无机材料学报 ›› 2024, Vol. 39 ›› Issue (2): 115-128.DOI: 10.15541/jim20230527 CSTR: 32189.14.10.15541/jim20230527
所属专题: 【信息功能】MAX、MXene及其他二维材料(202506)
丁浩明1,2( ), 陈科1,2, 李勉1,2, 李友兵3, 柴之芳1,2, 黄庆1,2(
), 陈科1,2, 李勉1,2, 李友兵3, 柴之芳1,2, 黄庆1,2( )
)
收稿日期:2023-11-10
修回日期:2023-12-14
出版日期:2023-12-19
网络出版日期:2023-12-19
通讯作者:
黄 庆, 研究员. E-mail: huangqing@nimte.ac.cn作者简介:丁浩明(1994-), 男, 博士. E-mail: dinghaoming@nimte.ac.cn
基金资助:
DING Haoming1,2( ), CHEN Ke1,2, LI Mian1,2, LI Youbing3, CHAI Zhifang1,2, HUANG Qing1,2(
), CHEN Ke1,2, LI Mian1,2, LI Youbing3, CHAI Zhifang1,2, HUANG Qing1,2( )
)
Received:2023-11-10
Revised:2023-12-14
Published:2023-12-19
Online:2023-12-19
Contact:
HUANG Qing, professor. E-mail: huangqing@nimte.ac.cnAbout author:DING Haoming (1994-), male, PhD. E-mail: dinghaoming@nimte.ac.cn
Supported by:摘要:
受到生物基因工程中“基因剪刀”的启发, “化学剪刀”作为一种重要的研究工具在材料结构编辑及应用研究中发挥着重要作用。本文对“化学剪刀”在材料结构编辑及应用方面的研究进展进行了评述。首先, 介绍了“化学剪刀”的概念和基本原理, 即指在保持初始材料主结构不变的条件下, 通过化学反应敲除、置换、修复或重构目标原子或结构单元, 从而定制化编辑材料晶格中的组成元素、晶体结构以及微观形貌, 最终实现特定的材料结构与功能。随后, 详细回顾了“化学剪刀”在材料结构编辑中的具体应用, 即如何利用化学剪切、化学修饰、化学合成和化学刻蚀与化学插层等结构编辑方法对材料结构进行精确调控和功能设计。最后, 对“化学剪刀”未来在材料结构编辑及应用的研究方向进行了展望。本评述详细介绍了“化学剪刀”在材料结构编辑及应用研究方面的研究进展和巨大潜力, 为探索和开发“化学剪刀”在材料领域的应用提供了有力的理论和实验支撑, 并有望推动相关材料领域的发展。
中图分类号:
丁浩明, 陈科, 李勉, 李友兵, 柴之芳, 黄庆. 无机材料的“化学剪刀”结构编辑策略[J]. 无机材料学报, 2024, 39(2): 115-128.
DING Haoming, CHEN Ke, LI Mian, LI Youbing, CHAI Zhifang, HUANG Qing. Chemical Scissor-mediated Structural Editing of Inorganic Materials[J]. Journal of Inorganic Materials, 2024, 39(2): 115-128.
| Regulation mechanism | Chemical scissor | Chemical mechanism | Target | Potential applications | Ref. |
|---|---|---|---|---|---|
| Chemical cutting | Plasma | Collision and reaction of plasma on graphene surface | Graphene | Electronic engineering | [ |
| O2/NH3 | Oxidation of edge carbon atoms | Graphene | Electronic engineering | [ | |
| Metal nanoparticles | Catalytic hydrogenation of carbon atoms | Graphene | Electronic engineering | [ | |
| TiO2 photocatalysis | Photoinduced oxidation of carbon atoms by highly active OH radicals | Graphene | Electronic engineering | [ | |
| HNO3/H2SO4/KClO3 | Thermal expansion caused by oxidation and decomposition of surface functional groups of graphite | Graphite | Energy storage | [ | |
| NH3/H2O2 | Oxidation of carbon atoms | g-C3N4 | Catalysis, Sensor | [ | |
| HNO3/H2SO4 | Oxidation of carbon atoms | g-C3N4 | Catalysis, Sensor | [ | |
| Chemical modification | FeCl3 | Oxidation of Pd atoms | Pd clusters | Catalysis, Sensor | [ |
