无机材料学报 ›› 2021, Vol. 36 ›› Issue (9): 981-990.DOI: 10.15541/jim20210020 CSTR: 32189.14.10.15541/jim20210020
所属专题: 【虚拟专辑】抗菌材料(2020~2021)
收稿日期:2021-01-11
修回日期:2021-01-22
出版日期:2021-09-20
网络出版日期:2021-03-01
通讯作者:
林 海, 副研究员. E-mail: linhai028@scu.edu.cn
作者简介:朱雨桐(1997-), 女, 硕士. E-mail: 876073900@qq.com
基金资助:
ZHU Yutong( ), TAN Peijie, LIN Hai(
), TAN Peijie, LIN Hai( ), ZHU Xiangdong, ZHANG Xingdong
), ZHU Xiangdong, ZHANG Xingdong
Received:2021-01-11
Revised:2021-01-22
Published:2021-09-20
Online:2021-03-01
Contact:
LIN Hai, associate professor. E-mail: linhai028@scu.edu.cn
About author:ZHU Yutong (1997-), female, master. E-mail: 876073900@qq.com
Supported by:摘要:
羟基磷灰石(HAP)具有优异的生物相容性、生物活性和缓慢的降解速率等特性, 有可能弥补透明质酸(HA)用作软组织填充材料的不足。本研究采用一步反应法制备均匀负载HAP颗粒的HA-HAP复合水凝胶, 分析了制备工艺参数对复合水凝胶理化性能的影响及调控方法, 评价了其细胞相容性。结果表明, 采用该方法制备的复合水凝胶含1.5%~3.0%HA、2.38%~15.8% HAP, 具有良好的灭菌稳定性。通过改变HAP含量和交联剂的用量可以调控复合水凝胶的力学性能、溶胀行为等理化性能。利用一阶指数衰减方程可以较好地拟合复合水凝胶的溶胀行为, 质量溶胀率在1 h时已达到平衡溶胀率的90%以上。复合水凝胶具有良好的HA酶降解性, 1500 U/mL的酶作用2 min后HA降解率超过95%, 复合水凝胶可以快速溃散; 加入HAP颗粒不仅使复合水凝胶有更好的可塑性, 而且更有利于面部软组织填充和轮廓修正。优化制备的可注射HA-HAP复合水凝胶具有可调的理化性能和良好的细胞相容性, 可以较长时间地保留在体内, 兼具促进组织修复或重建的潜力, 有望成为优良的软组织填充材料。
中图分类号:
朱雨桐, 谭佩洁, 林海, 朱向东, 张兴栋. 可注射透明质酸/羟基磷灰石复合材料: 制备、理化性能和细胞相容性[J]. 无机材料学报, 2021, 36(9): 981-990.
ZHU Yutong, TAN Peijie, LIN Hai, ZHU Xiangdong, ZHANG Xingdong. Injectable Hyaluronan/Hydroxyapatite Composite: Preparation, Physicochemical Property and Biocompatibility[J]. Journal of Inorganic Materials, 2021, 36(9): 981-990.
