无机材料学报 ›› 2020, Vol. 35 ›› Issue (8): 909-915.DOI: 10.15541/jim20190473 CSTR: 32189.14.10.15541/jim20190473
所属专题: 能源材料论文精选(二):超级电容器与储能电池(2020)
李雪渊1,2( ),王宏刚1,3,田柱1,朱建辉2,刘影2,贾兰1,尤东江2,李向明2,康利涛2(
),王宏刚1,3,田柱1,朱建辉2,刘影2,贾兰1,尤东江2,李向明2,康利涛2( )
)
收稿日期:2019-09-16
修回日期:2019-11-21
出版日期:2020-08-20
网络出版日期:2019-12-29
作者简介:李雪渊(1993–), 男, 硕士研究生. E-mail: 基金资助:
LI Xueyuan1,2( ),WANG Honggang1,3,TIAN Zhu1,ZHU Jianhui2,LIU Ying2,JIA Lan1,YOU Dongjiang2,LI Xiangming2,KANG Litao2(
),WANG Honggang1,3,TIAN Zhu1,ZHU Jianhui2,LIU Ying2,JIA Lan1,YOU Dongjiang2,LI Xiangming2,KANG Litao2( )
)
Received:2019-09-16
Revised:2019-11-21
Published:2020-08-20
Online:2019-12-29
Supported by:摘要:
锌锰(Zn-MnO2)电池具有高安全性、高环保性、高性价比的优点, 适用于大规模储能电池。然而, 金属锌负极在充放电中会因为“尖端效应”而产生锌枝晶, 造成电池容量衰减甚至短路失效。本研究通过添加亲水性纳米二氧化硅(SiO2)和海藻酸钠(SA)将电解质转化为准凝胶电解质, 有效抑制了锌负极表面的枝晶生长, 以及由之造成的Zn-MnO2电池性能衰减。恒流充放电测试结果表明, 采用准凝胶电解质的Zn-MnO2电池在1800次循环后容量保留率可达78%, 而使用普通电解质的Zn-MnO2电池在1000次循环后容量已基本衰减为0。进一步探究准凝胶电解质对锌沉积行为的影响, 发现准凝胶电解质的三维网络结构可以提高锌离子分布的均匀性, 降低电池容量衰减速度与失效风险。
中图分类号:
李雪渊,王宏刚,田柱,朱建辉,刘影,贾兰,尤东江,李向明,康利涛. 一种用于长寿命水系锌锰电池的海藻酸钠/二氧化硅准凝胶复合电解质[J]. 无机材料学报, 2020, 35(8): 909-915.
LI Xueyuan,WANG Honggang,TIAN Zhu,ZHU Jianhui,LIU Ying,JIA Lan,YOU Dongjiang,LI Xiangming,KANG Litao. A Quasi-gel SiO2/Sodium Alginate (SA) Composite Electrolyte for Long-life Zinc-manganese Aqueous Batteries[J]. Journal of Inorganic Materials, 2020, 35(8): 909-915.

