无机材料学报 ›› 2024, Vol. 39 ›› Issue (11): 1275-1282.DOI: 10.15541/jim20240075 CSTR: 32189.14.10.15541/jim20240075
所属专题: 【生物材料】骨骼与齿类组织修复(202506)
收稿日期:2024-02-20
修回日期:2024-03-30
出版日期:2024-11-20
网络出版日期:2024-05-31
通讯作者:
汪琦航, 助理研究员. E-mail: qhwang@whut.edu.cn;作者简介:蔡 豪(1999-), 男, 硕士研究生. E-mail: c17596125882@163.com
基金资助:
CAI Hao( ), WANG Qihang(
), WANG Qihang( ), ZOU Zhaoyong(
), ZOU Zhaoyong( )
)
Received:2024-02-20
Revised:2024-03-30
Published:2024-11-20
Online:2024-05-31
Contact:
WANG Qihang, lecturer. E-mail: qhwang@whut.edu.cn;About author:CAI Hao (1999-), male, Master candidate. E-mail: c17596125882@163.com
Supported by:摘要:
无定形碳酸钙(Amorphous Calcium Carbonate,ACC)在生物矿化中具有重要作用, 其结晶过程受到了人们广泛的关注。镁离子(Mg2+)能够有效调控ACC的结晶转变过程, 但其调控ACC转变为一水碳酸钙(Monohydrocalcite, MHC, CaCO3·H2O)晶体的作用机制并不清楚。