| H2 | Hydrogenation of N atoms | g-C3N4 | Catalysis, Sensor | [ | |
| CO2 | Oxidation of carbon atoms | Carbon materials | Energy storage | [ | |
| Chemical synthesis | C2H5OH | The breaking of organic bonds results in the dissolution of organic substances | 3-aminophenol formaldehyde resin | Catalysis | [ |
| Acetone | The breaking of organic bonds results in the dissolution of organic substances | 3-aminophenol formaldehyde resin | Catalysis, energy storage | [ | |
| Lone pair cations and halogen ions | Control of coordination environments | Sb2ZnO3Cl2 Sb16Cd8O25Cl14 Te6O11Cl2 Sb4O5Cl2 | Catalysis, energy storage | [ | |
| H+ | Control of reaction dynamics | Metal-benzene hexethiol (BHT) coordination polymer | Catalysis | [ | |
| Chemical etching and intercalation | Lewis acidic melts (CuCl2, ZnCl2) | Oxidation of A-site atoms in MAX phases | MAX phases | Catalysis, energy storage, electronic engineering | [ |
| HF, HCl/LiF | Acidic dissolution of A-site atoms in MAX phases | MAX phases | Catalysis, energy storage | [ | |
| Metal atoms (Mn, Fe, Co, Ni,Cu, Ag) | Regulating electronic structure by injecting electrons into host materials | Transition metal chalcogenides | Catalysis, energy storage | [ |
表1 典型“化学剪刀”的化学机制及其在材料结构编辑中的应用
Table 1 Chemical mechanism of typical "chemical scissors" and applications in structural editing
| Regulation mechanism | Chemical scissor | Chemical mechanism | Target | Potential applications | Ref. |
|---|---|---|---|---|---|
| Chemical cutting | Plasma | Collision and reaction of plasma on graphene surface | Graphene | Electronic engineering | [ |
| O2/NH3 | Oxidation of edge carbon atoms | Graphene | Electronic engineering | [ | |
| Metal nanoparticles | Catalytic hydrogenation of carbon atoms | Graphene | Electronic engineering | [ | |
| TiO2 photocatalysis | Photoinduced oxidation of carbon atoms by highly active OH radicals | Graphene | Electronic engineering | [ | |
| HNO3/H2SO4/KClO3 | Thermal expansion caused by oxidation and decomposition of surface functional groups of graphite | Graphite | Energy storage | [ | |
| NH3/H2O2 | Oxidation of carbon atoms | g-C3N4 | Catalysis, Sensor | [ | |
| HNO3/H2SO4 | Oxidation of carbon atoms | g-C3N4 | Catalysis, Sensor | [ | |
| Chemical modification | FeCl3 | Oxidation of Pd atoms | Pd clusters | Catalysis, Sensor | [ |
| H2 | Hydrogenation of N atoms | g-C3N4 | Catalysis, Sensor | [ | |