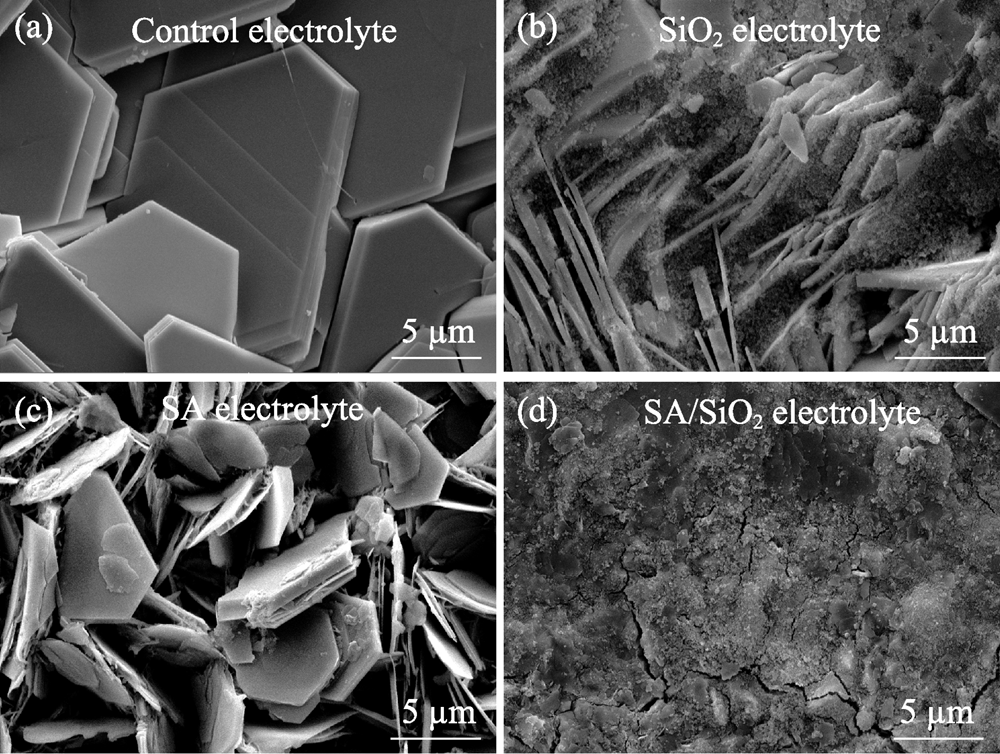
图1 不同电解质的光学照片(a~c)和不同添加剂在水中分散并冷冻干燥后的SEM照片(d~f)
Fig. 1 Optical photographs of different electrolytes (a-c) and SEM images of freeze-dried additives from their aqueous dispersions (d-f)
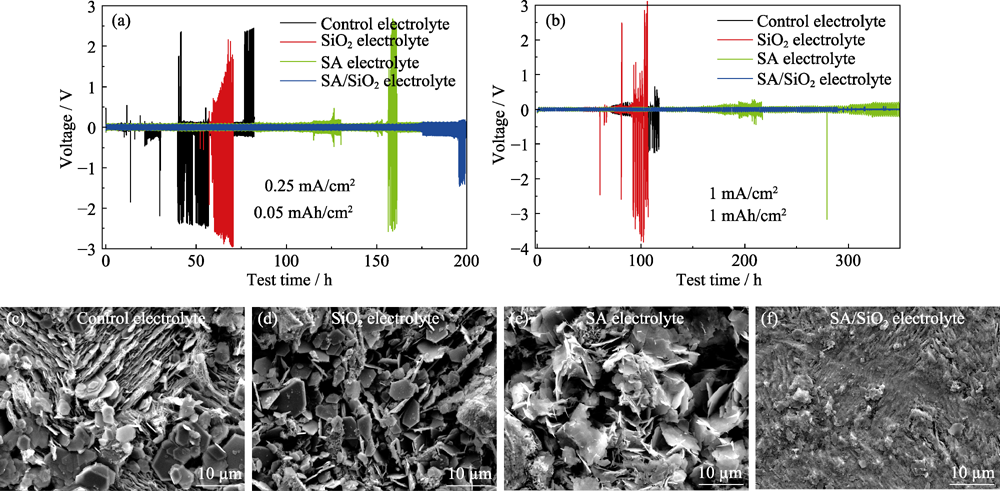
图3 采用不同电解质的Zn-Zn对称电池的恒电流充放电曲线(a~b)以及在1 mA?cm-2电流密度下循环50圈后锌电极的SEM照片(c~f)
Fig. 3 Galvanostatic charge/discharge curves of Zn-Zn symmetric cells in different electrolytes (a-b), and SEM images of Zn electrodes after 50 cycles at 1 mA?cm-2 (c-f)
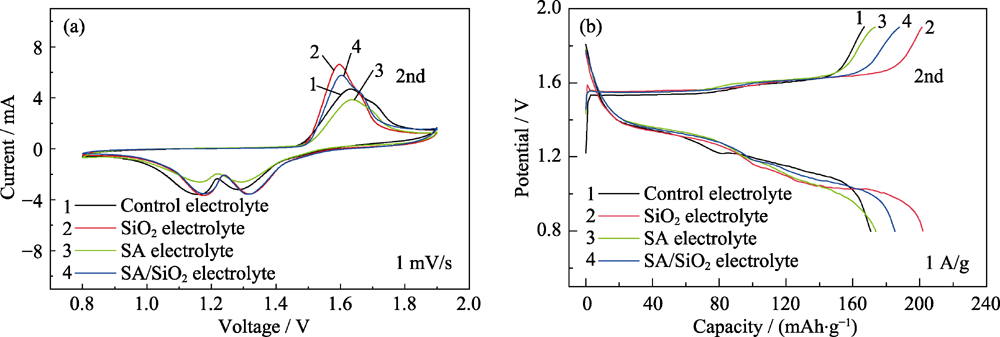