本研究使用Mg2+作为添加剂, 采用自动电位滴定系统, 原位研究了ACC到MHC的转变过程, 发现Mg2+能够提升ACC的稳定性, 抑制方解石和球霰石的形成。ACC向MHC转变的过程中, 首先发生部分溶解, 随着Ca2+被消耗, 溶液中Mg/Ca摩尔比提高。Mg2+进一步吸附在ACC颗粒表面, 抑制ACC表面溶解, 促使其从内部溶解, 形成富含Mg2+的中空结构以及尺寸更小的纳米颗粒。随后, MHC通过颗粒聚集的方式结晶生长。这些结果解释了Mg2+调控ACC通过非经典结晶方式转变为MHC的机理, 加深了对以ACC为前驱体的生物矿化机制的理解。
中图分类号:
蔡豪, 汪琦航, 邹朝勇. 镁离子调控无定形碳酸钙制备一水碳酸钙结晶过程[J]. 无机材料学报, 2024, 39(11): 1275-1282.
CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion[J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282.
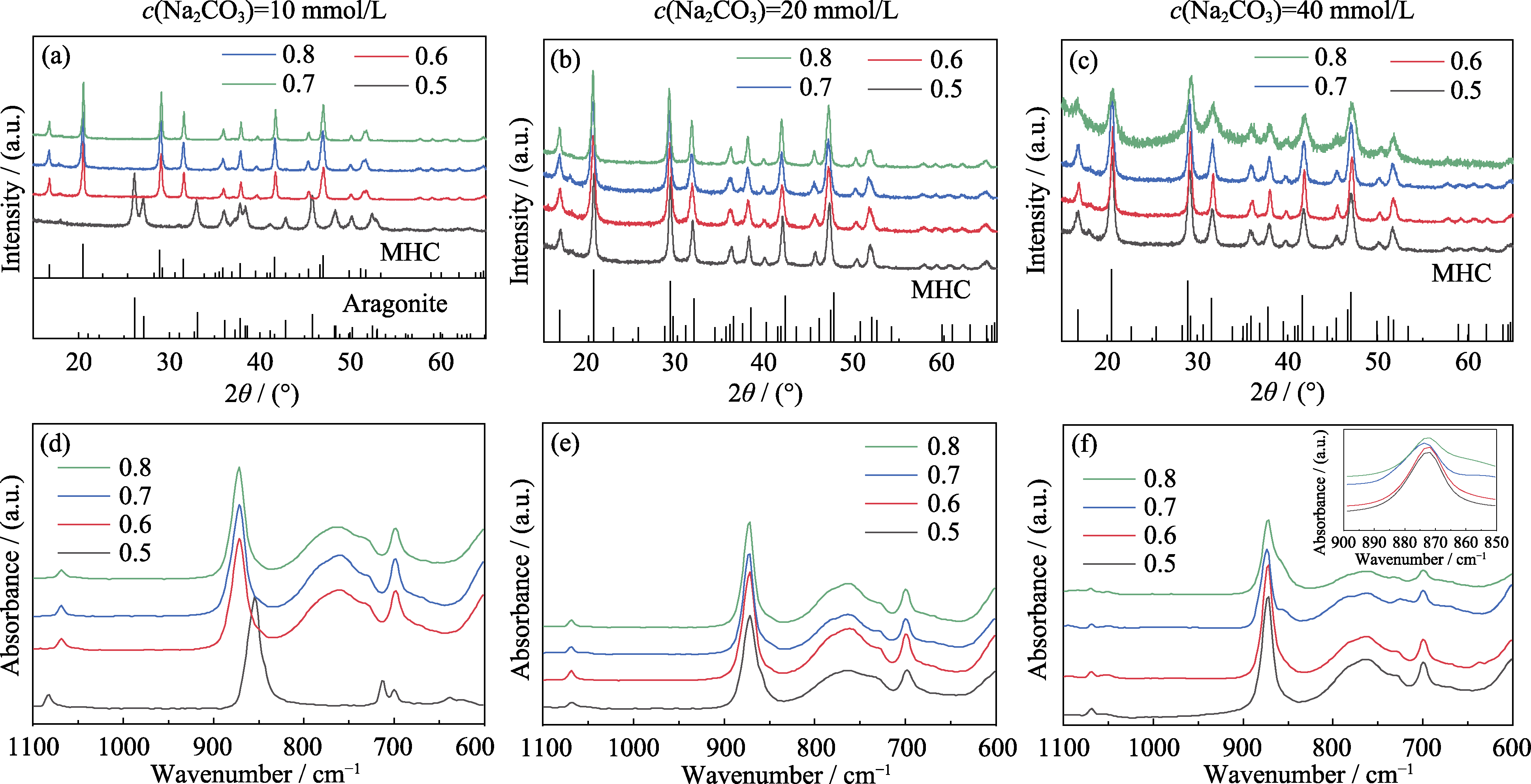
图1 (a, d) 10, (b, e) 20, (c, f) 40 mmol/L碳酸根和不同Mg/(Mg+Ca)比例下结晶产物的(a~c)XRD和(d~f)FT-IR图谱
Fig. 1 (a-c) XRD patterns and (d-f) FT-IR spectra of crystallized products prepared at different carbonate concentrations of (a, d) 10, (b, e) 20, (c, f) 40 mmol/L and different ratios of Mg/(Mg+Ca) Colorful figures are available on website