| CO2 | Oxidation of carbon atoms | Carbon materials | Energy storage | [ | |
| Chemical synthesis | C2H5OH | The breaking of organic bonds results in the dissolution of organic substances | 3-aminophenol formaldehyde resin | Catalysis | [ |
| Acetone | The breaking of organic bonds results in the dissolution of organic substances | 3-aminophenol formaldehyde resin | Catalysis, energy storage | [ | |
| Lone pair cations and halogen ions | Control of coordination environments | Sb2ZnO3Cl2 Sb16Cd8O25Cl14 Te6O11Cl2 Sb4O5Cl2 | Catalysis, energy storage | [ | |
| H+ | Control of reaction dynamics | Metal-benzene hexethiol (BHT) coordination polymer | Catalysis | [ | |
| Chemical etching and intercalation | Lewis acidic melts (CuCl2, ZnCl2) | Oxidation of A-site atoms in MAX phases | MAX phases | Catalysis, energy storage, electronic engineering | [ |
| HF, HCl/LiF | Acidic dissolution of A-site atoms in MAX phases | MAX phases | Catalysis, energy storage | [ | |
| Metal atoms (Mn, Fe, Co, Ni,Cu, Ag) | Regulating electronic structure by injecting electrons into host materials | Transition metal chalcogenides | Catalysis, energy storage | [ |
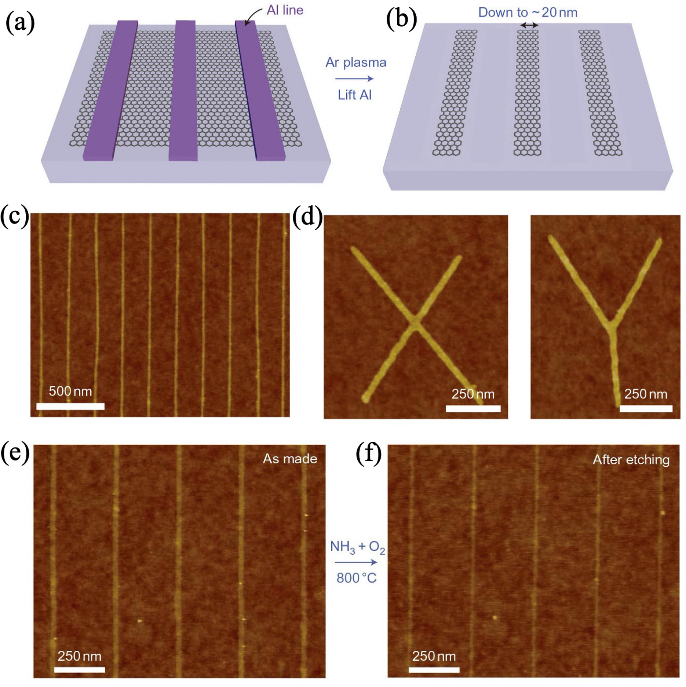
图1 光刻等离子刻蚀技术制备石墨烯纳米带[13]
Fig. 1 Preparation of graphene nanoribbons by photolithographic plasma etching technique[13] (a, b) Schematic illustrations of preparation of graphene nanoribbons by photolithographic plasma etching technique; (c, d) Atomic force microscope (AFM) images of graphene nanoribbons prepared by photolithographic plasma etching technique; AFM images of graphene nanoribbons, showing morphologies before (e) and after (f) cutting using NH3/O2 as chemical scissor
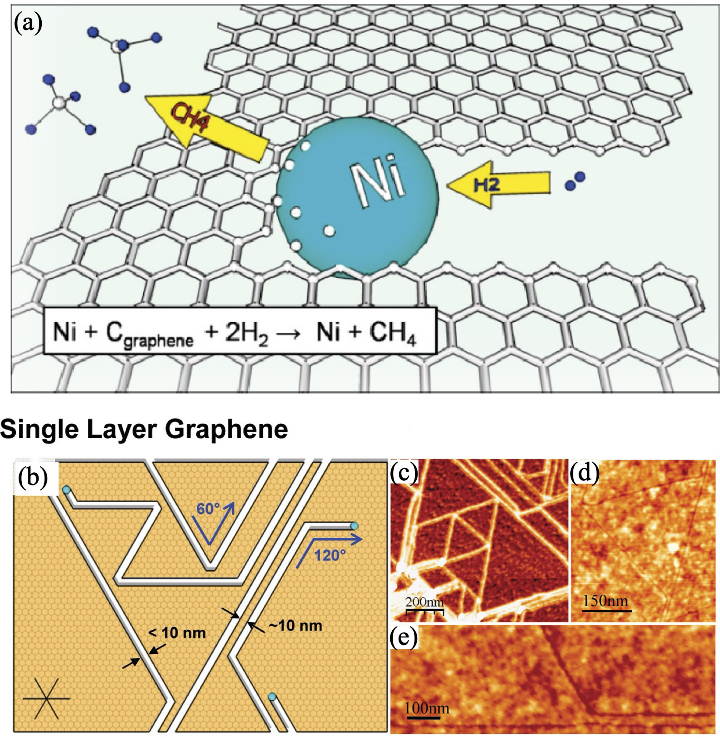
图2 金属纳米粒子作为催化剂“剪刀”切割石墨烯[19]
Fig. 2 Metal nanoparticles as catalyst scissors cutting graphene [19] (a) Schematic illustration of the cutting process of graphene using metal nanoparticles as catalyst scissors; (b) Schematic illustration of the cutting pathway of graphene; (c-e) AFM images of graphene after cutting, showing the cutting pathway

图3 光催化切割石墨烯[12]
Fig. 3 Photocatalytic cutting of graphene[12] (a) Schematic illustration of the photocatalytic approach to engineering single or few-layer graphene, in which the quartz plate/TiO2 thin film/ patterned Cr photomask is put into contact with graphene; (b) Optical microscope image of graphene ribbons obtained with a line-shape TiO2 photomask; (c) SEM image of a photo-catalytically patterned CVD-graphene with inset showing the photomask structure; (d) Optical microscope image of a patterned reduced graphene oxide (RGO) film, illustrating the feasibility of complex structural design; (e) SEM image of a periodically patterned RGO film; (f) SEM image of a series of CVD-graphene ribbons having different widths. Scale bars: 50 μm (b-e), 100 μm (inset in (c)), 10 μm (f)
