无机材料学报 ›› 2021, Vol. 36 ›› Issue (5): 552-560.DOI: 10.15541/jim20200395 CSTR: 32189.14.10.15541/jim20200395
• 研究快报 • 上一篇
收稿日期:2020-07-14
修回日期:2020-08-08
出版日期:2021-05-20
网络出版日期:2021-04-19
通讯作者:
朱向东, 研究员. E-mail: zhu_xd1973@scu.edu.cn
作者简介:吴永豪(1990-), 男, 博士研究生. E-mail:hpu11wyh@163.com
WU Yonghao( ), LI Xiangfeng, ZHU Xiangdong(
), LI Xiangfeng, ZHU Xiangdong( ), ZHANG Xingdong
), ZHANG Xingdong
Received:2020-07-14
Revised:2020-08-08
Published:2021-05-20
Online:2021-04-19
Contact:
ZHU Xiangdong, professor. E-mail: zhu_xd1973@scu.edu.cn
About author:WU Yonghao(1990-), male, PhD candidate. E-mail:hpu11wyh@163.com
Supported by:摘要:
本研究旨在研究羟基磷灰石(HA)前驱粉体与所制备陶瓷之间的关系, 制备具有优良力学性能及成骨活性的HA纳米陶瓷。采用三种HA前驱粉体, 即40 ℃合成的HA-40粉体、以PEG为模板40 ℃合成的HA-40PEG粉体和80 ℃合成的HA-80粉体, 系统研究了前驱粉体对陶瓷性能的影响。结果显示, HA-40、HA-40PEG和HA-80粉体制备的陶瓷晶粒尺寸分别为(217.87±57.53)、(123.22±20.16)和(316.65±68.91) nm, 表明HA-40PEG有利于HA纳米陶瓷的制备。烧结得到的HA-40PEG纳米晶陶瓷表现出良好的力学性能, 与另外两种亚微米晶陶瓷(HA-40和HA-80)相比, 其抗压强度更高(~300 MPa)。细胞研究结果显示, HA-40PEG比HA-40和HA-80更能促进MC3T3-E1前成骨细胞的铺展和增殖。由此可知, 前驱粉体合成是影响HA陶瓷性能的关键因素, 纳米晶构建有利于同时提高其力学性能和生物学性能。
中图分类号:
吴永豪, 李向锋, 朱向东, 张兴栋. 高强度羟基磷灰石纳米陶瓷的构建及其促成骨细胞活性研究[J]. 无机材料学报, 2021, 36(5): 552-560.
WU Yonghao, LI Xiangfeng, ZHU Xiangdong, ZHANG Xingdong. Construction of Hydroxyapatite Nanoceramics with High Mechanical Strength and Efficiency in Promoting the Spreading and Viability of Osteoblasts[J]. Journal of Inorganic Materials, 2021, 36(5): 552-560.
