无机材料学报 ›› 2020, Vol. 35 ›› Issue (7): 822-826.DOI: 10.15541/jim20190404 CSTR: 32189.14.10.15541/jim20190404
所属专题: 结构陶瓷论文精选(2020); 【虚拟专辑】分离膜,复相陶瓷(2020~2021)
收稿日期:2019-08-12
修回日期:2019-09-10
出版日期:2020-07-20
网络出版日期:2019-10-25
作者简介:李彦瑞(1992-), 男, 硕士研究生. E-mail: 205022738@qq.com基金资助:
LI Yanrui,LU Youjun( ),LIU Yang,YUAN Zhenxia,HUANG Zhenkun
),LIU Yang,YUAN Zhenxia,HUANG Zhenkun
Received:2019-08-12
Revised:2019-09-10
Published:2020-07-20
Online:2019-10-25
Supported by:摘要:
本工作研究了Si3N4-ZrO2-La2O3三元系统的相关系, 采用X射线衍射仪分析了物相组成。结果表明, 在1500 ℃/1 h/N2气氛条件下固相反应, 生成了ZrN和La4.67Si3O13、La5Si3NO12、La4Si2N2O7、LaSiNO2、La2Zr2O7等镧盐化合物的共存相。由于生成的氮化锆和硅酸镧等化合物不在Si3N4-ZrO2-La2O3三元系统内, 需引入SiO2测定SiO2-La2O3-ZrO2三元分系统相图, 进而扩大成Si3N4-ZrO2-La2O3-SiO2-ZrN五元系统, 本工作绘制并提出了此五元系统相图, 且提出了1570 ℃时SiO2-La2O3-ZrO2三元分系统实验相图。此外, 验证了La2O3在Si3N4-ZrO2-La2O3三元系统反应中促进Si3N4-ZrO2取代反应生成ZrN的作用。
中图分类号:
李彦瑞,陆有军,刘洋,袁振侠,黄振坤. Si3N4-ZrO2-La2O3系统反应合成ZrN及相图构建[J]. 无机材料学报, 2020, 35(7): 822-826.
LI Yanrui,LU Youjun,LIU Yang,YUAN Zhenxia,HUANG Zhenkun. Reaction Synthesizes of ZrN and Phase Diagram in the Si3N4-ZrO2-La2O3 System[J]. Journal of Inorganic Materials, 2020, 35(7): 822-826.
| Sample | SiO2/La2O3/ZrO2 | Phase composition (XRD analysis results*) |
|---|---|---|
| 0.3S′0.5Z0.2L | 0.3/0.5/0.2 | La2Zr2O7(s) La2SiO5(m) La2O3(w) |
| 0.43S′0.46Z0.11L | 0.43/0.46/0.11 | La2Zr2O7(s) La4.67Si3O13(s) La2SiO5(m) |
| 0.2S′0.3Z0.5L | 0.2/0.3/0.5 | La2Zr2O7(vs) La4.67Si3O13(vs) ZrO2(w) |
| 0.5S′0.3Z0.2L | 0.5/0.3/0.2 | La2Si2O7(vs) La4.67Si3O13(vs) ZrO2(w) |
| 0.4S′0.1Z0.5L | 0.4/0.1/0.5 | ZrSiO4(vs) La2Si2O7(s) ZrO2(m) |
| 0.7S′0.1Z0.2L | 0.7/0.1/0.2 | ZrSiO4(vs) La2Si2O7(m) SiO2(w) |
表1 SiO2-La2O3-ZrO2系统样品在1570 ℃烧结后的相组成
Table 1 Phase compositions of the SiO2-La2O3-ZrO2 system after sintered at 1570 ℃
| Sample | SiO2/La2O3/ZrO2 | Phase composition (XRD analysis results*) |
|---|---|---|
| 0.3S′0.5Z0.2L | 0.3/0.5/0.2 | La2Zr2O7(s) La2SiO5(m) La2O3(w) |
| 0.43S′0.46Z0.11L | 0.43/0.46/0.11 | La2Zr2O7(s) La4.67Si3O13(s) La2SiO5(m) |
| 0.2S′0.3Z0.5L | 0.2/0.3/0.5 | La2Zr2O7(vs) La4.67Si3O13(vs) ZrO2(w) |
| 0.5S′0.3Z0.2L | 0.5/0.3/0.2 | La2Si2O7(vs) La4.67Si3O13(vs) ZrO2(w) |
| 0.4S′0.1Z0.5L | 0.4/0.1/0.5 | ZrSiO4(vs) La2Si2O7(s) ZrO2(m) |
| 0.7S′0.1Z0.2L | 0.7/0.1/0.2 | ZrSiO4(vs) La2Si2O7(m) SiO2(w) |
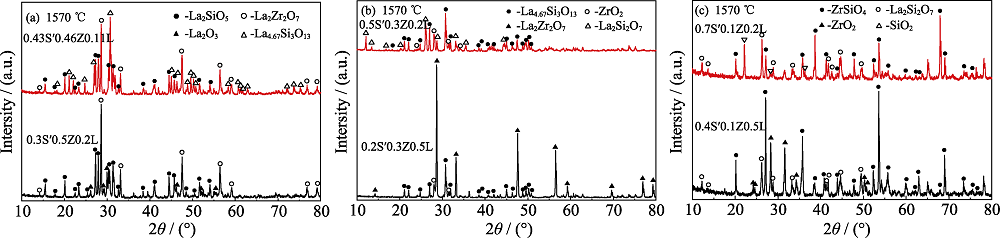
图1 (a) 0.3S′0.5Z0.2L和0.43S′0.46Z0.11L, (b) 0.2S′0.3Z0.5L和0.5S′0.3Z0.2L及(c) 0.4S′0.1Z0.5L和0.7S′0.1Z0.2L系统样品在1570 ℃恒温1 h的XRD图谱
