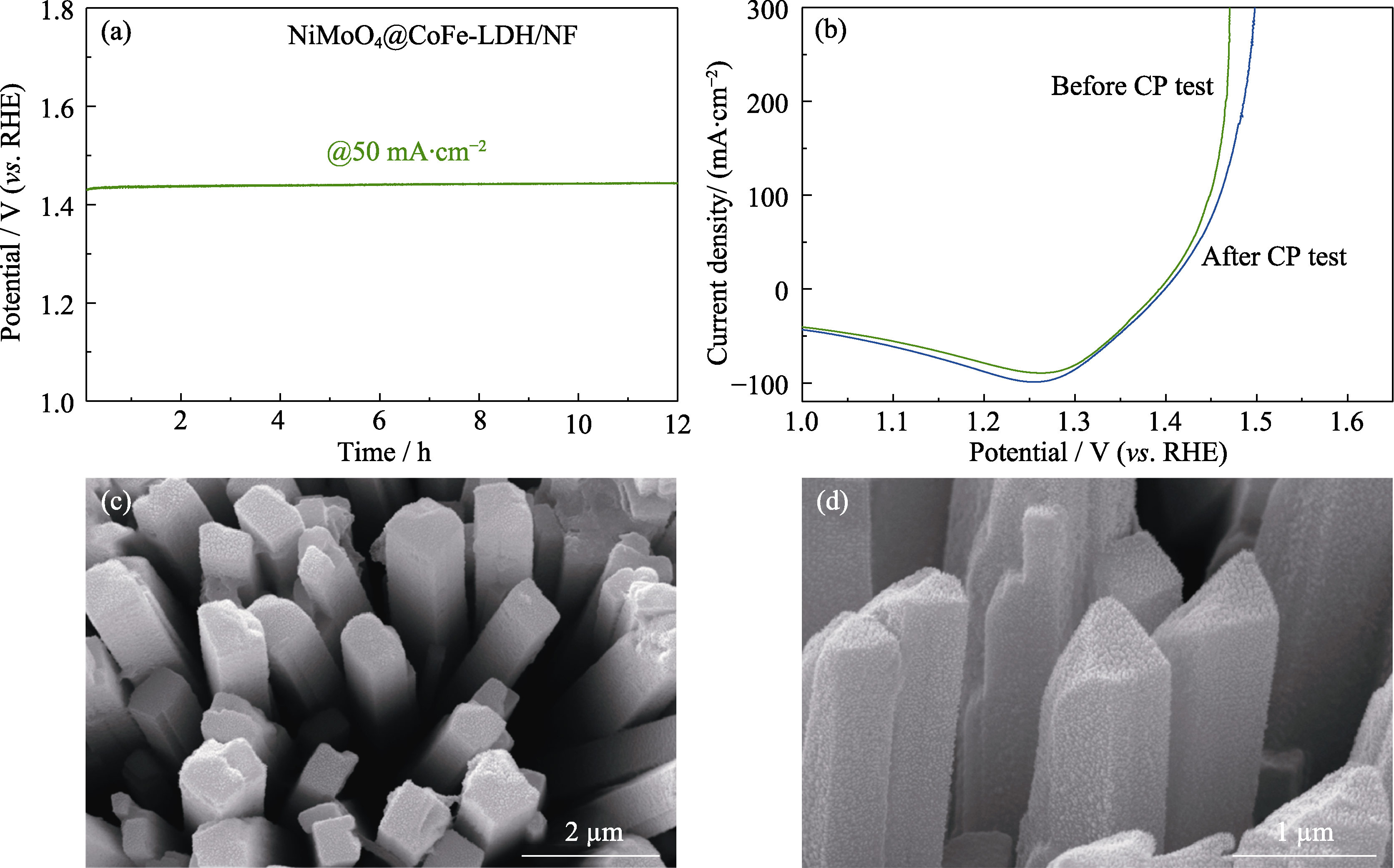
图7 NiMoO4@CoFe-LDH/NF的稳定性
Fig. 7 Stability of NiMoO4@CoFe-LDH/NF (a) Chrono-potential testing; (b) LSV polarization curves before and after 12 h of chrono-potential testing; (c, d) SEM images of NiMoO4@CoFe-LDH/NF after chrono-potential testing
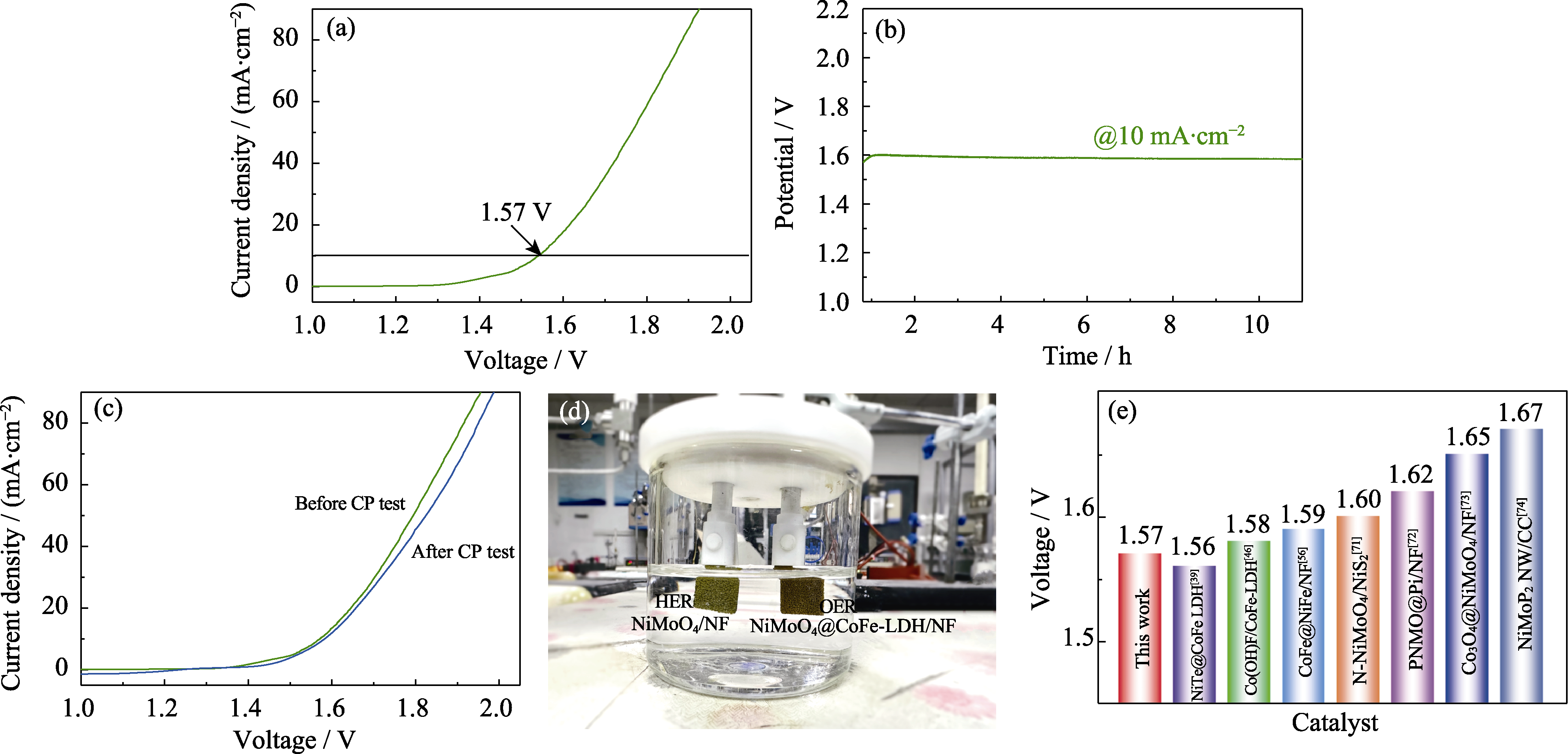
图9 NiMoO4@CoFe-LDH/NF||NiMoO4/NF双电极系统的全解水性能
Fig. 9 Overall water splitting performance of NiMoO4@CoFe-LDH/NF||NiMoO4/NF double electrode system (a) LSV curve; (b) Chrono-potential testing; (c) LSV curves before and after chrono-potential testing; (d) Photograph of the experimental setup; (e) Comparison of overall water splitting potential at 10 mA·cm−2 for currently reported electrocatalysts[39,46,56,71⇓⇓ -74]
| Catalyst | Overpotential/mV (at 10 mA·cm-2) | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|
| NiMoO4@CoFe-LDH/NF | 168 | 29 | This work |
| CoFe-LDH@NiFe | 190 | 45 | [ |
| NiSe@CoFe LDH/NF | 203 | 90 | [ |
| NiMoO4/NiFe | 188 | 39 | [ |
| Ni9S8/MoS2@NiMoO4 | 360 | 49 | [ |
| NiMoO4/Ni-MOF/NF | 218 | 67 | [ |
| Co3S4@NiMoO4 | 320 | 102 | [ |
| CeNiS@NiMoO4/NF | 187 | 35 | [ |
表S1 本实验制备的催化剂与其他文献报道的CoFe-LDH、NiMoO4相关电催化剂在1.0 mol·L-1 KOH中的过电位和Tafel斜率
Table S1 Overpotentials and Tafel slopes of this work, other reported CoFe-LDH, and NiMoO4 related electrocatalysts in 1.0 mol·L-1 KOH
| Catalyst | Overpotential/mV (at 10 mA·cm-2) | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|
| NiMoO4@CoFe-LDH/NF | 168 | 29 | This work |
| CoFe-LDH@NiFe | 190 | 45 | [ |
| NiSe@CoFe LDH/NF | 203 | 90 | [ |
| NiMoO4/NiFe | 188 | 39 | [ |
| Ni9S8/MoS2@NiMoO4 | 360 | 49 | [ |
| NiMoO4/Ni-MOF/NF | 218 | 67 | [ |
| Co3S4@NiMoO4 | 320 | 102 | [ |
| CeNiS@NiMoO4/NF | 187 | 35 | [ |
图S2 不同样品在不同扫描速率(40~120 mV·s-1)下的CV曲线
Fig. S2 CV plots for different samples at different sweep speeds (40-120 mV·s-1) (a) NiMoO4@CoFe-LDH/NF; (b) CoFe-LDH/NF; (c) NiMoO4/NF
| [1] | LAGIOIA G, SPINELLI M P, AMICARELLI V. Blue and green hydrogen energy to meet European Union decarbonisation objectives. an overview of perspectives and the current state of affairs. International Journal of Hydrogen Energy, 2023, 48(4): 1304. |