图2 碳酸根浓度为10 mmol/L, 不同Mg/(Mg+Ca)比例下结晶产物的SEM照片
Fig. 2 SEM images of crystallized products at carbonate concentration of 10 mmol/L and different ratios of Mg/(Mg+Ca) (a, b) 0.5; (c, d) 0.6; (e, f) 0.7; (g, h) 0.8
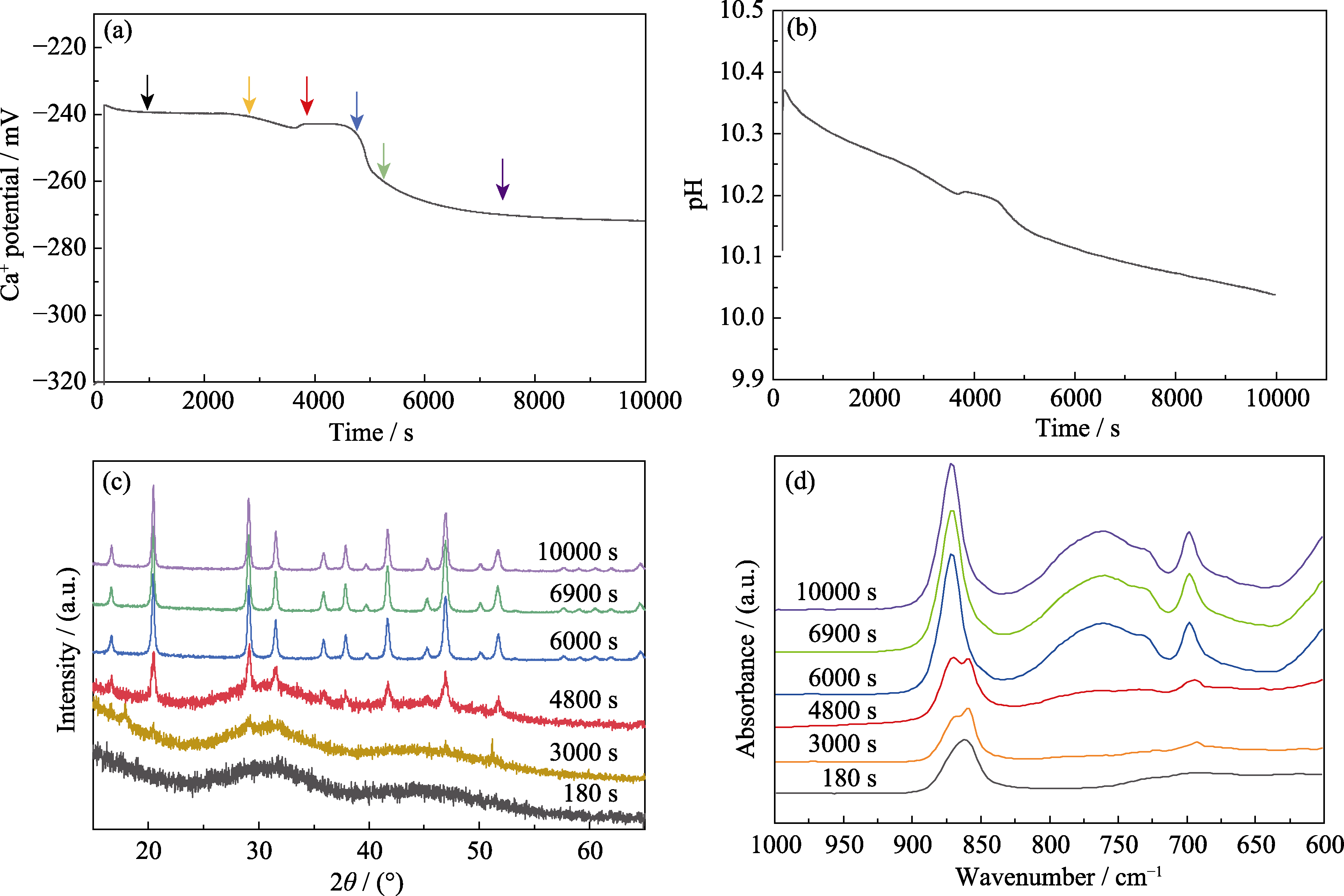
图3 含镁ACC结晶过程中溶液变化的原位监测以及不同时间点产物的结构表征
Fig. 3 In-situ monitoring of solution changes during crystallization of Mg-ACC and structural characterization of products at different time points (a) Ca2+ activity; (b) pH evolution; (c) XRD patterns and (d) FT-IR spectra of the crystallized products at different crystallization stages. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