| Sample | HA/wt% | HAP/wt% | BDDE equivalent |
|---|---|---|---|
| HA-1.0 | 10 | 0 | 1.0 |
| HAP15-0.5 | 10 | 15 | 0.5 |
| HAP30-0.5 | 10 | 30 | 0.5 |
| HAP30-1.0 | 10 | 30 | 1.0 |
| HAP45-1.0 | 10 | 45 | 1.0 |
表1 各复合水凝胶样品制备时HA、HAP与BDDE的用量
Table 1 Amount of HA, HAP and BDDE in preparing composite hydrogels
| Sample | HA/wt% | HAP/wt% | BDDE equivalent |
|---|---|---|---|
| HA-1.0 | 10 | 0 | 1.0 |
| HAP15-0.5 | 10 | 15 | 0.5 |
| HAP30-0.5 | 10 | 30 | 0.5 |
| HAP30-1.0 | 10 | 30 | 1.0 |
| HAP45-1.0 | 10 | 45 | 1.0 |
| Sample | Before dialysis | After dialysis | ||
|---|---|---|---|---|
| HA/% | HAP/% | HA/% | HAP/% | |
| HA-1.0 | 10 | 0 | 2.01 | - |
| HAP15-0.5 | 10 | 15 | 1.58 | 2.38 |
| HAP30-0.5 | 10 | 30 | 1.54 | 4.63 |
| HAP30-1.0 | 10 | 30 | 2.19 | 7.73 |
| HAP45-1.0 | 10 | 45 | 3.00 | 15.8 |
表2 复合水凝胶透析前后的HA与HAP含量
Table 2 Contents of HA and HAP in different composites before and after dialysis
| Sample | Before dialysis | After dialysis | ||
|---|---|---|---|---|
| HA/% | HAP/% | HA/% | HAP/% | |
| HA-1.0 | 10 | 0 | 2.01 | - |
| HAP15-0.5 | 10 | 15 | 1.58 | 2.38 |
| HAP30-0.5 | 10 | 30 | 1.54 | 4.63 |
| HAP30-1.0 | 10 | 30 | 2.19 | 7.73 |
| HAP45-1.0 | 10 | 45 | 3.00 | 15.8 |

图2 复合水凝胶灭菌前后的形态
Fig. 2 Appearance of composite hydrogels before and after autoclaving (A) Fully dialyzed; (B) Not dialyzed. From left to right: HA-1.0, HAP15-0.5, HAP30-0.5, HAP30-1.0, and HAP45-1.0

图3 含残留交联剂的复合水凝胶HAP45-1.0灭菌后的形态
Fig. 3 Appearance of composite hydrogels HAP45-1.0 with remained crosslinking reagents From left to right: fully dialyzed, alkali-contained, cross linker-contained
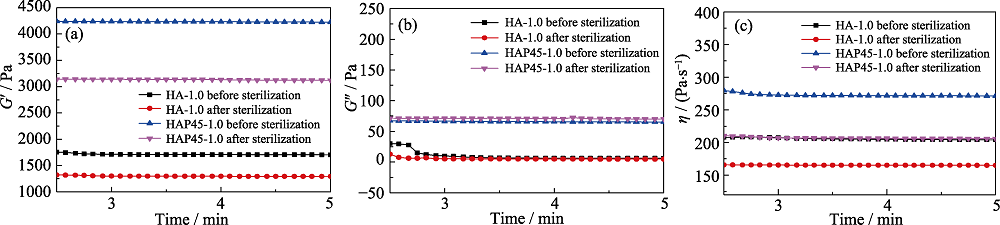
图4 水凝胶材料灭菌前后储能模量(a)、损耗模量(b)及复合黏度(c)的变化
Fig. 4 Storage modulus (a), loss modulus (b) and composite viscosity (c) of hydrogels before and after sterilization
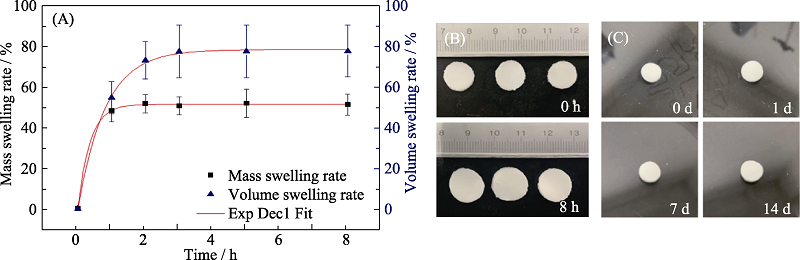
图7 复合水凝胶HAP45-1.0的溶胀行为及稳定性
Fig. 7 Swelling behavior of composite hydrogel HAP45-1.0 and stabilization in vitro (A) Mass and volume swelling rate; (B) Sizes of samples before and after swelling for 8 h; (C) Stability of the samples immersed in PBS for different periods
| A | y0 | R2 | |
|---|---|---|---|
| Mass swelling rate | -51.64 | 51.64 | 0.9992 |
| Volume swelling rate | -78.81 | 78.66 | 0.9984 |
表3 复合水凝胶HAP45-1.0溶胀率的一阶指数衰减拟合参数
Table 3 The 1st order exponential decay equation fitting results of hydrogel HAP45-1.0 swelling behavior
| A | y0 | R2 | |
|---|---|---|---|
| Mass swelling rate | -51.64 | 51.64 | 0.9992 |
| Volume swelling rate | -78.81 | 78.66 | 0.9984 |
| [1] |
DE VRIES C G, GEERTSMA R E. Clinical data on injectable tissue fillers: a review. Expert Review of Medical Devices, 2013, 10(6):835-853.