图4 采用不同电解质的Zn-MnO2电池的循环伏安曲线(a)和恒电流充放电曲线(b)
Fig. 4 CV (a) and galvanostatic charge/discharge curves (b) of Zn-MnO2 batteries with different electrolytes
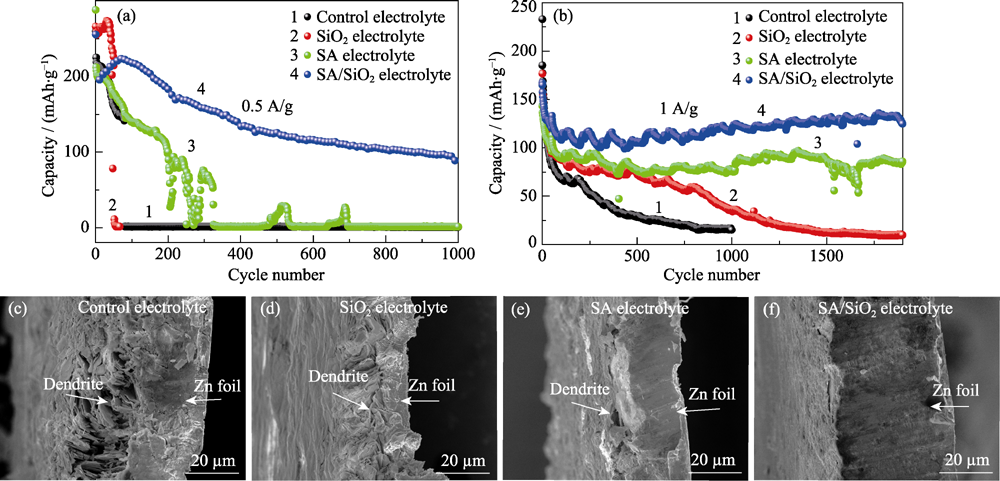
图5 使用不同电解质的Zn-MnO2电池在0.5(a)和1 A?g-1(b)电流密度下循环稳定性曲线; 在1 A?g-1循环100次后锌负极的横截面SEM照片(c~f)
Fig. 5 Cycling performance of Zn-MnO2 batteries using different electrolytes at 0.5 (a) and 1 A?g-1 (b); Cross sectional SEM images of zinc electrodes after 100 cycles at 1 A?g-1 (c-f)
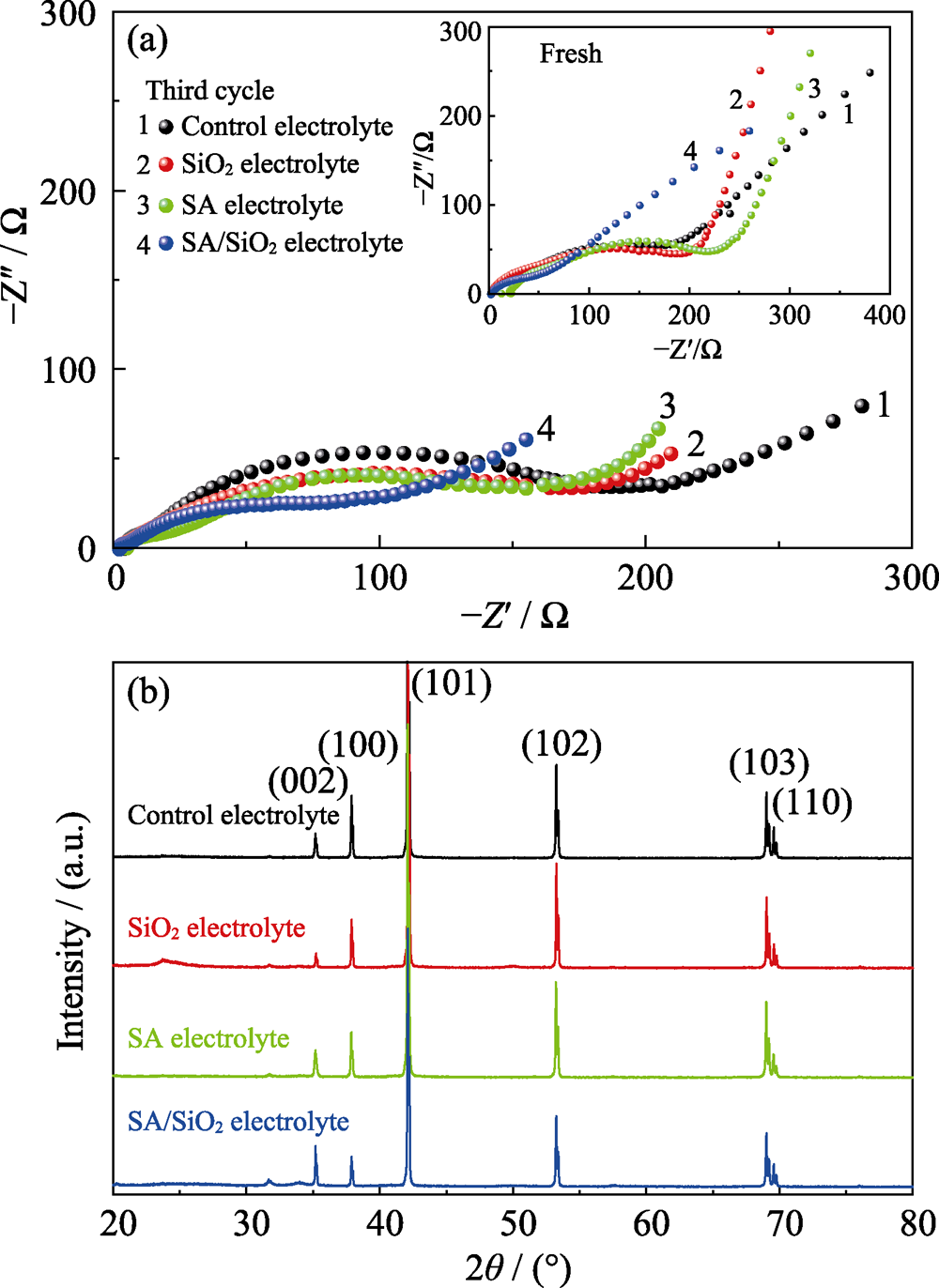
图7 采用不同电解质的Zn-MnO2电池的电化学阻抗图谱(a)以及100次循环后锌负极的XRD图谱(b)
Fig. 7 EIS plots of Zn-MnO2 battery with different electrolytes (a) and XRD patterns of Zn anode after 100 cycles (b)
图S1 压制在两片泡沫镍中间的海藻酸钠粉末在循环伏安测试前后的光学照片
Fig. S1 Optical image of sodium alginate powder pressed between two pieces of nickel foams before and after cyclic voltammetry test
图S3 使用不同电解质的Zn-MnO2电池100次充放电循环后的锌负极SEM照片
Fig. S3 SEM images of zinc electrodes for Zn-MnO2 batteries after 100 charge/discharge cycles in different electrolytes
| [1] |
PANG Q, SUN C, YUY, et al. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Advanced Energy Materials, 2018,8(19):1800144.