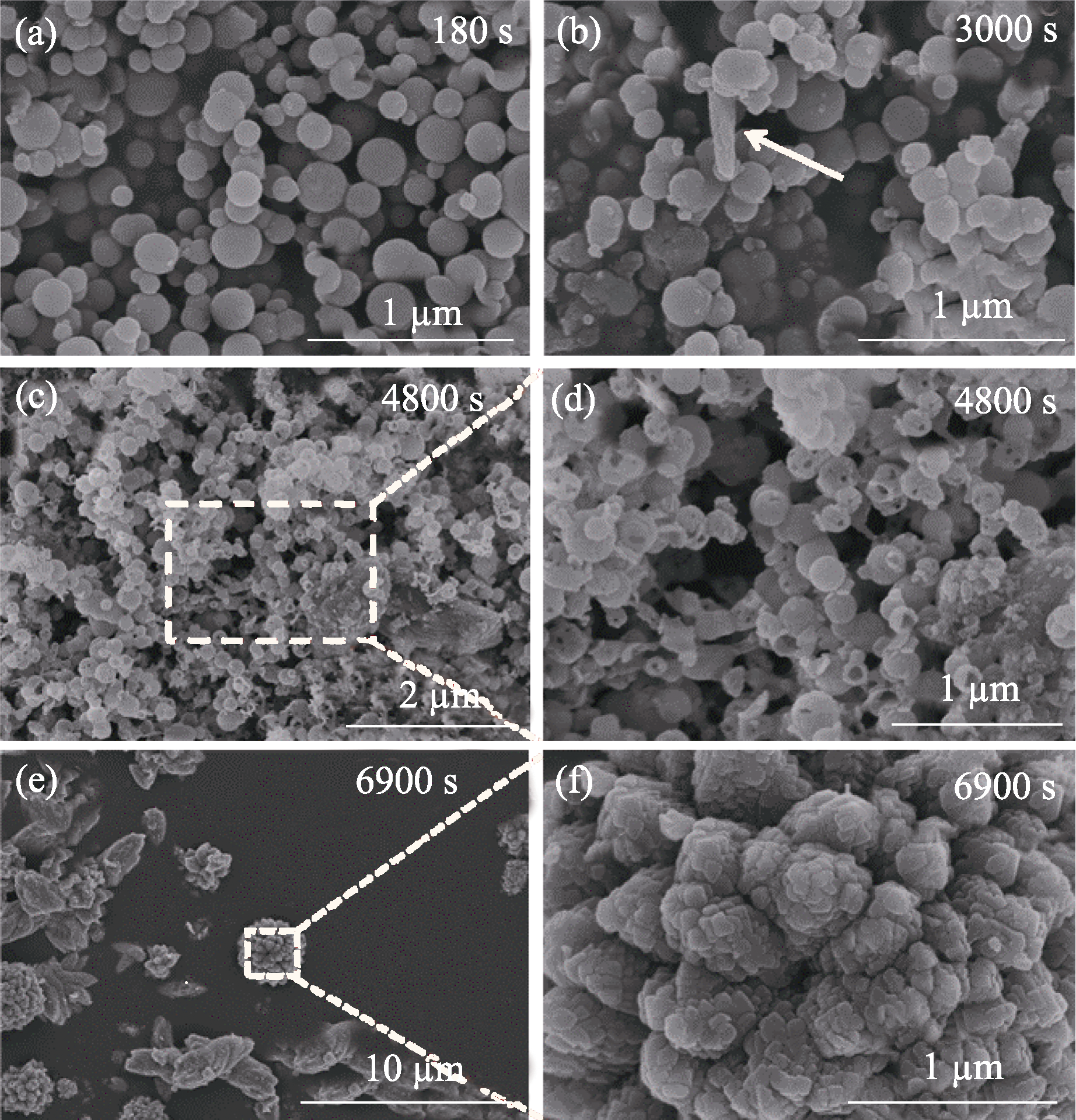
图4 ACC结晶转化过程中不同阶段产物的SEM照片
Fig. 4 SEM images of the products at different stages during ACC crystallization (a) 180 s; (b) 3000 s; (c, d) 4800 s; (e, f) 6900 s. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6

图5 ACC结晶转化过程中不同阶段产物的TEM照片
Fig. 5 TEM images of the products at different stages during ACC crystallization (a) 180 s; (b) 3000 s; (c, d) 4800 s; (e, f) 6900 s. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
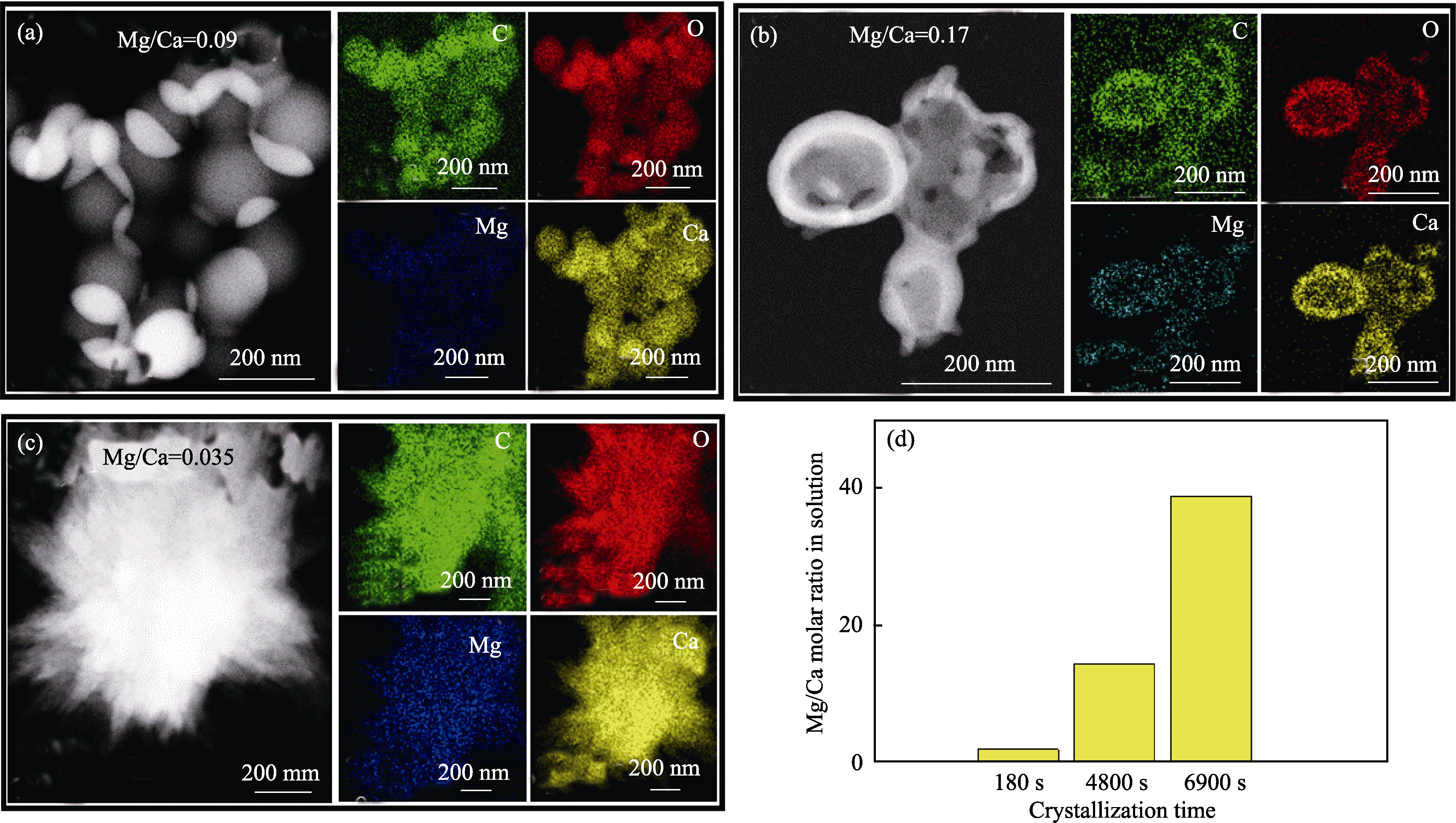
图6 ACC结晶转化过程中不同阶段产物和溶液中的Mg/Ca摩尔比
Fig. 6 Mg/Ca molar ratios of the products and solutions at different stages during ACC crystallization (a-c) HAADF images and their corresponding EDS mappings of products during crystallization for |(a) 180, (b) 4800 and (c) 6900 s; (d) Mg/Ca molar ratio in solution after different crystallization time. Concentration of carbonate: 10 mmol/L; Mg/(Mg+Ca) ratio: 0.6
| [1] | FERMANI S, DŽAKULA B N, REGGI M, et al. Effects of magnesium and temperature control on aragonite crystal aggregation and morphology. CrystEngComm, 2017, 19(18): 2451. |
| [2] | RODRIGUEZ-BLANCO J D, SHAW S, BOTS P, et al. The role of Mg in the crystallization of monohydrocalcite. Geochimica et Cosmochimica Acta, 2014, 127: 204. |
| [3] | WANG C Y, XU Y, LIU Y L, et al. Synthesis and characterization of lamellar aragonite with hydrophobic property. Materials Science & Engineering: C, 2009, 29(3): 843. |
| [4] |
GOWER L B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chemical Reviews, 2008, 108(11): 4551.