| [2] | PANCHENKO V A, DAUS Y V, KOVALEV A A, et al. Prospects for the production of green hydrogen: review of countries with high potential. International Journal of Hydrogen Energy, 2023, 48(12): 4551. |
| [3] | LI X, RAORANE C J, XIA C, et al. Latest approaches on green hydrogen as a potential source of renewable energy towards sustainable energy: spotlighting of recent innovations, challenges, and future insights. Fuel, 2023, 334: 126684. |
| [4] | QURESHI F, YUSUF M, KHAN M A, et al. A state-of-the-art review on the latest trends in hydrogen production, storage, and transportation techniques. Fuel, 2023, 340: 127574. |
| [5] | KARACA A E, DINCER I. Development of a new photoelectrochemical system for clean hydrogen production and a comparative environmental impact assessment with other production methods. Chemosphere, 2023, 337: 139367. |
| [6] | JAYAPRABAKAR J, HARI N S S, BADREENATH M, et al. Nano materials for green hydrogen production: technical insights on nano material selection, properties, production routes and commercial applications. International Journal of Hydrogen Energy, 2024, 52: 674. |
| [7] | HOTA P, DAS A, MAITI D K. A short review on generation of green fuel hydrogen through water splitting. International Journal of Hydrogen Energy, 2023, 48(2): 523. |
| [8] | VAN DER ZALM J M, QUINTAL J, HIRA S A, et al. Recent trends in electrochemical catalyst design for hydrogen evolution, oxygen evolution, and overall water splitting. Electrochimica Acta, 2023, 439: 141715. |
| [9] | LI H, GUO J, LI Z, et al. Research progress of hydrogen production technology and related catalysts by electrolysis of water. Molecules, 2023, 28(13): 5010. |
| [10] | PLEVOVA M, HNAT J, BOUZEK K. Electrocatalysts for the oxygen evolution reaction in alkaline and neutral media. A comparative review. Journal of Power Sources, 2021, 507: 230072. |
| [11] | LAKSHMI K C S, VEDHANARAYANAN B, LIN T W. Electrocatalytic hydrogen and oxygen evolution reactions: role of two-dimensional layered materials and their composites. Electrochimica Acta, 2023, 447: 142119. |
| [12] | HOANG A L, BALAKRISHNAN S, HODGES A, et al. High- performing catalysts for energy-efficient commercial alkaline water electrolysis. Sustainable Energy & Fuels, 2023, 7(1): 31. |