DOI URL |
| [2] |
ORTIZ A E, AHLUWALIA J, SONG S S, et al. Analysis of U.S. food and drug administration data on soft-tissue filler complications. Dermatologic Surgery, 2020, 46(7):958-961.
DOI URL |
| [3] | BUCK D W, ALAM M, KIM J Y S. Injectable fillers for facial rejuvenation: a review. Journal of Plastic, Reconstructive & Aesthetic Surgery, 2009, 62(1):11-18. |
| [4] |
ALIJOTAS-REIG J, FERNÁNDEZ-FIGUERAS M T, PUIG L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermalfillers. Seminars in Arthritis and Rheumatism, 2013, 43(2):241-258.
DOI URL |
| [5] |
BRANDT F S, CAZZANIGA A. Hyaluronic acid gel fillers in the management of facial aging. Clinical Interventions in Aging, 2008, 3(1):153-159.
DOI URL |
| [6] |
SHARMA P, SHARMA S. Comparative study of a new dermal filler uma jeunesse and juvéderm. Journal of Cosmetic Dermatology, 2011, 10(2):118-125.
DOI URL |
| [7] |
YANG R, TAN L, CEN L, et al. An injectable scaffold based on crosslinked hyaluronic acid gel for tissue regeneration. RSC Advances, 2016, 6(20):16838-16850.
DOI URL |
| [8] |
CHOI S C, YOO M A, LEE S Y, et al. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. Journal of Biomedical Materials Research Part A, 2015, 103(9):3072-3080.
DOI URL |
| [9] |
YEOM J, BHANG S H, KIM B S, et al. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjugate Chemistry, 2010, 21(2):240-247.
DOI URL |
| [10] | LOGHEM J V, YUTSKOVSKAYA Y A, WERSCHLER W P. Calcium hydroxylapatite: over a decade of clinical experience. Journal of Clinical and Aesthetic Dermatology, 2015, 8(1):38-49. |
| [11] | GRAIVIER M H, BASS L S, BUSSO M, et al. Calcium hydroxylapatite (radiesse) for correction of the mid- and lower face: consensus recommendations. Plastic & Reconstructive Surgery, 2007, 120(Suppl. 6):55S-66S. |
| [12] | EMER J, SUNDARAM H. Aesthetic applications of calcium hydroxylapatite volumizing filler: an evidence-based review and discussion of current concepts. Journal of Drugs in Dermatology Jdd., 2013, 12(12):1345-1354. |
| [13] |
LIU Y, WU Y H, LIN H, et al. Study on an injectable biomedical paste using cross-linked sodium hyaluronate as a carrier of hydroxyapatite particles. Carbohydrate Polymers, 2018, 195:378-386.
DOI URL |
| [14] |
REDBORD K P, BUSSO M, HANKO C W. Soft-tissue augmentation with hyaluronic acid and calcium hydroxyl apatite fillers. Dermatologic Therapy, 2011, 24(1):71-81.
DOI URL |
| [15] | EVIATAR J, LO C, KIRSZROT J. Radiesse: advanced techniques and applications for a unique and versatile implant. Plastic and Reconstructive Surgery, 2015, 136(Suppl. 5):164S-170S. |
| [16] |
JEONG S H, FAN Y F, BAEK J U, et al. Long-lasting and bioactive hyaluronic acid-hydroxyapatite composite hydrogels for injectable dermal fillers: physical properties and in vivo durability. Journal of Biomaterials Applications, 2016, 31(3):464-474.