DOI URL |
| [2] | HILDER M, WINTHER-JENSEN B, CLARK N B. The effect of binder and electrolyte on the performance of thin zinc-air battery. Electrochimica Acta, 2012,69(3):8-14. |
| [3] | DAI X, WAN F, ZHANG L, et al. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Materials, 2019,17(1):43-50. |
| [4] |
ZHANG X, WU S, DENG S, et al. 3D CNTs networks enable MnO2 cathodes with high capacity and superior rate capability for flexible rechargeable Zn-MnO2 batteries. Small Methods, 2019,3(12):1900525.
DOI URL |
| [5] |
ZHANG H, LIU Q, FANG Y, et al. Boosting Zn-ion energy storage capability of hierarchically porous carbon by promoting chemical adsorption. Advanced Materials, 2019,31(44):1904948.
DOI URL |
| [6] |
GALLAWAY J W, DESAI D, GAIKWAD A, et al. A lateral microfluidic cell for imaging electrodeposited zinc near the shorting condition. Journal of The Electrochemical Society, 2010,157(12):A1279-A1286.
DOI URL |
| [7] |
CHENG X B, HOU T Z, ZHANG R, et al. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Advanced Materials, 2016,28(15):2888-2895.
DOI URL PMID |
| [8] |
HIGASHI S, LEE S W, LEE J S, et al. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nature Communications, 2016,7:11801.
DOI URL PMID |
| [9] |
PARKER J F, CHERVIN C N, PALA I R, et al. Rechargeable nickel-3D zinc batteries: an energy-dense, safer alternative to lithium- ion. Science, 2017,356(6336):415.
DOI URL PMID |
| [10] |
ZENG Y, ZHANG X, QIN R, et al. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Advanced Materials, 2019,31(36):1903675.
DOI URL |
| [11] |
WOOD K N, KAZYAK E, CHADWICK A F, et al. Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Central Science, 2016,2(11):790-801.
DOI URL PMID |
| [12] |
SLATER M D, KIM D, LEE E, et al. Sodium-ion batteries. Advanced Functional Materials, 2013,23(8):947-58.
DOI URL |
| [13] |
XUE L, GAO H, ZHOU W, et al. Liquid K-Na alloy anode enables dendrite-free potassium batteries. Advanced Materials, 2016,28(43):9608-9612.
DOI URL PMID |
| [14] |
KANG L, CUI M, JIANG F, et al. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Advanced Energy Materials, 2018,8(25):1801090.
DOI URL |
| [15] | CHEN N, DAI Y, XING Y, et al. Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy & Environmental Science, 2017,10(7):1660-1667. |
| [16] |
ZHANG R, CHEN X R, CHEN X, et al. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angewandte Chemie International Edition, 2017,56(27):7764-7768.
DOI URL PMID |
| [17] |
CUI M, XIAO Y, KANG L, et al. Quasi-isolated Au particles as heterogeneous seeds to guide uniform Zn deposition for aqueous zinc-ion batteries. ACS Applied Energy Materials, 2019,2(9):6490-6496.
DOI URL |
| [18] |
DING F, XU W, GRAFF G L, et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. Journal of the American Chemical Society, 2013,135(11):4450-4456.
DOI URL PMID |
| [19] |
WANG F, BORODIN O, GAO T, et al. Highly reversible zinc metal anode for aqueous batteries. Nature Materials, 2018,17(6):543-549.
DOI URL PMID |
| [20] |
XU W, ZHAO K, HUO W, et al. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy, 2019,62:275-281.