| [13] | GUO R H, MO Y J, AN S L, et al. Cerium oxide hollow sphere: controllable synthesis and its effect on electrocatalytic performance of Pt-based catalysts. Journal of Inorganic Materials, 2018, 33(7): 779. |
| [14] | IQBAL M Z, ZAHID R, KHAN M W, et al. Exploration of catalytically active materials for efficient electrochemical hydrogen and oxygen evolution reactions. International Journal of Hydrogen Energy, 2023, 48(22): 8045. |
| [15] | NAGAPPAN S, KARMAKAR A, MADHU R, et al. Tuning the active sites and optimizing the d-spacing value in CoFe-LDH by ex situ intercalation of guest anions: an innovative electrocatalyst for overall water splitting reaction. Catalysis Science & Technology, 2023, 13(22): 6377. |
| [16] | HOU Z, FAN F, WANG Z, et al. Heterostructural NiSe-CoFe LDH as a highly effective and stable electrocatalyst for the oxygen evolution reaction. Dalton Transactions, 2023, 52(29): 10064. |
| [17] |
DENG B, LIANG J, YUE L, et al. CoFe-LDH nanowire arrays on graphite felt: a high-performance oxygen evolution electrocatalyst in alkaline media. Chinese Chemical Letters, 2022, 33(2): 890.
DOI |
| [18] | GUO T, LI L, WANG Z. Recent development and future perspectives of amorphous transition metal-based electrocatalysts for oxygen evolution reaction. Advanced Energy Materials, 2022, 12(24): 2200827. |
| [19] |
GUO C, SHI Y, LU S, et al. Amorphous nanomaterials in electrocatalytic water splitting. Chinese Journal of Catalysis, 2021, 42(8): 1287.
DOI |
| [20] | KARKEH-ABADI F, GHIYASIYAN-ARANI M, DAWI E A, et al. Microstructural study of sonochemical synthesized belt-like Zr (MoO4)2/MoO3 composites for efficient energy storage. Journal of Energy Storage, 2023, 70: 107896. |
| [21] | DELICE S, ISIK M, GASANLY N M, et al. Growth and temperature-tuned band gap characteristics of LiGd (MoO4)2 single crystals for optoelectronic applications. Ceramics International, 2023, 49(15): 25840. |
| [22] | GAJRAJ V, DEVI P, KUMAR R, et al. Fabrication of nanocluster- aggregated dense Ce2(MoO4)3 microspherical architectures for high-voltage energy storage and high catalytic energy conversion applications. Energy & Fuels, 2022, 36(14): 7841. |