| Sample | Particle size, D50/nm | Crystallinity, Xc/% | Specific surface area/(m2·g-1) | Crystallinity index | Maturity index |
|---|---|---|---|---|---|
| HA-40 | (124.62±28.71) | 31.76 | (89.76±3.96) | 5.91 | 2.02 |
| HA-40PEG | (65.16±31.23) | 39.48 | (81.40±0.66) | 5.31 | 1.91 |
| HA-80 | (221.50±48.82) | 77.94 | (41.76±0.71) | 7.45 | 1.86 |
Table 1 Physicochemical properties of the three HA precursor powders
| Sample | Particle size, D50/nm | Crystallinity, Xc/% | Specific surface area/(m2·g-1) | Crystallinity index | Maturity index |
|---|---|---|---|---|---|
| HA-40 | (124.62±28.71) | 31.76 | (89.76±3.96) | 5.91 | 2.02 |
| HA-40PEG | (65.16±31.23) | 39.48 | (81.40±0.66) | 5.31 | 1.91 |
| HA-80 | (221.50±48.82) | 77.94 | (41.76±0.71) | 7.45 | 1.86 |
| Sample | Crystallinity, Xc/% | Surface roughness, Ra/nm | Contact angle/(°) | Grain size/nm | Relative density/% |
|---|---|---|---|---|---|
| HA-40 | 97.48 | (61.49±5.65) | (69.20±6.94) | (217.87±57.53) | (94.90±2.27) |
| HA-40PEG | 96.92 | (66.70±2.81) | (56.07±0.42) | (123.22±20.16) | (93.45±3.32) |
| HA-80 | 98.33 | (57.81±3.44) | (79.83±1.99) | (316.65±68.91) | (85.57±0.91) |
Table 2 Physicochemical properties of the three HA ceramics
| Sample | Crystallinity, Xc/% | Surface roughness, Ra/nm | Contact angle/(°) | Grain size/nm | Relative density/% |
|---|---|---|---|---|---|