DOI PMID |
| [5] | WANG Q, YUAN B, HUANG W, et al. Bioprocess inspired formation of calcite mesocrystals by cation-mediated particle attachment mechanism. National Science Review, 2023, 10(4): nwad014. |
| [6] | SU J T, ZHU F J, ZHANG G Y, et al. Transformation of amorphous calcium carbonate nanoparticles into aragonite controlled by ACCBP. CrystEngComm, 2016, 18(12): 2125. |
| [7] | KARTHIKA S, RADHAKRISHNAN T K, KALAICHELVI P. A review of classical and nonclassical nucleation theories. Crystal Growth & Design, 2016, 16(11): 6663. |
| [8] | WANG Q, HU L, WANG X, et al. Expanding from materials to biology inspired by biomineralization. Interdisciplinary Materials, 2024, 3(2): 165. |
| [9] |
ZOU Z, HABRAKEN W J E M, MATVEEVA G, et al. A hydrated crystalline calcium carbonate phase: calcium carbonate hemihydrate. Science, 2019, 363(6425): 396.
DOI PMID |
| [10] | 解晶晶, 邹朝勇, 傅正义. 无定形碳酸钙的稳定性和结晶转化过程研究进展. 中国材料进展, 2020(4): 261. |
| [11] | LU Z, RICKABY R E M, KENNEDY H, et al. An ikaite record of late holocene climate at the antarctic peninsula. Earth and Planetary Science Letters, 2012, 325: 108. |
| [12] | POLITI Y, ARAD T, KLEIN E, et al. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science, 2004, 306(5699): 1161. |
| [13] |
POLITI Y, METZLER R A, ABRECHT M, et al. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(45): 17362.
DOI PMID |
| [14] |
AIZENBERG J, LAMBERT G, WEINER S, et al. Factors involved in the formation of amorphous and crystalline calcium carbonate: a study of an ascidian skeleton. Journal of the American Chemical Society, 2002, 124(1): 32.
PMID |
| [15] | KHAIROUN I, MAGNE D, GAUTHIER O, et al. In vitro characterization and in vivo properties of a carbonated apatite bone cement. Journal of Biomedical Materials Research, 2002, 60(4): 633. |
| [16] | HABRAKEN W J E M, MASIC A, BERTINETTI L, et al. Layered growth of crayfish gastrolith: about the stability of amorphous calcium carbonate and role of additives. Journal of Structural Biology, 2014, 189(1): 28. |
| [17] | KRAUSS F, SCHRIEVER W. Die hydrate des calcium carbonats. Zeitschrift Für Anorganische Und Allgemeine Chemie, 2004, 188(1): 259. |
| [18] | DAHL K, BUCHARDT B. Monohydrocalcite in the arctic Ikka Fjord, SW Greenland: first reported marine occurrence. Journal of Sedimentary Research, 2006, 76(3): 460. |
| [19] | SWAINSON I P. The structure of monohydrocalcite and the phase composition of the beachrock deposits of Lake Butler and Lake Fellmongery, South Australia. American Mineralogist, 2008, 93(7): 1014. |
| [20] | YAMAMOTO G, ATSUSHI K, SATORU O. Structural variations of amorphous magnesium carbonate during nucleation, crystallization, and decomposition of nesquehonite MgCO3·3H2O. Physics and Chemistry of Minerals, 2022, 50(5): 305. |
| [21] | SON S, LI W Q, LEE J Y, et al. On the coordination of Mg2+ in aragonite: ab-initio absorption spectroscopy and isotope fractionation study. Geochimica et Cosmochimica Acta, 2020, 286: 324. |
| [22] | PURGSTALLER B, KONRAD F, DIETZEL M, et al. Control of Mg2+/Ca2+ activity ratio on the formation of crystalline carbonate minerals via an amorphous precursor. Crystal Growth & Design, 2017, 17(3): 1069. |
| [23] | MATSUMOTO M, FUKUNAGA T, ONOE K. Polymorph control of calcium carbonate by reactive crystallization using microbubble technique. Chemical Engineering Research and Design, 2010, 88(12): 1624. |
| [24] | TADIER S, ROKIDI S, REY C, et al. Crystal growth of aragonite in the presence of phosphate. Journal of Crystal Growth, 2017, 458: 44. |
| [25] | WILLINGER M G, POLLEUX J, ANTONIETTI M, et al. Structural evolution of aragonite superstructures obtained in the presence of the siderophore deferoxamine. CrystEngComm, 2015, 17(21): 3927. |