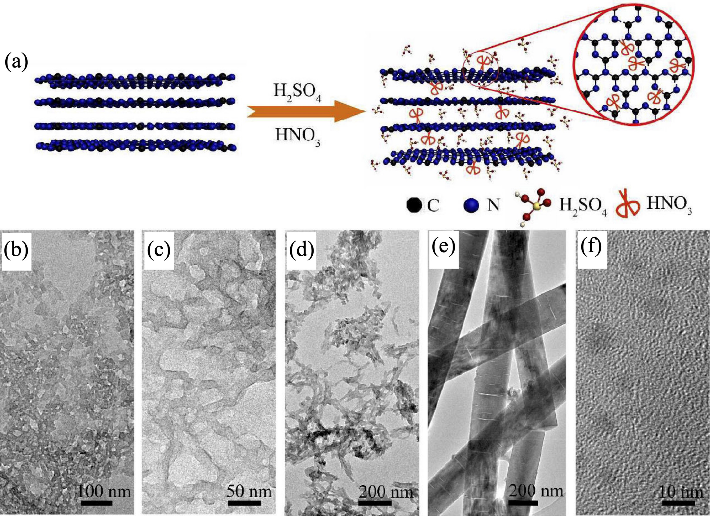
图5 H2SO4/HNO3作为“化学剪刀”剪切g-C3N4[22]
Fig. 5 H2SO4/HNO3 as chemical scissors cutting g-C3N4 [22] (a) Schematic illustration of cutting g-C3N4 using H2SO4/HNO3 as chemical scissors; (b-f) TEM images of samples with different mixed acid volume ratios (V(HNO3)∶V(H2SO4)= (b) 1∶3, (c) 1∶2, (d) 1∶1, (e) 2∶1, (f) 3∶1)
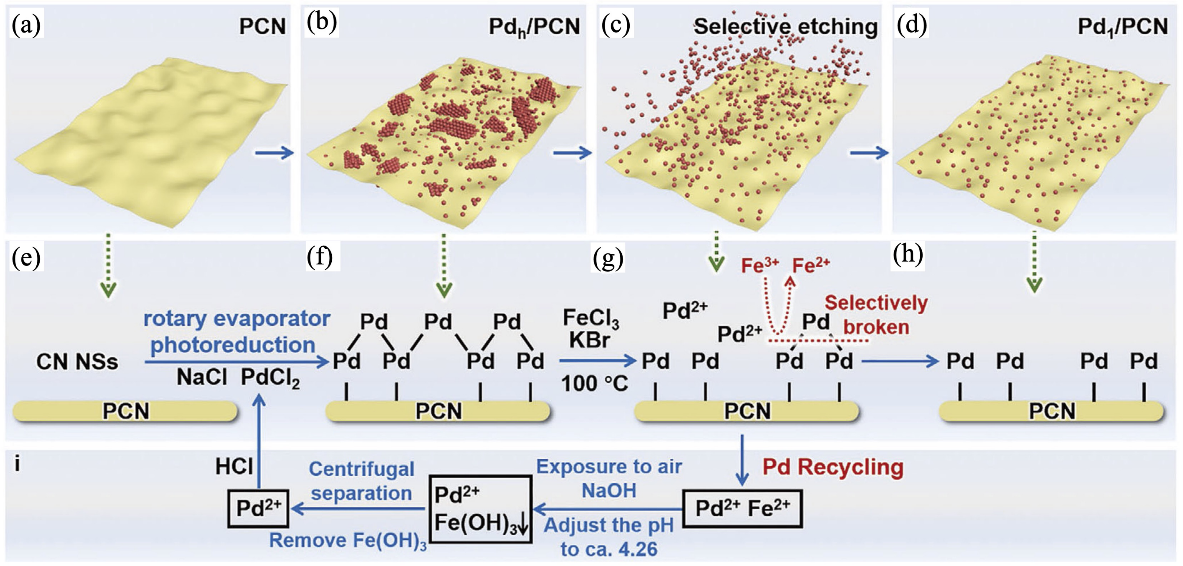
图6 “化学剪刀”选择性修剪聚合氮化碳(PCN)表面Pd原子实现高载量Pd单原子修饰[23]
Fig. 6 Pd single-atom modification by selectively trimming the Pd atoms on the PCN surface using chemical scissors[23] (a-d) Schematic illustration of Pd single-atom modification by selectively trimming the Pd atoms on the PCN surface using chemical scissors; (e-h) Chemical mechanism and reaction process of Pd single-atom modification by selectively trimming the Pd atoms on the PCN surface using chemical scissors
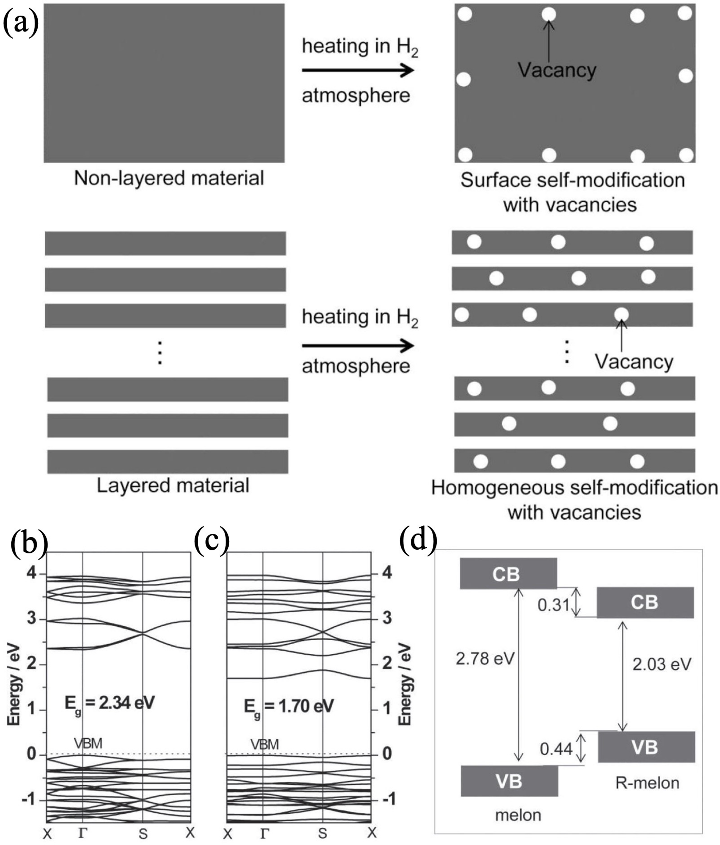
图7 H2作为“化学剪刀”实现g-C3N4中N原子空位的修饰[24]
Fig. 7 N atomic vacancy modification of g-C3N4 realized by cutting of H2 chemical scissor[24] (a) Schematic illustration of N atomic vacancy modification of g-C3N4 realized by cutting of H2 chemical scissor; (b-d) Band structures of g-C3N4 before and after modification
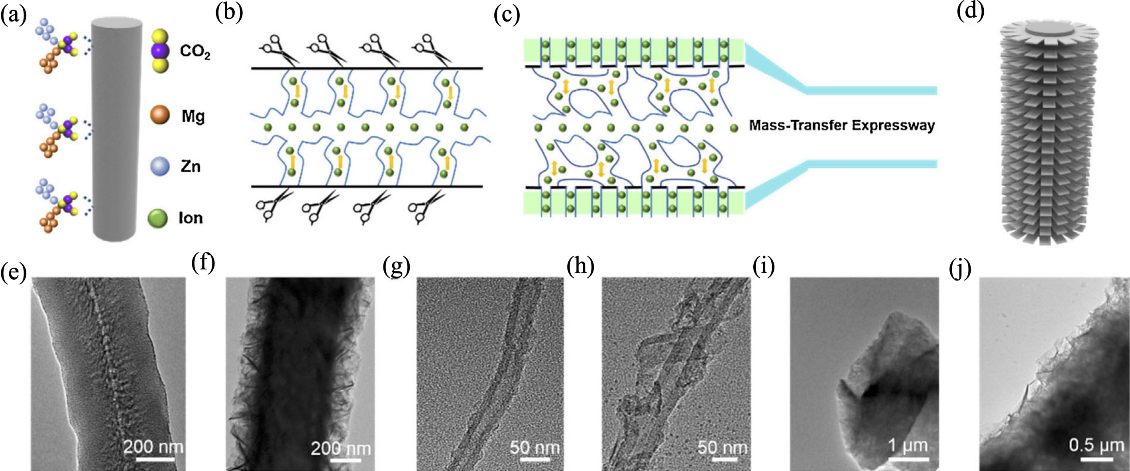
图8 Mg/Zn/CO2作为“化学剪刀”剪切碳纳米材料[25]
Fig. 8 Mg/Zn/CO2 scissors rationally tailoring the carbon nanomaterials[25] (a, b) Molecular scissors acting on the surface of carbon nanomaterials; (c, d) Highly interconnected microstructure of tubular superstructure of nanocarbon (TSNC) facilitating substantial ion-reserved accommodation and rapid mass-transfer expressway; (e-j) TEM images of the obtained carbon nanomaterials, showing different cutting morphologies
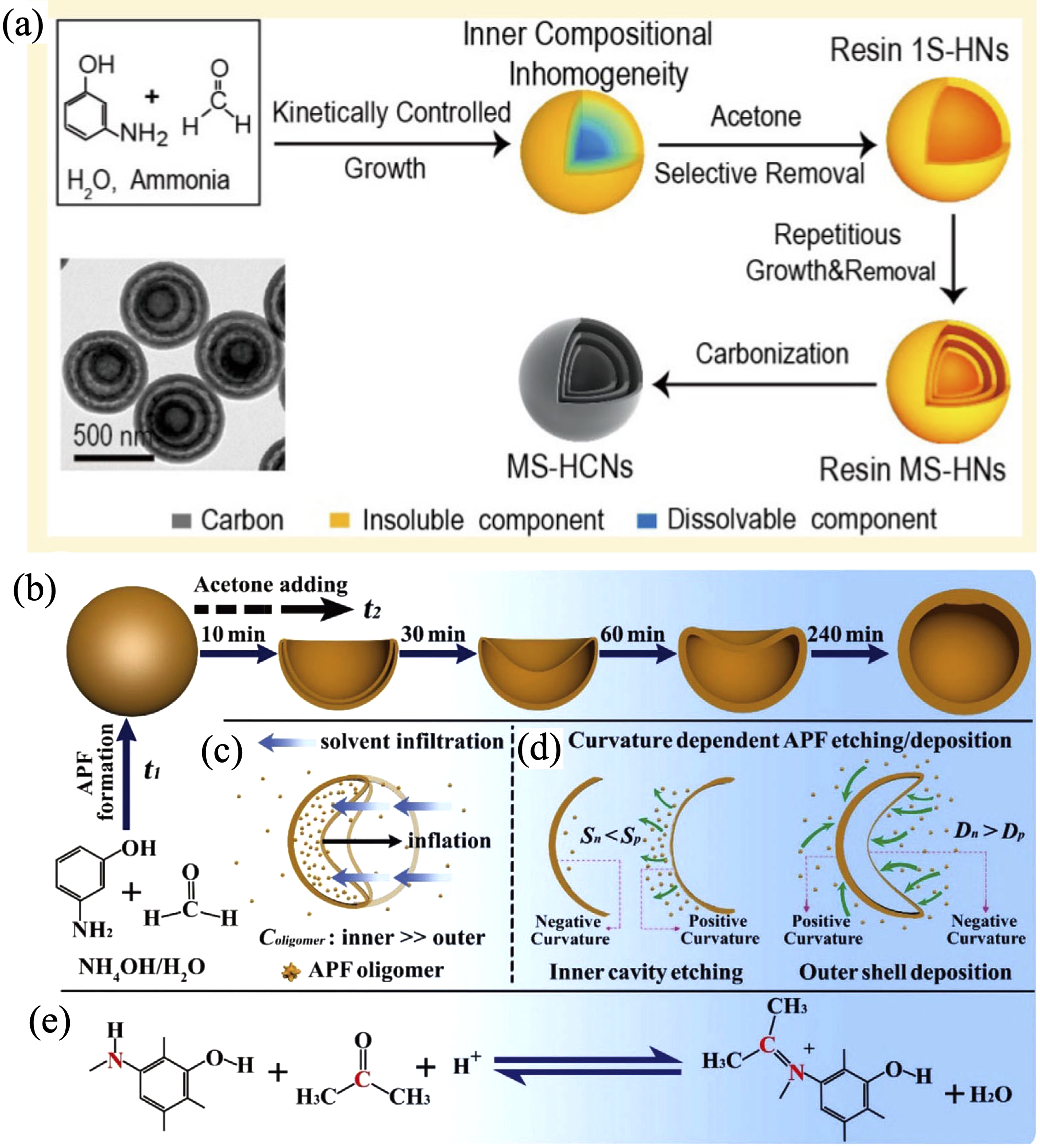
图9 “化学剪刀”制备的中空纳米结构
Fig. 9 Preparation of hollow nanostructure using chemical scissors (a) Schematic illustration and reaction mechanism of modulation of nanostructure and chemical composition by using ethanol as chemical scissors to precisely cut formaldehyde resin[26]; (b-e) Illustrations of the deflation-inflation asymmetric growth (DIAG) process and mechanism[27]