| [23] | RAY S K, HUR J. A critical review on modulation of NiMoO4- based materials for photocatalytic applications. Journal of Environmental Management, 2021, 278: 111562. |
| [24] | EDA K, KATO Y, OHSHIRO Y, et al. Synthesis, crystal structure, and structural conversion of Ni molybdate hydrate NiMoO4·nH2O. Journal of Solid State Chemistry, 2010, 183(6): 1334. |
| [25] | ZHAO X, MENG J, YAN Z, et al. Nanostructured NiMoO4 as active electrocatalyst for oxygen evolution. Chinese Chemical Letters, 2019, 30(2): 319. |
| [26] | RAMMAL M B, OMANOVIC S. Synthesis and characterization of NiO, MoO3, and NiMoO4 nanostructures through a green, facile method and their potential use as electrocatalysts for water splitting. Materials Chemistry and Physics, 2020, 255: 123570. |
| [27] | DABIR M P, MASOUDPANAH S M, MAMIZADEH M. CTAB- assisted hydrothermal synthesis of platelike and nanorod-like NiMoO4 morphologies for supercapacitor and hydrogen evolution applications. Journal of Energy Storage, 2023, 70: 107951. |
| [28] | SILVA M M S, RAIMUNDO R A, SILVA T R, et al. Morphology- controlled NiFe2O4nanostructures: influence of calcination temperature on structural, magnetic and catalytic properties towards OER. Journal of Electroanalytical Chemistry, 2023, 933: 117277. |
| [29] | LI M X, XIAO B, ZHAO Z Y, et al. Morphology evolution regulation of dual-doped S, Fe-NiMoO4 microrods based on precipitation-dissolution equilibrium for oxygen evolution. Fuel, 2023, 336: 126769. |
| [30] | PRASAD K, MAHATO N, YOO K, et al. Morphology regulated hierarchical rods-, buds-, and sheets-like CoMoO4 for electrocatalytic oxygen evolution reaction. Energies, 2023, 16(5): 2441. |
| [31] | GAO G, WANG K, WANG X. 2D/2D core/shell structure of FeCo2O4@NiMn LDH for efficient oxygen evolution reaction. Journal of Alloys and Compounds, 2023, 937: 168478. |
| [32] | ZHANG C, XU W, LI S, et al. Core-shell heterojunction engineering of Ni0.85Se-O/CN electrocatalyst for efficient OER. Chemical Engineering Journal, 2023, 454: 140291. |
| [33] |
CHEN J, LI X, MA B, et al. CoP@Ni core-shell heterostructure nanowire array: a highly efficient electrocatalyst for hydrogen evolution. Journal of Colloid and Interface Science, 2023, 637: 354.
DOI PMID |