| HA-40 | 97.48 | (61.49±5.65) | (69.20±6.94) | (217.87±57.53) | (94.90±2.27) |
| HA-40PEG | 96.92 | (66.70±2.81) | (56.07±0.42) | (123.22±20.16) | (93.45±3.32) |
| HA-80 | 98.33 | (57.81±3.44) | (79.83±1.99) | (316.65±68.91) | (85.57±0.91) |
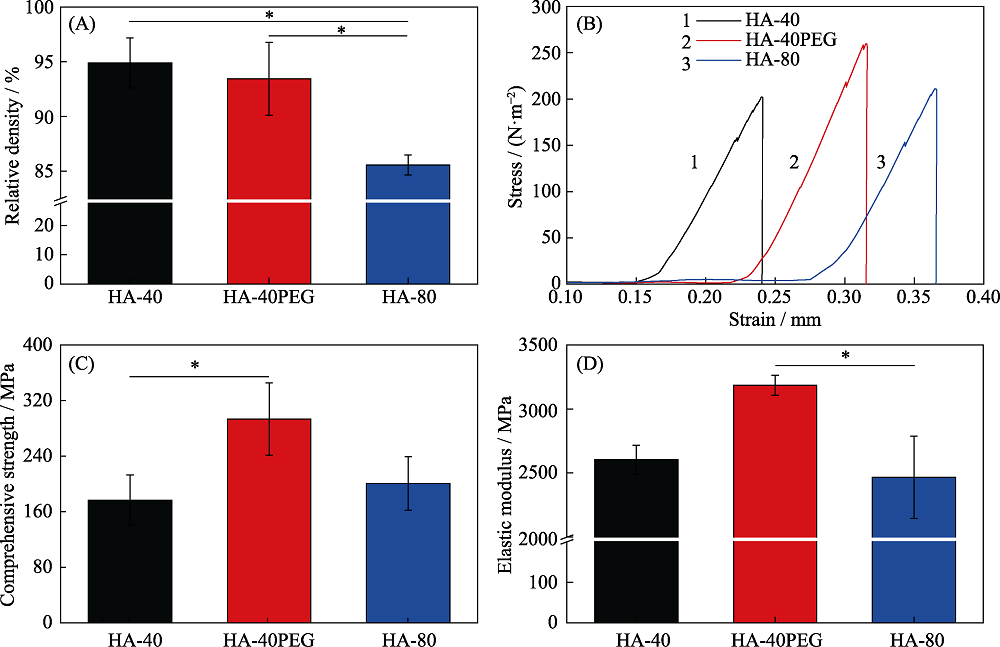
Fig. 5 (A) Relative densities, (B) strain-stress curves, (C) compressive strengths and (D) elastic modulus of the three kinds of HA ceramics (HA-40, HA-40PEG and HA-80) Values are expressed as the mean ± SD (n = 3); *p< 0.05 and **p< 0.01
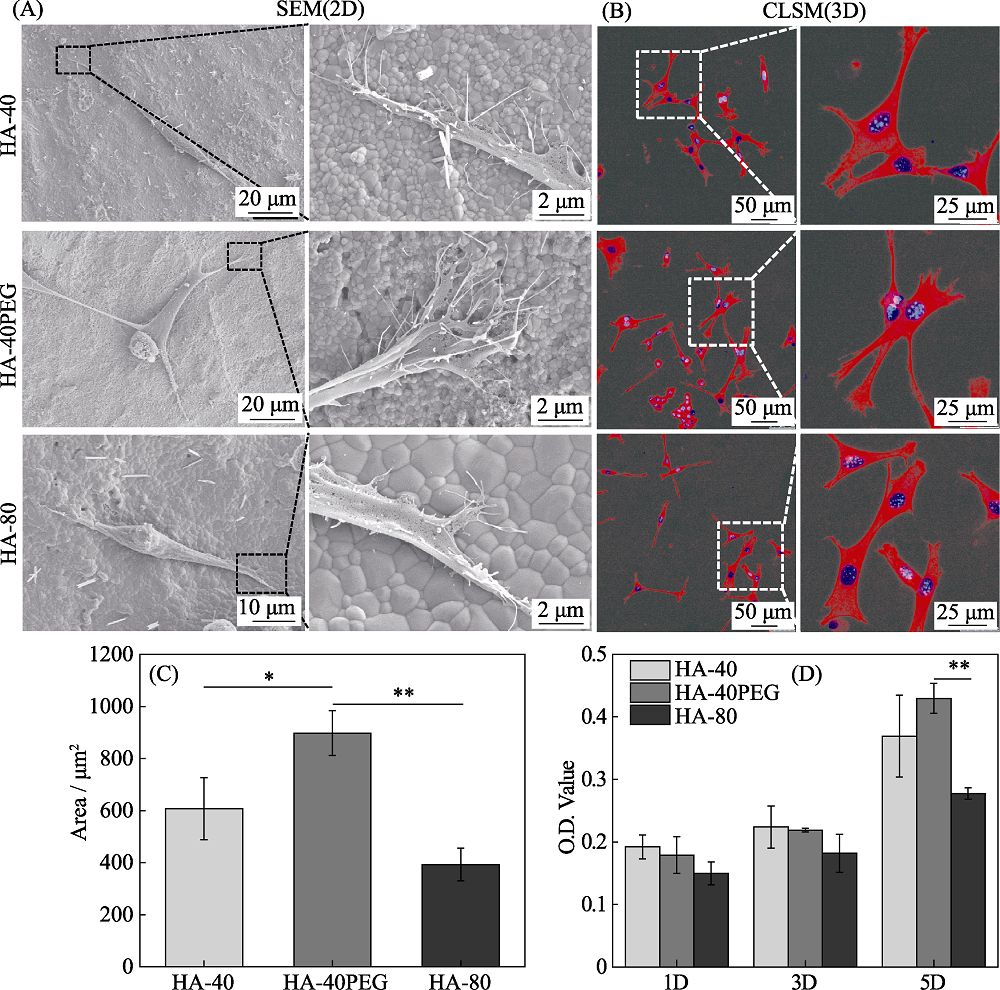
Fig. 6 (A) SEM images, (B) CLSM observations of cytoskeleton, (C) cell area, and (D) CCK-8 results of MC3T3-E1 cultured on HA ceramics Values are expressed as the mean ± SD (n=3); *p<0.05 and **p<0.01
| [1] | HONG Y, FAN H, LI B, et al. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Materials Science & Engineering R, 2010,70(3):225-242. |
| [2] | DOROZHKIN S V. Calcium orthophosphates in nature. Biology and Medicine Materials, 2009,2(1):399-498. |
| [3] | NASIRI-TABRIZI B, HONARMANDI P, EBRAHIMI-KAHRIZSANGI R, et al. Synthesis of nanosize single-crystal hydroxyapatite via mechanochemical method. Materials Letters, 2009,63(5):543-546. |
| [4] |
WANG J, SHAW L L. Nanocrystalline hydroxyapatite with simultaneous enhancements in hardness and toughness. Biomaterials, 2009,30(34):6565-6572.