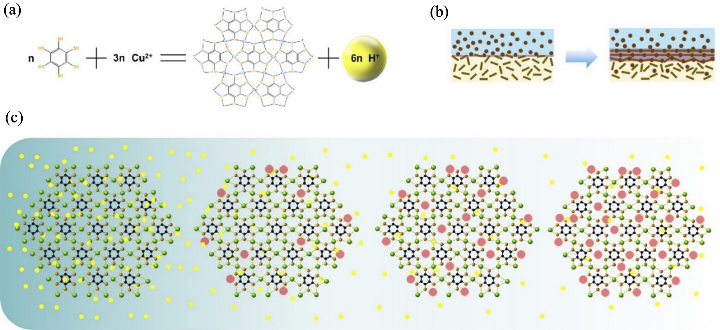
图11 质子浓度调节实现“化学剪刀”效应合成二维MOF材料的示意图[30]
Fig. 11 Two-dimensional MOF materials synthesized by proton concentration modulation with chemical scissor effect[30] (a) Chemical reaction mechanism of Cu-BHT; (b) Interfacial self-assembly growth process, and (c) schematic illustration of metal vacancy engineering via precise pH regulation (yellow sphere: proton; black sphere: C atom; gold sphere: S atom; green sphere: Cu atom; red circle: Cu vacancy)
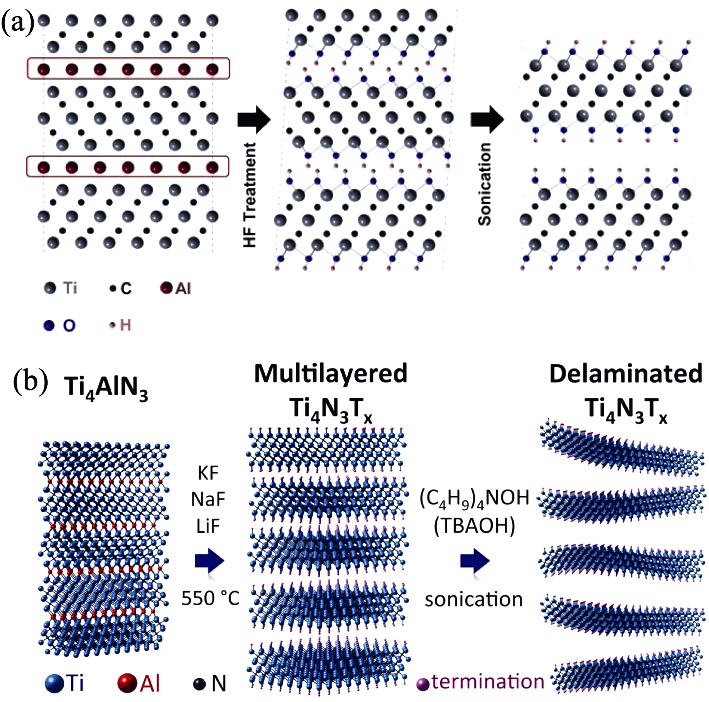
图12 不同的MXene化学刻蚀制备方法的示意图
Fig. 12 Schematic illustrations of different etching methods for preparation of MXene (a) HF as echant[36]; (b) KF-NaF-LiF melt as echant[68]
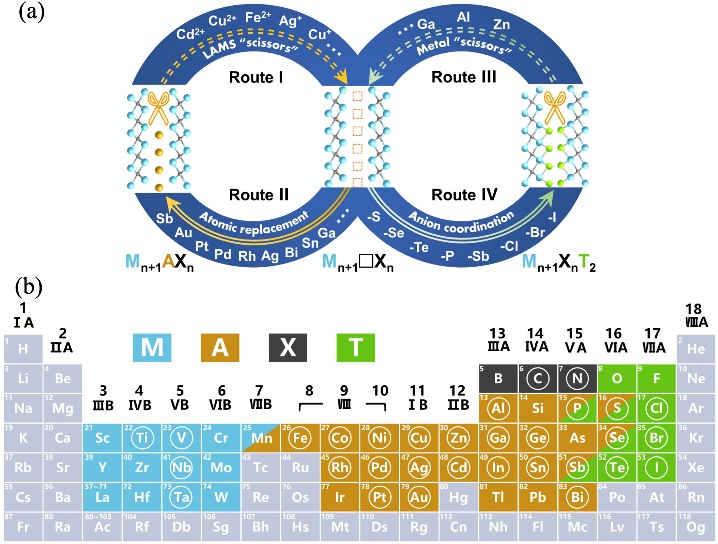
图13 “化学剪刀”辅助的层状过渡金属碳/氮化物的结构编辑策略[10]
Fig. 13 Chemical-scissors assisted structural editing strategy for layered transition metal carbides/nitrides[10] (a) Schematic illustration of chemical-scissors assisted structural editing strategy for layered transition metal carbides/nitrides; (b) Periodic table showing elements involved in the formation of MAX phases and MXenes. Light blue: M elements; brown: A elements; black: X elements; green: ligand (T) elements; circled: elements studied in the present work
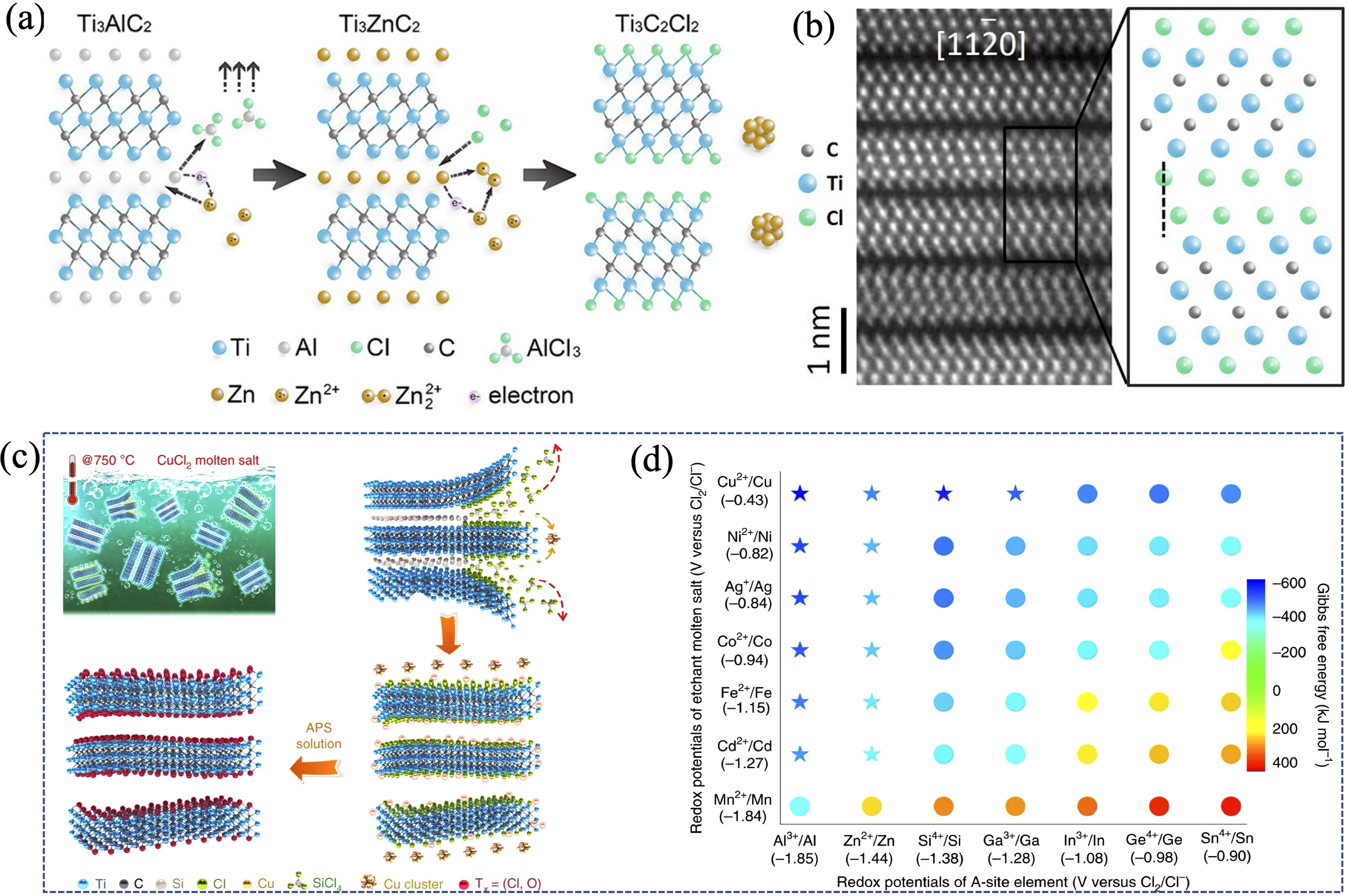
图14 路易斯酸熔盐法制备MAX相和MXenes
Fig. 14 Lewis-acidic-melt method for the preparation of MAX phases and MXenes (a) Schematic illustration of preparing novel MAX phases and MXene based on Lewis-acidic-molten-salt route[31]; (b) STEM image of Ti3C2Cl2 prepared by Lewis-acidic-molten-salt route, and its corresponding atomic model[31]; (c) Schematic illustration of preparing Ti3C2Tx MXene via a reaction between Ti3SiC2 and CuCl2; (d) Redox potential/Gibbs free energy between Lewis acid cations and A-site atoms in molten salts[34]
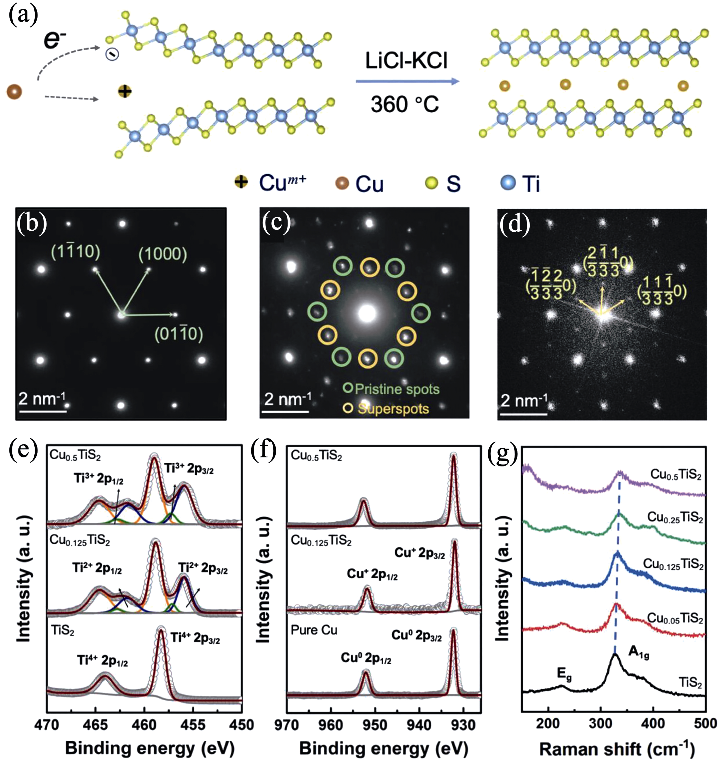
图15 “化学剪刀”辅助的过渡金属硫属化合物的原子插层[38]
Fig. 15 Chemical scissor-assisted atomic intercalation of transition metal dichalcogenides[38] (a) Intercalation schematic of Cu-TiS2 in LiCl-KCl eutectic molten salt at 360 ℃; (b-d) FFT patterns of pristine TiS2 diffraction spots, Cu0.125TiS2, and Cu0.5TiS2 diffraction superspots taken along the [0001] zone-axis direction; (e, f) Ti2p and Cu2p XPS analysis of CuxTiS2 (x=0, 0.125, and 0.5); (g) Raman analysis of CuxTiS2 (x=0, 0.05, 0.125, 0.25, and 0.5)
| [1] |
EDELSTEIN A, MURDAY J, RATH B. Challenges in nanomaterials design. Progress in Materials Science, 1997, 42: 5.
DOI URL |
| [2] |