| [34] | YANG S, TIWARI S K, ZHU Z, et al. In situ fabrication of Mn-doped NiMoO4 rod-like arrays as high performance OER electrocatalyst. Nanomaterials, 2023, 13(5): 827. |
| [35] | ZHANG Y, YAO R, WU Y, et al. In situ rapid and deep self-reconstruction of Fe-doped hydrate NiMoO4 for stable water oxidation at high current densities. Chemical Engineering Journal, 2023, 461: 142081. |
| [36] | WU X, ZHANG T, WEI J, et al. Facile synthesis of Co and Ce dual-doped Ni3S2 nanosheets on Ni foam for enhanced oxygen evolution reaction. Nano Research, 2020, 13: 2130. |
| [37] | YU L, REN Z. Systematic study of the influence of iR compensation on water electrolysis. Materials Today Physics, 2020, 14: 100253. |
| [38] | ZHENG W. iR compensation for electrocatalysis studies: considerations and recommendations. ACS Energy Letters, 2023, 8(4): 1952. |
| [39] | YAO L, LI R, ZHANG H, et al. Interface engineering of NiTe@CoFe LDH for highly efficient overall water-splitting. International Journal of Hydrogen Energy, 2022, 47(76): 32394. |
| [40] |
LI H B, YU M H, WANG F X, et al. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nature Communications, 2013, 4: 1894.
DOI PMID |
| [41] | LIU W, LIU H, DANG L, et al. Amorphous cobalt-iron hydroxide nanosheet electrocatalyst for efficient electrochemical and photo- electrochemical oxygen evolution. Advanced Functional Materials, 2017, 27(14): 1603904. |
| [42] | JIANG G, ZHENG C, JIN Z. Hexagonal CdS assembled with lamellar NiCo LDH form S-scheme heterojunction for photocatalytic hydrogen evolution. Materials Science in Semiconductor Processing, 2021, 135: 106128. |
| [43] | BO X, LI Y, CHEN X, et al. Operando Raman spectroscopy reveals Cr-induced-phase reconstruction of NiFe and CoFe oxyhydroxides for enhanced electrocatalytic water oxidation. Chemistry of Materials, 2020, 32(10): 4303. |
| [44] | SHEN Y, DASTAFKAN K, SUN Q, et al. Improved electrochemical performance of nickel-cobalt hydroxides by electrodeposition of interlayered reduced graphene oxide. International Journal of Hydrogen Energy, 2019, 44(7): 3658. |
| [45] |
YANG F, SLIOZBERG K, SINEV I, et al. Synergistic effect of cobalt and iron in layered double hydroxide catalysts for the oxygen evolution reaction. ChemSusChem, 2017, 10(1): 156.