DOI URL PMID |
| [5] |
BOSE S, DASGUPTA S, TARAFDER S, et al. Microwave-processed nanocrystalline hydroxyapatite: simultaneous enhancement of mechanical and biological properties. Acta Biomater., 2010,6(9):3782-3790.
URL PMID |
| [6] | FANG Z, FENG Q, TAN R. In-situ grown hydroxyapatite whiskers reinforced porous HA bioceramic. Ceramics International, 2013,39(8):8847-8852. |
| [7] | PROKOPIEV O, SEVOSTIANOV I. Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. Materials Science & Engineering A, 2006,431(1):218-227. |
| [8] |
HABIBOVIC P, YUAN H, VAN DER VALK C M, et al. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials, 2005,26(17):3565-3575.
DOI URL PMID |
| [9] |
KIM H M, HIMENO T, KOKUBO T, et al. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials, 2005,26(21):4366-4373.
URL PMID |
| [10] | RICE R W, WU C C, BOICHELT F. Hardness-grain-size relations in ceramics. Journal of the American Ceramic Society, 1994,77(10):2539-2553. |
| [11] | MOSHTAGHIOUN B M, GOMEZ-GARCIA D, DOMINGUEZ- RODRIGUEZ A, et al. Grain size dependence of hardness and fracture toughness in pure near fully-dense boron carbide ceramics. Journal of the European Ceramic Society, 2016,36(7):1829-1834. |
| [12] |
GU Y W, LOH N H, KHOR K A, et al. Spark plasma sintering of hydroxyapatite powders. Biomaterials, 2002,23(1):37-43.
DOI URL PMID |
| [13] | KIM B N, PRAJATELISTIA E, HAN Y H, et al. Transparent hydroxyapatite ceramics consolidated by spark plasma sintering. Scripta Materialia, 2013,69(5):366-369. |
| [14] | GUO X, XIAO P, JING L, et al. Fabrication of nanostructured hydroxyapatite via hydrothermal synthesis and spark plasma sintering. Journal of the American Ceramic Society, 2005,88(4):1026-1029. |
| [15] | RAMESH S, TAN C Y, BHADURI S B, et al. Rapid densification of nanocrystalline hydroxyapatite for biomedical applications. Ceramics International, 2007,33(7):1363-1367. |
| [16] | VELJOVIC D, JOKIC B, PETROVIĆ R, et al. Processing of dense nanostructured HAP ceramics by sintering and hot pressing. Ceramics International, 2009,35:1407-1413. |
| [17] | WANG J, SHAW L L. Transparent nanocrystalline hydroxyapatite by pressure-assisted sintering. Scripta Materialia, 2010,63(6):593-596. |
| [18] |
CHEN I W, WANG X H. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature, 2000,404(6774):168-171.
URL PMID |
| [19] | LIN K, CHEN L, CHANG J. Fabrication of dense hydroxyapatite nanobioceramics with enhanced mechanical properties via two-step sintering process. International Journal of Applied Ceramic Technology, 2012,9(3):479-485. |
| [20] | LUKIĆ M J, ŠKAPIN S D, MARKOVIĆ S, et al. Processing route to fully dense nanostructured HAp bioceramics: from powder synthesis to sintering. Journal of the American Ceramic Society, 2012,95(11):3394-3402. |
| [21] | THUAULT A, SAVARY E, HORNEZ J C, et al. Improvement of the hydroxyapatite mechanical properties by direct microwave sintering in single mode cavity. Journal of the European Ceramic Society, 2014,34(7):1865-1871. |
| [22] |
LI X, SONG T, CHEN X, et al. Osteoinductivity of porous biphasic calcium phosphate ceramic spheres with nanocrystalline and their efficacy in guiding bone regeneration. ACS Applied Materials & Interfaces, 2019,11(4):3722-3736.