DOI URL |
| [21] |
HUANG J, CHI X, HAN Q, et al. Thickening and homogenizing aqueous electrolyte towards highly efficient and stable Zn metal batteries. Journal of The Electrochemical Society, 2019,166(6):A1211-A1216.
DOI URL |
| [22] |
MARTHA S K, HARIPRAKASH B, GAFFOOR S A, et al. Performance characteristics of a gelled-electrolyte valve-regulated lead- acid battery. Bulletin of Materials Science, 2003,26(5):465-469.
DOI URL |
| [23] |
HOU X, XUE Z, XIA Y, et al. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. International Journal of Biological Macromolecules, 2019,125:1289-1298.
DOI URL PMID |
| [24] |
YADAV M, RHEE K Y, PARK S J. Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohydrate Polymers, 2014,110:18-25.
DOI URL |
| [25] |
GÓMEZ-ORD EZ E, RUP REZ P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids, 2011,25(6):1514-1520.
DOI URL |
| [26] |
DAEMI H, BARIKANI M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Scientia Iranica, 2012,19(6):2023-2028.
DOI URL |
| [27] |
SUN J Y, ZHAO X, ILLEPERUMA W R, et al. Highly stretchable and tough hydrogels. Nature, 2012,489(7414):133-136.
DOI URL |
| [28] | YANG M, XIA Y, WANG Y, et al. Preparation and property investigation of crosslinked alginate/silicon dioxide nanocomposite films. Journal of Applied Polymer Science, 2016,133(22):15-27. |
| [29] |
YAN J, WANG J, LIU H, et al. Rechargeable hybrid aqueous batteries. Journal of Power Sources, 2012,216:222-226.
DOI URL |
| [30] |
WEI X, DESAI D, YADAV G G, et al. Impact of anode substrates on electrodeposited zinc over cycling in zinc-anode rechargeable alkaline batteries. Electrochimica Acta, 2016,212:603-613.
DOI URL |
| [31] |
WANG Z, HUANG J, GUO Z, et al. A metal-organic framework host for highly reversible dendrite-free zinc metal anodes. Joule, 2019,3(5):1289-1300.
DOI URL |
| [32] |
SUN W, WANG F, HOU S, et al. Zn/MnO2 Battery Chemistry with H + and Zn 2+ coinsertion . Journal of the American Chemical Society, 2017,139(29):9775-9778.
DOI URL PMID |
| [33] |
HUANG J, WANG Z, HOU M, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nature Communications, 2018,9(1):2906.
DOI URL PMID |
| [34] | HOANG TUAN K A, DOAN. T N L, LU C Y, et al. Performance of thixotropic gel electrolytes in the rechargeable aqueous Zn/ LiMn2O4 battery. ACS Sustainable Chemistry & Engineering, 2016,5(2):1804-1811. |
| [35] |
ZHANG N, CHENG F, LIU J, et al. Rechargeable aqueous zinc- manganese dioxide batteries with high energy and power densities. Nature Communications, 2017,8(1):405.
DOI URL PMID |
| [36] | ZENG K, LI X H, WANG Z, et al. Cave-embedded porous Mn2O3 hollow microsphere as anode material for lithium ion batteries. Electrochimica Acta, 2017, ( Supplement C):795-802. |
| [37] |
CHEN W C, WEN T C. Electrochemical and capacitive properties of polyaniline-implanted porous carbon electrode for supercapacitors. Journal of Power Sources, 2003,117(1/2):273-282.
DOI URL |
| [38] | SUN K E K, HOANG T K A, DOAN T N L, et al. Highly sustainable zinc anodes for a rechargeable hybrid aqueous battery. Chemistry - A European Journal, 2018,24(7):1667-1673. |
| [39] |
LI N, WEI W, XIE K, et al. Suppressing dendritic lithium formation using porous media in lithium metal-based batteries. Nano Letters, 2018,18(3):2067-2073.