YAN L, ZHAO F, WANG J, et al. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Advanced Materials, 2019, 31(45): 1805391.
DOI URL |
| [3] |
LIN S, YU T, YU Z, et al. Nanomaterials safer-by-design: an environmental safety perspective. Advanced Materials, 2018, 30(17): 1705691.
DOI URL |
| [4] |
BAIG N, KAMMAKAKAM I, FALATH W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Materials Advances, 2021, 2(6): 1821.
DOI URL |
| [5] |
JINEK M, CHYLINSKI K, FONFARA I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 2012, 337(6096): 816.
DOI PMID |
| [6] |
BARRANGOU R, FREMAUX C, DEVEAU H, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science, 2007, 315(5819): 1709.
DOI PMID |
| [7] |
WIEDENHEFT B, STERNBERG S H, DOUDNA J A. RNA- guided genetic silencing systems in bacteria and archaea. Nature, 2012, 482(7385): 331.
DOI |
| [8] |
DELTCHEVA E, CHYLINSKI K, SHARMA C M, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 2011, 471(7340): 602.
DOI |
| [9] |
BHAYA D, DAVISON M, BARRANGOU R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annual Review of Genetics, 2011, 45: 273.
DOI PMID |
| [10] |
DING H, LI Y, LI M, et al. Chemical scissor-mediated structural editing of layered transition metal carbides. Science, 2023, 379(6637): 1130.
DOI PMID |
| [11] |
FUJII S, ENOKI T. Cutting of oxidized graphene into nanosized pieces. Journal of the American Chemical Society, 2010, 132(29): 10034.
DOI PMID |
| [12] |
ZHANG L, DIAO S, NIE Y, et al. Photocatalytic patterning and modification of graphene. Journal of the American Chemical Society, 2011, 133(8): 2706.
DOI PMID |
| [13] |
WANG X, DAI H. Etching and narrowing of graphene from the edges. Nature Chemistry, 2010, 2(8): 661.
DOI PMID |
| [14] |
PI Y, MA Y, WANG X, et al. Multilevel hollow phenolic resin nanoreactors with precise metal nanoparticles spatial location toward promising heterogeneous hydrogenations. Advanced Materials, 2022, 34(43): 2205153.
DOI URL |
| [15] |
JO V, KIM M K, LEE D W, et al. Lone pairs as chemical scissors in new antimony oxychlorides, Sb2ZnO3Cl2 and Sb16Cd8O25Cl14. Inorganic Chemistry, 2010, 49(6): 2990.
DOI URL |
| [16] |
HAN M Y, ÖZYILMAZ B, ZHANG Y, et al. Energy band-gap engi-neering of graphene nanoribbons. Physical Review Letters, 2007, 98(20): 206805..
DOI URL |
| [17] |
WANG X, TABAKMAN S M, DAI H. Atomic layer deposition of metal oxides on pristine and functionalized graphene. Journal of the American Chemical Society, 2008, 130(26): 8152.