URL PMID |
| [23] | LIU D, WU Y, WU H, et al. Effect of process parameters on the microstructure and property of hydroxyapatite precursor powders and resultant sintered bodies. International Journal of Applied Ceramic Technology, 2018,16(2):444-454. |
| [24] | SONG J, YONG L, YING Z, et al. Mechanical properties of hydroxyapatite ceramics sintered from powders with different morphologies. Materials Science & Engineering A, 2011,528(16/17):5421-5427. |
| [25] | LANDI E, TAMPIERI A, CELOTTI G, et al. Densification behaviour and mechanisms of synthetic hydroxyapatites. Journal of the European Ceramic Society, 2000,20(14):2377-2387. |
| [26] | WEINER S, BAR-YOSEF O. States of preservation of bones from prehistoric sites in the Near East: a survey. Journal of Archaeological Science, 1990,17(2):187-196. |
| [27] |
FARLAY D, PANCZER G, REY C, et al. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. Journal of Bone and Mineral Metabolism, 2010,28:433-445.
DOI URL PMID |
| [28] |
LI X, DENG Y, WANG M, et al. Stabilization of Ca-deficient hydroxyapatite in biphasic calcium phosphate ceramics by adding alginate to enhance their biological performances. Journal of Materials Chemistry B, 2017,6(1):84-97.
URL PMID |
| [29] | MAZAHERI M, HAGHIGHATZADEH M, ZAHEDI A M, et al. Effect of a novel sintering process on mechanical properties of hydroxyapatite ceramics. Journal of Alloys & Compounds, 2009,471(1):180-184. |
| [30] |
DASGUPTA S, TARAFDER S, BANDYOPADHYAY A, et al. Effect of grain size on mechanical, surface and biological properties of microwave sintered hydroxyapatite. Materials Science & Engineering C Materials for Biological Applications, 2013,33(5):2846-2854.
URL PMID |
| [31] | PANG Y X, BAO X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. Journal of the European Ceramic Society, 2003,23(10):1697-1704. |
| [32] |
KUMAR R, PRAKASH K, CHEANG P, et al. Temperature driven morphological changes of chemically precipitated hydroxyapatite nanoparticles. Langmuir, 2004,20:5196-5200.
URL PMID |
| [33] | TSENG Y H, KUO C S, LI Y Y, et al. Polymer-assisted synthesis of hydroxyapatite nanoparticle. Materials Science and Engineering: C, 2009,29(3):819-822. |
| [34] | LI H, XUE F, WAN X, et al. Polyethylene glycol-assisted preparation of beta-tricalcium phosphate by direct precipitation method. Powder Technology, 2016,301:255-260. |
| [35] | AKAO M, AOKI H, KATO K. Mechanical properties of sintered hydroxyapatite for prosthetic applications. Journal of Materials Science, 1981,16:809-812. |
| [36] | ARIFVIANTO B, MAHARDIKA M, DEWO P, et al. Effect of surface mechanical attrition treatment (SMAT) on microhardness, surface roughness and wettability of AISI 316L. Materials Chemistry and Physics, 2011,125(3):418-426. |
| [37] |
DOS SANTOS E, FARINA M, SOARES G, et al. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. Journal of Materials Science: Materials in Medicine, 2008,19(6):2307-2316.
DOI URL PMID |
| [38] |
LI B, CHEN X, GUO B, et al. Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomaterialia, 2009,5(1):134-143.
URL PMID |
| [39] |
GUO X, GOUGH J E, XIAO P, et al. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. Journal of Biomedical Materials Research Part A, 2007,82(4):1022-1032.
DOI URL PMID |
| [40] |
MICHIARDI A, APARICIO C, RATNER B D, et al. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials, 2007,28(4):586-594.