DOI URL |
| [17] |
FAKHARI A, BERKLAND C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomaterialia, 2013, 9(7):7081-7092.
DOI URL |
| [18] |
BOULLE K, GLOGAU R, KONO T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatologic Surgery, 2013, 39(12):1758-1766.
DOI URL |
| [19] | PAN H H, TAO J H, WU T, et al. Molecular simulation of water behaviours on hydroxyapatite crystal faces. Chinese Journal of Inorganic Chemistry, 2006, 22(8):1392-1400. |
| [20] |
DAI Z, RONHOLM J, TIAN Y P, et al. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng., 2016, 7:1-13.
DOI URL |
| [21] | GALANTE R, PINTO T J A, COLACO R, et al. Sterilization of hydrogels for biomedical applications: a review. J. Biomed. Mater. Res., 2017, B6:106. |
| [22] |
WENDE F J, COHIL S, NORD L I, et al. 1D NMR methods for determination of degree of cross-linking and BDDE substitution positions in HA hydrogels. Carbohyd. Polym., 2017, 157:1525-1530.
DOI URL |
| [23] |
HARIDAS J, ROSEMARY M J. Effect of steam sterilization and biocompatibility studies of hyaluronic acid hydrogel for viscosupplementation. Polymer Degradation and Stability, 2019, 163:220-227.
DOI URL |
| [24] | FENG X D. Study on the mechanism of initial dark oxidation of ether. Chemical Journal of Chinese Universities, 1998, 19(7):1181-1183. |
| [25] |
LÓPEZ J F, DEGLESNE P A, ARROYO R, et al. Detection of a new reaction by-product in BDDE cross-linked autoclaved hyaluronic acid hydrogels by LC-MS analysis. Medical Devices: Evidence and Research, 2018, 11:367-376.
DOI URL |
| [26] |
BAEK J, FAN Y F, JEONG S H, et al. Facile strategy involving low-temperature chemical cross-linking to enhance the physical and biological properties of hyaluronic acid hydrogel. Carbohyd. Polym., 2018, 202:545-553.
DOI URL |
| [27] | FERRY J D. Viscoelastic Properties of Polymers, 2nd edition. New York: Wiley-Interscience, 1970, 8:595. |
| [28] |
BARBUCCI R, LAMPONI S, BORZACCHIELLO A, et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials, 2002, 23:4503-4513.
DOI URL |
| [29] |
CHANG J W, KOO W Y, KIM E K, et al. Facial rejuvenation using a mixture of calcium hydroxylapatite filler and hyaluronic acid filler. Journal of Craniofacial Surgery, 2020, 31(1):e18-e21.
DOI URL |
| [30] |
WIBOWO A, KAPOOR K M, PHILIPP-DORMSTON W G. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthetic Plastic Surgery, 2019, 43(5):1337-1344.
DOI URL |
| [31] | KIM D W, YOON E S, JI Y H, et al. Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. Journal of Plastic, Reconstructive & Aesthetic Surgery, 2011, 64:1590-1595. |
| [32] | TANO T, ONO K, HIRATSUKA Y, et al. Retinal vessel diameters in a Japanese population: the locomotive syndrome and health outcome in Aizu cohort study. Acta Ophthalmologica, 2016, 94(6):431-441. |
| [33] |
WANG M, LI W, ZHANG Y, et al. Comparison of intra-arterial and subcutaneous testicular hyaluronidase injection treatments and the vascular complications of hyaluronic acid filler. Dermatologic Surgery, 2017, 43(2):246-254.
DOI URL |
| [34] |
OHBA S, SUMITA Y, UMEBAYASHI M, et al. Onlay bone augmentation on mouse calvarial bone using a hydroxyapatite/ collagen composite material with total blood or platelet-rich plasma. Arch. Oral. Biol., 2016, 61:23-27.