DOI PMID |
| [46] | QIN M, WANG Y, ZHANG H, et al. Hierarchical Co(OH)F/CoFe-LDH heterojunction enabling high-performance overall water-splitting. CrystEngComm, 2022, 24(34): 6018. |
| [47] | FANG D, HE F, XIE J, et al. Calibration of binding energy positions with C1s for XPS results. Journal of Wuhan University of Technology-Materials Science Edition, 2020, 35: 711. |
| [48] | JIN L, WANG Q, WANG K, et al. Engineering NiMoO4/NiFe LDH/rGO multicomponent nanosheets toward enhanced electrocatalytic oxygen evolution reaction. Dalton Transactions, 2022, 51(16): 6448. |
| [49] | YAO B, ZHANG W W, SHE L, et al. Controlled direct electrodeposition of crystalline NiFe/amorphous NiFe-(oxy) hydroxide on NiMo alloy as a highly efficient bifunctional electrocatalyst for overall water splitting. Chemical Engineering Journal, 2022, 446: 137420. |
| [50] | DURAI L, GOPALAKRISHNAN A, BADHULIKA S. A low-cost and facile electrochemical sensor for the trace-level recognition of flutamide in biofluids using large-area bimetallic NiCo2O4 micro flowers. New Journal of Chemistry, 2022, 46(7): 3383. |
| [51] | GUO L, CHI J, ZHU J, et al. Dual-doping NiMoO4 with multi-channel structure enable urea-assisted energy-saving H2 production at large current density in alkaline seawater. Applied Catalysis B: Environmental, 2023, 320: 121977. |
| [52] |
LI Y, ZHAO Q, ZHANG M, et al. Fabricating heterostructures for boosting the structure stability of Li-rich cathodes. ACS Omega, 2023, 8(7): 6720.
DOI PMID |
| [53] | WANG J, CHEN K, PENG R, et al. Synergistically enhanced alkaline hydrogen evolution reaction by coupling CoFe layered double hydroxide with NiMoO4 prepared by two-step electrodeposition. New Journal of Chemistry, 2021, 45(44): 20825. |
| [54] | PAYNE B P, BIESINGER M C, MCINTYRE N S. X-ray photoelectron spectroscopy studies of reactions on chromium metal and chromium oxide surfaces. Journal of Electron Spectroscopy and Related Phenomena, 2011, 184(1/2): 29. |
| [55] | YI T, SHI L, HAN X, et al. Approaching high-performance lithium storage materials by constructing hierarchical CoNiO2@CeO2 nanosheets. Energy & Environmental Materials, 2021, 4(4): 586. |
| [56] | YANG R, ZHOU Y, XING Y, et al. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Applied Catalysis B: Environmental, 2019, 253: 131. |
| [57] | XU H, WU J, LI C, et al. Investigation of polyaniline films doped with Fe3+ as the electrode material for electrochemical supercapacitors. Electrochimica Acta, 2015, 165: 14. |
| [58] | PENG L, YANG N, YANG Y, et al. Atomic cation-vacancy engineering of NiFe-layered double hydroxides for improved activity and stability towards the oxygen evolution reaction. Angewandte Chemie International Edition, 2021, 60(46): 24612. |
| [59] | NIE F, LI Z, DAI X, et al. Interfacial electronic modulation on heterostructured NiSe@CoFe LDH nanoarrays for enhancing oxygen evolution reaction and water splitting by facilitating the deprotonation of OH to O. Chemical Engineering Journal, 2022, 431: 134080. |
| [60] | ZHANG S, CEN M, WANG Q, et al. Complete reconstruction of NiMoO4/NiFe LDH for enhanced oxygen evolution reaction. Chemical Communications, 2023, 59(23): 3427. |
| [61] | CHEN L, DENG Z, CHEN Z, et al. Building Ni9S8/MoS2nanosheets decorated NiMoO4 nanorods heterostructure for enhanced water splitting. Advanced Materials Interfaces, 2021, 8(21): 2101483. |