| [26] | ZHU F J, NISHIMURA T, SAKAMOTO T, et al. Tuning the stability of CaCO3 crystals with magnesium ions for the formation of aragonite thin films on organic polymer templates. Chemistry-An Asian Journal, 2013, 8(12): 3002. |
| [27] | SUZUKI M, KOGURE T, WEINER S, et al. Formation of aragonite crystals in the crossed lamellar microstructure of limpet shells. Crystal Growth & Design, 2011, 11(11): 4850. |
| [28] | PARK W K, KO S J, LEE S W, et al. Effects of magnesium chloride and organic additives on the synthesis of aragonite precipitated calcium carbonate. Journal of Crystal Growth, 2008, 310(10): 2593. |
| [29] | HEYWOOD B R, MANN S. Molecular construction of oriented inorganic materials: controlled nucleation of calcite and aragonite under compressed langmuir monolayers. Chemistry of Materials, 1994, 6(3): 311. |
| [30] | LEVI-KALISMAN Y, RAZ S, WEINER S, et al. X-ray absorption spectroscopy studies on the structure of a biogenic “amorphous” calcium carbonate phase. Journal of the Chemical Society, Dalton Transactions, 2000(21): 3977. |
| [31] | LAM R S K, CHARNOCK J M, LENNIE A, et al. Synthesis-dependant structural variations in amorphous calcium carbonate. CrystEngComm, 2007, 9(12): 1226. |
| [32] | SUN W, JAYARAMAN S, CHEN W, et al. Nucleation of metastable aragonite CaCO3 in seawater. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(11): 3199. |
| [33] | HUANG Z, ZHANG G. Biomimetic synthesis of aragonite nanorod aggregates with unusual morphologies using a novel template of natural fibrous proteins at ambient condition. Crystal Growth & Design, 2012, 12(4): 1816. |
| [34] | MUNEMOTO T, FUKUSHI K. Transformation kinetics of monohydrocalcite to aragonite in aqueous solutions. Journal of Mineralogical and Petrological Sciences, 2008, 103(5): 345. |
| [35] | NEUMANN M, EPPLE M. Monohydrocalcite and its relationship to hydrated amorphous calcium carbonate in biominerals. European Journal of Inorganic Chemistry, 2007, 2007(14): 1953. |
| [36] | CHEN T, NEVILLE A, YUAN M. Assessing the effect of Mg2+ on CaCO3 scale formation-bulk precipitation and surface deposition. Journal of Crystal Growth, 2005, 275(1): e1341. |
| [37] | BOTS P, BENNING L G, RODRIGUEZ-BLANCO J D, et al. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Crystal Growth & Design, 2012, 12(7): 3806. |
| [38] | ANDERSEN F A, BREČEVIĆ L J C. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chemica Scandinavica, 1991, 45: 1018. |
| [39] | COLEYSHAW E E, CRUMP G, GRIFFITH W P. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2003, 59(10): 2231. |
| [40] | LOSTE E, WILSON R M, SESHADRI R, et al. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. Journal of Crystal Growth, 2003, 254(1): 206. |
| [41] | JU Y M, HUANG F, DING X, et al. Phase transformation-induced Mg isotope fractionation in Mg-mediated CaCO3 mineralization. Nano Research, 2023, 16(2): 3597. |
| [42] | POLITI Y, BATCHELOR D R, ZASLANSKY P, et al. Role of magnesium ion in the stabilization of biogenic amorphous calcium carbonate: a structure-function investigation. Chemistry of Materials, 2010, 22(1): 161. |
| [43] | RODRIGUEZ-BLANCO J D, SHAW S, BOTS P, et al. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. Journal of Alloys and Compounds, 2012, 536: S477. |
| [44] | YAGI S, FUKUSHI K. Phosphate sorption on monohydrocalcite. Journal of Mineralogical and Petrological Sciences, 2011, 106(2): 109. |
| [45] |
DI TOMMASO D, DE LEEUW N H. Structure and dynamics of the hydrated magnesium ion and of the solvated magnesium carbonates: insights from first principles simulations. Physical Chemistry Chemical Physics, 2010, 12(4): 894.