DOI PMID |
| [18] |
CI L, XU Z, WANG L, et al. Controlled nanocutting of graphene. Nano Research, 2008, 1: 116.
DOI URL |
| [19] |
CAMPOS L C, MANFRINATO V R, SANCHEZ-YAMAGISHI J D, et al. Anisotropic etching and nanoribbon formation in single-layer graphene. Nano Letters, 2009, 9(7): 2600.
DOI PMID |
| [20] |
MCALLISTER M J, LI J L, ADAMSON D H, et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chemistry of Materials, 2007, 19(18): 4396.
DOI URL |
| [21] | BAI X, YAN S, WANG J, et al. A simple and efficient strategy for the synthesis of a chemically tailored gC3N4 material. Journal of Materials Chemistry A, 2014, 2(41): 175219. |
| [22] |
BU X, BU Y, YANG S, et al. Graphitic carbon nitride nanoribbon for enhanced visible-light photocatalytic H2 production. RSC Advances, 2016, 6(113): 112210.
DOI URL |
| [23] |
LI A, KAN E, CHEN S, et al. Enabling high loading in single-atom catalysts on bare substrate with chemical scissors by saturating the anchoring sites. Small, 2022, 18(19): 2200073.
DOI URL |
| [24] |
NIU P, YIN L C, YANG Y Q, et al. Increasing the visible light absorption of graphitic carbon nitride (Melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Advanced Materials, 2014, 26(47): 8046.
DOI URL |
| [25] |
WANG Q, ZHOU Y, ZHAO X, et al. Tailoring carbon nanomaterials via a molecular scissor. Nano Today, 2021, 36: 101033.
DOI URL |
| [26] |
BIN D S, CHI Z X, LI Y, et al. Controlling the compositional chemistry in single nanoparticles for functional hollow carbon nanospheres. Journal of the American Chemical Society, 2017, 139(38): 13492.
DOI URL |
| [27] |
YU R, HUANG X, LIU Y, et al. Shaping nanoparticles for interface catalysis: concave hollow spheres via deflation-inflation asymmetric growth. Advanced Science, 2020, 7(13): 2000393.
DOI URL |
| [28] |
GIESTER G. Te6O11Cl2-a revision of crystal symmetry. Acta Crystallographica Section C: Crystal Structure Communications, 1994, 50(1): 3.
DOI URL |
| [29] |
SÄRNSTRAND C. The crystal structure of antimony (III) chloride oxide Sb4O5Cl2. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1978, 34(8): 2402.
DOI URL |
| [30] |
LUO Y, WU Y, BRAUN A, et al. Defect engineering to tailor metal vacancies in 2D conductive metal-organic frameworks: an example in electrochemical sensing. ACS Nano, 2022, 16(12): 20820.
DOI PMID |
| [31] |
LI M, LU J, LUO K, et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. Journal of the American Chemistry Society, 2019, 141(11): 4730.
DOI URL |
| [32] |
DING H, LI Y, LU J, et al. Synthesis of MAX phases Nb2CuC and Ti2(Al0.1Cu0.9)N by A-site replacement reaction in molten salts. Materials Research Letters, 2019, 7(12): 510.
DOI URL |
| [33] |
LI Y, LIANG J, DING H, et al. Near-room temperature ferromagnetic behavior of single-atom-thick 2D iron in nanolaminated ternary MAX phases. Applied Physics Reviews, 2021, 8(3): 031418.
DOI URL |
| [34] |
LI Y, SHAO H, LIN Z, et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nature Materials, 2020, 19(8): 894.
DOI PMID |
| [35] |
LI M, LI X, QIN G, et al. Halogenated Ti3C2 MXenes with electrochemically active terminals for high-performance zinc ion batteries. ACS Nano, 2021, 15(1): 1077.
DOI URL |
| [36] |
NAGUIB M, KURTOGLU M, PRESSER V, et al. Two- dimensional nanocrystals produced by exfoliation of Ti3AlC2. Advanced Materials, 2011, 23(37): 4248.
DOI URL |
| [37] |
GHIDIU M, LUKATSKAYA M R, ZHAO M Q, et al. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature, 2014, 516(7529): 78.
DOI |
| [38] | GAO L, LI M, FAN Q, et al. Intercalation of metal into transition metal dichalcogenides in molten salts. Small, 2023: 2304281. |
| [39] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films. Science, 2004, 306(5696): 666.
DOI PMID |
| [40] |
WEISS N O, ZHOU H, LIAO L, et al. Graphene: an emerging electronic material. Advanced Materials, 2012, 24(43): 5782.
DOI URL |
| [41] | KATSNELSON M I. Graphene: carbon in two dimensions. Materials Today, 2007, 10(1/2): 20. |
| [42] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Two- dimensional gas of massless Dirac fermions in graphene. Nature, 2005, 438(7065): 197.
DOI |
| [43] |
ZHANG Y, TAN Y W, STORMER H L, et al. Experimental observation of the quantum Hall effect and Berry's phase in graphene. Nature, 2005, 438(7065): 201.
DOI |
| [44] |
VAN NOORDEN R. Moving towards a graphene world. Nature, 2006, 442(7100): 228.
DOI |
| [45] |
LI X, WANG X, ZHANG L, et al. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science, 2008, 319(5867): 1229.
DOI PMID |
| [46] |
WANG X, OUYANG Y, LI X, et al. Room-temperature all- semiconducting sub-10-nm graphene nanoribbon field-effect transistors. Physical Review Letters, 2008, 100(20): 206803.
DOI URL |
| [47] | JIAO L, ZHANG L, WANG X, et al. Narrow graphene nanoribbons from carbon nanotubes. Nature, 2009, 458(7240): 8770. |
| [48] |
BAI J, DUAN X, HUANG Y. Rational fabrication of graphene nanoribbons using a nanowire etch mask. Nano Letters, 2009, 9(5): 2083.
DOI PMID |
| [49] |
NAKADA K, FUJITA M, DRESSELHAUS G, et al. Edge state in graphene ribbons: nanometer size effect and edge shape dependence. Physical Review B, 1996, 54(24): 17954.
PMID |
| [50] |
WU Z S, REN W, GAO L, et al. Efficient synthesis of graphene nanoribbons sonochemically cut from graphene sheets. Nano Research, 2010, 3: 16.
DOI URL |
| [51] |
LI J L, KUDIN K N, MCALLISTER M J, et al. Oxygen-driven unzipping of graphitic materials. Physical Review Letters, 2006, 96(17): 176101.
DOI URL |
| [52] |
AJAYAN P M, YAKOBSON B I. Oxygen breaks into carbon world. Nature, 2006, 441(7095): 818.
DOI |
| [53] |
DATTA S S, STRACHAN D R, KHAMIS S M, et al. Crystallographic etching of few-layer graphene. Nano Letters, 2008, 8(7): 1912.
DOI PMID |
| [54] |
LIU P, ZHAO Y, QIN R, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science, 2016, 352(6287): 797.