URL PMID |
| [41] | YAO C, PERLA V, MCKENZIE J L, et al. Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesion. Journal of Biomedical Nanotechnology, 2005,1(1):68-73. |
| [42] |
WEBSTER T J, SIEGEL R W, BIZIOS R. Osteoblast adhesion on nanophase ceramics. Biomaterials, 1999,20(13):1221-1227.
DOI URL PMID |
| [1] | 安然, 林锶, 郭世刚, 张冲, 祝顺, 韩颖超. 铁掺杂纳米羟基磷灰石的制备及紫外吸收性能研究[J]. 无机材料学报, 2025, 40(5): 457-465. |
| [2] | 李承瑜, 丁自友, 韩颖超. 锰掺杂纳米羟基磷灰石的体外抗菌-促成骨性能研究[J]. 无机材料学报, 2024, 39(3): 313-320. |
| [3] | 刘妍, 张宇帆, 王茜蔓, 李婷, 马文婷, 杨富巍, 陈靓, 赵东月, 严小琴. 基于羟基磷灰石材料的风化脆弱骨质文物加固保护研究[J]. 无机材料学报, 2023, 38(11): 1345-1354. |
| [4] | 李榅凯, 赵宁, 毕志杰, 郭向欣. 钠离子电池Na3Zr2Si2PO12陶瓷电解质的喷雾干燥法制备及性能优化[J]. 无机材料学报, 2022, 37(2): 189-196. |
| [5] | 陈亚玲, 舒松, 王劭鑫, 李建军. Mn-HAP基低温SCR催化剂的制备及抗硫中毒性能[J]. 无机材料学报, 2022, 37(10): 1065-1072. |
| [6] | 朱雨桐, 谭佩洁, 林海, 朱向东, 张兴栋. 可注射透明质酸/羟基磷灰石复合材料: 制备、理化性能和细胞相容性[J]. 无机材料学报, 2021, 36(9): 981-990. |
| [7] | 林子扬, 常宇辰, 吴章凡, 包荣, 林文庆, 王德平. 不同模拟体液对硼硅酸盐生物活性玻璃基骨水泥矿化性能的影响[J]. 无机材料学报, 2021, 36(7): 745-752. |
| [8] | 宋可可, 黄浩, 鲁梦婕, 杨安春, 翁杰, 段可. 水热制备锌、硅、镁、铁等元素掺杂羟基磷灰石及其表征[J]. 无机材料学报, 2021, 36(10): 1091-1096. |
| [9] | 邵悦婷, 朱英杰, 董丽颖, 蔡安勇. 羟基磷灰石超长纳米线/植物纤维纳米复合“宣纸”及其防霉性能[J]. 无机材料学报, 2021, 36(1): 107-112. |
| [10] | 孙团伟,朱英杰. 一步溶剂热法合成锶掺杂羟基磷灰石超长纳米线[J]. 无机材料学报, 2020, 35(6): 724-728. |
| [11] | 刘子阳, 耿振, 李朝阳. 牡蛎壳为原料制备医用CaCO3/HA复合生物材料[J]. 无机材料学报, 2020, 35(5): 601-607. |
| [12] | 代钊,王铭,王双,李静,陈翔,汪大林,祝迎春. 氧化锆基微量元素共掺杂羟基磷灰石增韧涂层研究[J]. 无机材料学报, 2020, 35(2): 179-186. |
| [13] | 付亚康,翁杰,刘耀文,张科宏. 钛网表面含hBMP-2的复合涂层制备及hBMP-2的释放研究[J]. 无机材料学报, 2020, 35(2): 173-178. |
| [14] | 周子航, 王群, 葛翔, 李朝阳. 掺锶羟基磷灰石纳米颗粒的合成、表征及模拟研究[J]. 无机材料学报, 2020, 35(11): 1283-1289. |
| [15] | 肖文谦,张静,李克江,邹新宇,蔡昱东,李波,刘雪,廖晓玲. 荔枝状CaCO3@HA/Fe3O4磁性介孔多级微球的制备[J]. 无机材料学报, 2019, 34(9): 925-932. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||