DOI PMID |
| [46] | MOOMAW A S, MAGUIRE M E. The unique nature of Mg2+channels. Physiology, 2008, 23: 275. |
| [47] | ZOU Z, XIE J, MACÍAS-SÁNCHEZ E, et al. Nonclassical crystallization of amorphous calcium carbonate in the presence of phosphate ions. Crystal Growth Design, 2021, 21(1): 414. |
| [48] | ZOU Z, BERTINETTI L, POLITI Y, et al. Control of polymorph selection in amorphous calcium carbonate crystallization by poly (aspartic acid): two different mechanisms. Small, 2017, 13(21): 1603100. |
| [49] | HUANG W, WANG Q, CHI W, et al. Multiple crystallization pathways of amorphous calcium carbonate in the presence of poly (aspartic acid) with a chain length of 30. CrystEngComm, 2022, 24(26): 4809. |
| [1] | 李宪珂, 张超逸, 黄林, 孙鹏, 刘波, 徐军, 唐慧丽. 高质量铟掺杂氧化镓单晶浮区法生长研究[J]. 无机材料学报, 2024, 39(12): 1384-1390. |
| [2] | 郝永鑫, 秦娟, 孙军, 杨金凤, 李清连, 黄贵军, 许京军. 坩埚底角形状对提拉法生长同成分铌酸锂晶体的影响[J]. 无机材料学报, 2024, 39(10): 1167-1174. |
| [3] | 秦娟, 梁丹丹, 孙军, 杨金凤, 郝永鑫, 李清连, 张玲, 许京军. 提拉法生长平肩同成分铌酸锂晶体的研究[J]. 无机材料学报, 2023, 38(8): 978-986. |
| [4] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [5] | 杨佳雪, 李雯, 王燕, 朱昭捷, 游振宇, 李坚富, 涂朝阳. Dy3+: Y3Al5O12晶体的光谱与黄色激光性能[J]. 无机材料学报, 2023, 38(3): 350-356. |
| [6] | 吴振, 李慧芳, 张中晗, 张振, 李阳, 蓝江河, 苏良碧, 武安华. 面向磁光应用的CeF3晶体生长与性能表征[J]. 无机材料学报, 2023, 38(3): 296-302. |
| [7] | 齐雪君, 张健, 陈雷, 王绍涵, 李翔, 杜勇, 陈俊锋. 坩埚下降法生长大尺寸Bi12GeO20晶体的宏观缺陷[J]. 无机材料学报, 2023, 38(3): 280-287. |
| [8] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [9] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [10] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [11] | 王海东, 王燕, 朱昭捷, 李坚富, LAKSHMINARAYANA Gandham, 涂朝阳. Dy3+掺杂SrGdGa3O7晶体的晶体生长, 结构、光学和可见光荧光特性[J]. 无机材料学报, 2023, 38(12): 1475-1482. |
| [12] | 徐家跃, 李志超, 潘芸芳, 周鼎, 温丰, 马文军. 超化学计量比氧化铀晶体的研究进展[J]. 无机材料学报, 2020, 35(11): 1183-1192. |
| [13] | 李荣辉, 郏义征, 胡楠楠. 三维层级花状活性氧化铝纳米材料的制备及其除砷性能研究[J]. 无机材料学报, 2019, 34(5): 553-559. |
| [14] | 王东海, 薛艳艳, 李纳, 周仕明, 徐晓东, 李东振, 徐军, 王庆国. 导模法生长微孔蓝宝石晶体工艺及性能研究[J]. 无机材料学报, 2019, 34(12): 1290-1294. |
| [15] | 陈巧玲, 罗敏, 林晨升. 新型紫外非线性光学晶体KNa5Ca5(CO3)8的合成、表征及性能的研究[J]. 无机材料学报, 2018, 33(6): 667-672. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||