DOI PMID |
| [55] |
WAN J, CHEN W, JIA C, et al. Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties. Advanced Materials, 2018, 30(11): 1705369.
DOI URL |
| [56] |
BROUNS S J, JORE M M, LUNDGREN M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science, 2008, 321(5891): 960.
DOI PMID |
| [57] |
YAN H, CHENG H, YI H, et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. Journal of the American Chemical Society, 2015, 137(33): 10484.
DOI URL |
| [58] |
JOHNSSON M, TÖRNROOS K, MILA F, et al. Tetrahedral clusters of copper (II): crystal structures and magnetic properties of Cu2Te2O5X2 (X= Cl, Br). Chemistry of Materials, 2000, 12(10): 2853.
DOI URL |
| [59] |
JOHNSSON M, TÖRNROOS K W, LEMMENS P, et al. Crystal structure and magnetic properties of a new two-dimensional S= 1 quantum spin system Ni5(TeO3)4X2(X= ClBr). Chemistry of Materials, 2003, 15(1): 68.
DOI URL |
| [60] |
TSENG Y M, LIAO J H, CHIU T H, et al. Superatom pruning by diphosphine ligands as a chemical scissor. Inorganic Chemistry, 2023, 62(9): 3866.
DOI URL |
| [61] |
BARSOUM M W. The MN+1AXN phases: a new class of solids: thermodynamically stable nanolaminates. Progress in Solid State Chemistry, 2000, 28: 201.
DOI URL |
| [62] |
SOKOL M, NATU V, KOTA S, et al. On the chemical diversity of the MAX phases. Trends in Chemistry, 2019, 1(2): 210.
DOI URL |
| [63] |
CHING W Y, MO Y, ARYAL S, et al. Intrinsic mechanical properties of 20 MAX-phase compounds. Journal of the American Ceramic Society, 2013, 96(7): 2292.
DOI URL |
| [64] |
SUN Z M. Progress in research and development on MAX phases: a family of layered ternary compounds. International Materials Reviews, 2013, 56(3): 143.
DOI URL |
| [65] |
DING H, LI M, LI Y, et al. Progress in structural tailoring and properties of ternary layered ceramics. Journal of Inorganic Materials, 2023, 38(8): 845.
DOI URL |
| [66] |
LYU J, KASHKAROV E B, TRAVITZKY N, et al. Sintering of MAX-phase materials by spark plasma and other methods. Journal of Materials Science, 2020, 56(3): 1980.
DOI |
| [67] | LI M, HUANG Q. Recent progress and prospects of ternary layered carbides/nitrides MAX phases and their derived two-dimensional nanolaminates MXenes. Journal of Inorganic Materials, 2020, 35(1): 1. |
| [68] |
URBANKOWSKI P, ANASORI B, MAKARYAN T, et al. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale, 2016, 8(22): 11385.
DOI URL |
| [69] |
RAHMAN M A, RAHAMAN M Z. Study on structural, electronic, optical and mechanical properties of MAX phase compounds and applications review article. American Journal of Modern Physics, 2015, 4(2): 75.
DOI URL |
| [70] |
HALIM J, LUKATSKAYA M R, COOK K M, et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chemistry of Materials, 2014, 26(7): 2374.
PMID |
| [71] |
MA G, SHAO H, XU J, et al. Li-ion storage properties of two-dimensional titanium-carbide synthesized via fast one-pot method in air atmosphere. Nature Communications, 2021, 12: 5085.
DOI |
| [72] |
SHEN M, JIANG W, LIANG K, et al. One-pot green process to synthesize MXene with controllable surface terminations using molten salts. Angewandte Chemie International Edition, 2021, 60(52): 27013.
DOI URL |
| [73] |
BU F, SUN Z, ZHOU W, et al. Reviving Zn0 dendrites to electroactive Zn2+ by mesoporous MXene with active edge sites. Journal of the American Chemical Society, 2023, 145(44):24284.
DOI URL |
| [74] | GAO L, LI M, WANG L M, et al. Chemical scissor medicated intercalation of NbS2by transition metal for electronmagnetic properties tuning. Advanced Functional Materials, https://doi.org/10.1002/adfm.202313243. |
| [1] | 费玲, 雷蕾, 汪德高. 二维MXene材料在新型薄膜太阳能电池技术中的研究进展[J]. 无机材料学报, 2024, 39(2): 215-224. |
| [2] | 万胡杰, 肖旭. MXenes及其复合物的太赫兹电磁屏蔽与吸收[J]. 无机材料学报, 2024, 39(2): 129-144. |
| [3] | 陈泽, 支春义. MXene在锌离子电池中的应用: 研究进展与展望[J]. 无机材料学报, 2024, 39(2): 204-214. |
| [4] | 邓顺桂, 张传芳. 多功能MXene油墨:面向印刷能源及电子器件的新视角[J]. 无机材料学报, 2024, 39(2): 195-203. |
| [5] | 刘艳艳, 谢曦, 刘增乾, 张哲峰. MAX相陶瓷增强金属基复合材料: 制备、性能与仿生设计[J]. 无机材料学报, 2024, 39(2): 145-152. |
| [6] | 尹建宇, 刘逆霜, 高义华. MXene在压力传感中的研究进展[J]. 无机材料学报, 2024, 39(2): 179-185. |
| [7] | 巴坤, 王建禄, 韩美康. MXene的红外特性及其应用研究展望[J]. 无机材料学报, 2024, 39(2): 162-170. |
| [8] | 李腊, 沈国震. 二维MXenes材料在柔性光电探测器中的应用展望[J]. 无机材料学报, 2024, 39(2): 186-194. |
| [9] | 徐向明, Husam N ALSHAREEF. MXetronics—MXene电子学[J]. 无机材料学报, 2024, 39(2): 171-178. |
| [10] | 李雷, 程群峰. 高性能MXenes纳米复合材料研究进展[J]. 无机材料学报, 2024, 39(2): 153-161. |
| [11] | 陶顺衍, 杨加胜, 邵芳, 吴应辰, 赵华玉, 董绍明, 张翔宇, 熊瑛. 航机CMC热端部件用热喷涂涂层的机遇与挑战[J]. 无机材料学报, 2024, 39(10): 1077-1083. |
| [12] | 郑嘉乾, 卢霄, 鲁亚杰, 王迎军, 王臻, 卢建熙. 医用生物陶瓷的功能性生物适配机制及应用[J]. 无机材料学报, 2024, 39(1): 1-16. |
| [13] | 刘云鹏, 盛伟繁, 吴忠华. 同步辐射及其在无机材料中的应用进展[J]. 无机材料学报, 2021, 36(9): 901-918. |
| [14] | 张翔, 李文杰, 王乐滨, 陈曦, 赵九蓬, 李垚. 无机电致变色材料反射特性研究进展[J]. 无机材料学报, 2021, 36(5): 451-460. |
| [15] | 杨浏鑫,罗文华,汪长安,徐晨. 新型无机二维材料在气体分离膜领域的研究进展[J]. 无机材料学报, 2020, 35(9): 959-971. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||