DOI URL PMID |
| [40] |
LIU W, LI W, ZHUO D, et al. Core-shell nanoparticle coating as an interfacial layer for dendrite-free lithium metal anodes. ACS Cent. Sci., 2017,3(2):135-140.
DOI URL PMID |
| [1] | 王月月, 黄佳慧, 孔红星, 李怀珠, 姚晓红. 载银放射状介孔二氧化硅的制备及其在牙科树脂中的应用[J]. 无机材料学报, 2025, 40(1): 77-83. |
| [2] | 曹青青, 陈翔宇, 吴健豪, 王筱卓, 王乙炫, 王禹涵, 李春颜, 茹菲, 李兰, 陈智. SiO2增强自敏性氮化碳微球可见光降解盐酸四环素的研究[J]. 无机材料学报, 2024, 39(7): 787-792. |
| [3] | 王晓俊, 许文, 刘润路, 潘辉, 朱申敏. 水凝胶负载的纳米银/氮化碳光催化剂的制备及性能研究[J]. 无机材料学报, 2022, 37(7): 731-740. |
| [4] | 李远洋,江波. 具有超亲水光催化性能的λ/4-λ/2型两层宽频增透膜的制备和性能研究[J]. 无机材料学报, 2019, 34(2): 159-163. |
| [5] | 何前军, 陈丹阳, 范明俭. 精准纳米气体治疗研究进展[J]. 无机材料学报, 2018, 33(8): 811-824. |
| [6] | 宋晶晶, 陈波, 林开利. 核壳结构羟基磷灰石/介孔二氧化硅纳米颗粒的制备及其药物释放研究[J]. 无机材料学报, 2018, 33(6): 623-628. |
| [7] | 王亚斌, 刘忠, 史时辉, 呼科科, 张琰图, 郭敏. 树枝状纤维形二氧化硅纳米粒子的研究进展[J]. 无机材料学报, 2018, 33(12): 1274-1288. |
| [8] | 白家峰, 王希庆, 陈义昌, 邓勇辉, 李志华, 刘 鸿, 刘绍华. 聚多巴胺修饰的介孔二氧化硅微球的合成及其用于负载左旋薄荷醇凉味剂[J]. 无机材料学报, 2017, 32(8): 845-850. |
| [9] | 童 琴, 董亚梅, 严 良, 何丹农. 以海藻酸钠为基体的Ag/AgBr/TiO2整体式光催化剂的高效制备及其光催化性能[J]. 无机材料学报, 2017, 32(6): 637-642. |
| [10] | 马洪兵, 白 华, 薛 晨, 陶鹏飞, 徐群峰, 江 南. 一种链珠状SiO2纳米线的制备及分析[J]. 无机材料学报, 2016, 31(5): 523-528. |
| [11] | 宋聪, 喻晓蔚, 钱丹, 孙振忠, 江波. KH560修饰的溶胶-凝胶法二氧化硅载体用于脂肪酶的固定化[J]. 无机材料学报, 2016, 31(3): 311-316. |
| [12] | 马 丽, 朱建华, 黄 磊. 海藻酸钠调控羟基磷灰石纳米棒的低温快速合成[J]. 无机材料学报, 2015, 30(3): 311-317. |
| [13] | 孙志娟, 陈雪莲, 蒋春跃. 自组装法制备中空二氧化硅纳米粒子减反射薄膜[J]. 无机材料学报, 2014, 29(9): 947-955. |
| [14] | 盛 晨, 于 云, 于 洋, 米 乐, 唐根初, 宋力昕. 基于二氧化硅气凝胶制备的多层隔热材料微观结构及热性能研究[J]. 无机材料学报, 2013, 28(7): 790-794. |
| [15] | 张 倩, 单 锋, 陆学民, 路庆华. 金纳米线-介孔二氧化硅薄膜的制备和非线性光学性能研究[J]. 无机材料学报, 2013, 28(10): 1087-1092. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||