| [62] | LI Q, ZHANG K, LI X, et al. Enhanced electrocatalytic performance of uniformly spherical Ni-MOF decorated with NiMoO4 nanorods for oxygen evolution reaction. Journal of Alloys and Compounds, 2022, 920: 165941. |
| [63] | CHEN L, XU G C, XU G, et al. Co-based coordination polymer-derived Co3S4 nanotube decorated with NiMoO4 nanosheets for effective oxygen evolution reaction. International Journal of Hydrogen Energy, 2020, 45(55): 30463. |
| [64] | WANG F, LIU Z, ZHANG K, et al. Ce-doped Ni-S nanosheets on Ni foam supported NiMoO4 micropillars: fast electrodeposition, improved electrocatalytic activity and ultralong durability for the oxygen evolution reaction in various electrolytes. Dalton Transactions, 2021, 50(47): 17774. |
| [65] | YUAN J, CHEN S, ZHANG Y, et al. Structural regulation of coupled phthalocyanine-porphyrin covalent organic frameworks to highly active and selective electrocatalytic CO2 reduction. Advanced Materials, 2022, 34(30): 2203139. |
| [66] | ZHENG W, LIU M, LEE L Y S. Best practices in using foam-type electrodes for electrocatalytic performance benchmark. ACS Energy Letters, 2020, 5(10): 3260. |
| [67] | REN Y, WANG J, WANG W, et al. Boride-mediated synthesis of a highly active cobalt-based electrocatalyst for alkaline hydrogen evolution reaction. Journal of Materials Chemistry A, 2023, 11(23): 1328. |
| [68] | WANG J, LI L, MENG L, et al. Morphology engineering of nickel molybdate hydrate nanoarray for electrocatalytic overall water splitting: from nanorod to nanosheet. RSC Advances, 2018, 8(61): 35131. |
| [69] | YANG F, LUO Y, YU Q, et al. A durable and efficient electrocatalyst for saline water splitting with current density exceeding 2000 mA·cm-2. Advanced Functional Materials, 2021, 31(21): 2010367. |
| [70] | AHMED J, UBIADULLAH M, ALHOKBANY N, et al. Synthesis of ultrafine NiMoO4 nano-rods for excellent electro-catalytic performance in hydrogen evolution reactions. Materials Letters, 2019, 257: 126696. |
| [71] | AN L, FENG J, ZHANG Y, et al. Epitaxial heterogeneous interfaces on N-NiMoO4/NiS2 nanowires/nanosheets to boost hydrogen and oxygen production for overall water splitting. Advanced Functional Materials, 2019, 29(1): 1805298. |
| [72] | JIANG R, ZHAO D, FAN H, et al. Phosphorus doping and phosphates coating for nickel molybdate/nickel molybdate hydrate enabling efficient overall water splitting. Journal of Colloid and Interface Science, 2022, 606: 384. |
| [73] | DU X, LI N, ZHANG X. Controlled synthesis of Co3O4@NiMoO4 core-shell nanorod arrays for efficient water splitting. Dalton Transactions, 2018, 47(35): 12071. |
| [74] | WANG X D, CHEN H Y, XU Y F, et al. Self-supported NiMoP2 nanowires on carbon cloth as an efficient and durable electrocatalyst for overall water splitting. Journal of Materials Chemistry A, 2017, 5(15): 7191. |
| [1] | 李家琪, 李小松, 李煊赫, 朱晓兵, 朱爱民. 暖等离子体合成过渡金属掺杂氧化锰析氧电催化剂[J]. 无机材料学报, 2024, 39(7): 835-844. |
| [2] | 张文宇, 郭瑞华, 岳全鑫, 黄雅荣, 张国芳, 关丽丽. 高熵磷化物双功能催化剂的制备及高效电解水性能[J]. 无机材料学报, 2024, 39(11): 1265-1274. |
| [3] | 王鹏, 靳遵龙, 陈宁光, 刘勇豪. Mo掺杂α-MnO2电催化析氧反应的理论研究[J]. 无机材料学报, 2022, 37(5): 541-546. |
| [4] | 付永胜, 毕敏, 李春, 孙敬文, 汪信, 朱俊武. 非贵金属/碳氮复合材料电催化析氧反应的研究进展[J]. 无机材料学报, 2022, 37(2): 163-172. |
| [5] | 张盛, 蒋亿, 纪媛媛, 杜莹, 盛振环, 殷竟洲, 李乔琦, 张莉莉. 凹凸棒石/g-C3N4复合材料的制备及其电催化析氧性能研究[J]. 无机材料学报, 2019, 34(8): 803-810. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||