DOI URL |
| [35] | FLINT P W, CORIO R L, CUMMINGS C W. Comparison of soft tissue response in rabbits following laryngeal implantation with hydroxylapatite, silicone rubber, and Teflon. Ann. Oto. Rhinol. Laryn., 1997, 106(5):399-407. |
| [36] | REBELLATO P R O, TORRE D S, RASTELLI G J C, et al. Calcium hydroxylapatite for collagen biostimulation in the neck. Int. J. Dermatol. Venereology Leprosy Sci., 2020, 3(1):27-31. |
| [1] | 安然, 林锶, 郭世刚, 张冲, 祝顺, 韩颖超. 铁掺杂纳米羟基磷灰石的制备及紫外吸收性能研究[J]. 无机材料学报, 2025, 40(5): 457-465. |
| [2] | 李承瑜, 丁自友, 韩颖超. 锰掺杂纳米羟基磷灰石的体外抗菌-促成骨性能研究[J]. 无机材料学报, 2024, 39(3): 313-320. |
| [3] | 刘妍, 张宇帆, 王茜蔓, 李婷, 马文婷, 杨富巍, 陈靓, 赵东月, 严小琴. 基于羟基磷灰石材料的风化脆弱骨质文物加固保护研究[J]. 无机材料学报, 2023, 38(11): 1345-1354. |
| [4] | 陈亚玲, 舒松, 王劭鑫, 李建军. Mn-HAP基低温SCR催化剂的制备及抗硫中毒性能[J]. 无机材料学报, 2022, 37(10): 1065-1072. |
| [5] | 林子扬, 常宇辰, 吴章凡, 包荣, 林文庆, 王德平. 不同模拟体液对硼硅酸盐生物活性玻璃基骨水泥矿化性能的影响[J]. 无机材料学报, 2021, 36(7): 745-752. |
| [6] | 王恩典, 常江. 钼掺杂铜硅钙石的制备及其抗菌性能和细胞相容性研究[J]. 无机材料学报, 2021, 36(7): 738-744. |
| [7] | 吴永豪, 李向锋, 朱向东, 张兴栋. 高强度羟基磷灰石纳米陶瓷的构建及其促成骨细胞活性研究[J]. 无机材料学报, 2021, 36(5): 552-560. |
| [8] | 宋可可, 黄浩, 鲁梦婕, 杨安春, 翁杰, 段可. 水热制备锌、硅、镁、铁等元素掺杂羟基磷灰石及其表征[J]. 无机材料学报, 2021, 36(10): 1091-1096. |
| [9] | 邵悦婷, 朱英杰, 董丽颖, 蔡安勇. 羟基磷灰石超长纳米线/植物纤维纳米复合“宣纸”及其防霉性能[J]. 无机材料学报, 2021, 36(1): 107-112. |
| [10] | 孙团伟,朱英杰. 一步溶剂热法合成锶掺杂羟基磷灰石超长纳米线[J]. 无机材料学报, 2020, 35(6): 724-728. |
| [11] | 刘子阳, 耿振, 李朝阳. 牡蛎壳为原料制备医用CaCO3/HA复合生物材料[J]. 无机材料学报, 2020, 35(5): 601-607. |
| [12] | 代钊,王铭,王双,李静,陈翔,汪大林,祝迎春. 氧化锆基微量元素共掺杂羟基磷灰石增韧涂层研究[J]. 无机材料学报, 2020, 35(2): 179-186. |
| [13] | 付亚康,翁杰,刘耀文,张科宏. 钛网表面含hBMP-2的复合涂层制备及hBMP-2的释放研究[J]. 无机材料学报, 2020, 35(2): 173-178. |
| [14] | 周子航, 王群, 葛翔, 李朝阳. 掺锶羟基磷灰石纳米颗粒的合成、表征及模拟研究[J]. 无机材料学报, 2020, 35(11): 1283-1289. |
| [15] | 肖文谦,张静,李克江,邹新宇,蔡昱东,李波,刘雪,廖晓玲. 荔枝状CaCO3@HA/Fe3O4磁性介孔多级微球的制备[J]. 无机材料学报, 2019, 34(9): 925-932. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||