Fig. 1 XRD patterns of the samples of 0.3S′0.5Z0.2L and 0.43S′0.46Z0.11L (a), 0.2S′0.3Z0.5L and 0.5S′0.3Z0.2L (b),0.4S′0.1Z0.5L and 0.7S′0.1Z0.2L (c) sintered at 1570 ℃ for 1 h
| Sample | Si3N4/ZrO2/La2O3 | Phase composition (XRD analysis results*) |
|---|---|---|
| 1S3Z3L | 1/3/3 | La5Si3NO12(s) La2Zr2O7(s) ZrN(m) |
| 2S6Z3L | 2/6/3 | La4.67Si3O13 (vs) ZrN(vs) |
| ZrO2(vw) | ||
| 3S9Z7L | 3/9/7 | La4.67Si3O13(vs) ZrN(m), |
| 4S9Z10L | 4/9/10 | La5Si3NO12(vs) ZrN(m) |
| 4S3Z6L | 4/3/6 | LaSiNO2 (vs) ZrN(w) La5Si3NO12(w) |
| 4S3Z12L | 4/3/12 | La4Si2N2O7(vs) ZrN(w) La5Si3NO12(w) |
| 8S3Z8L (1550 ℃) | 8/3/8 | LaSiNO2(vs) ZrN(w) La2Si6N8O3(w) |
表2 Si3N4-ZrO2-La2O3系统1500 ℃烧结样品的相组成
Table 2 Phase compositions of the Si3N4-ZrO2-La2O3 system samples at 1500 ℃
| Sample | Si3N4/ZrO2/La2O3 | Phase composition (XRD analysis results*) |
|---|---|---|
| 1S3Z3L | 1/3/3 | La5Si3NO12(s) La2Zr2O7(s) ZrN(m) |
| 2S6Z3L | 2/6/3 | La4.67Si3O13 (vs) ZrN(vs) |
| ZrO2(vw) | ||
| 3S9Z7L | 3/9/7 | La4.67Si3O13(vs) ZrN(m), |
| 4S9Z10L | 4/9/10 | La5Si3NO12(vs) ZrN(m) |
| 4S3Z6L | 4/3/6 | LaSiNO2 (vs) ZrN(w) La5Si3NO12(w) |
| 4S3Z12L | 4/3/12 | La4Si2N2O7(vs) ZrN(w) La5Si3NO12(w) |
| 8S3Z8L (1550 ℃) | 8/3/8 | LaSiNO2(vs) ZrN(w) La2Si6N8O3(w) |
| T/℃ | ΔG/(kJ·mol-1) | T/℃ | ΔG/(kJ·mol-1) |
|---|---|---|---|
| 1400 | -122.513 | 1600 | -139.531 |
| 1500 | -130.893 | 1700 | -148.436 |
表3 反应(4)的热力学计算结果
Table 3 (4)Thermodynamic calculation results of reaction formula
| T/℃ | ΔG/(kJ·mol-1) | T/℃ | ΔG/(kJ·mol-1) |
|---|---|---|---|
| 1400 | -122.513 | 1600 | -139.531 |
| 1500 | -130.893 | 1700 | -148.436 |
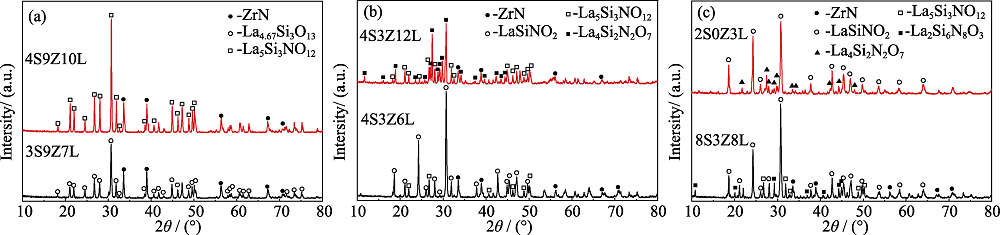
图5 (a) 3S9Z7L和4S9Z10L、(b) 4S3Z6L和4S3Z12L系统样品在1500 ℃恒温1 h后, 以及(c) 8S3Z8L系统样品在1550 ℃和2S0Z3L系统样品在1500 ℃恒温1 h后的XRD图谱
Fig. 5 XRD patterns of the samples (a) 3S9Z7L and 4S9Z10L, (b) 4S3Z6L and 4S3Z12L after sintered at 1500 ℃ for 1 h, and (c) 8S3Z8L after sintered at 1550 ℃ for 1 h and the samples 2S0Z3L after sintered at 1500 ℃ for 1 h
| [1] |
CHEN Y, DENG C, YU C, et al. Molten-salt nitridation synthesis of cubic ZrN nanopowders at low temperature via magnesium thermal reduction. Ceramics International, 2018,44(7):8710-8715.
DOI URL |
| [2] |
FU B, GAO L. Synthesis of nanocrystalline zirconium nitride powders by reduction-nitridation of zirconium oxide. Journal of the American Ceramic Society, 2004,87(4):696-698.
DOI URL |
| [3] | BRUGNON C, LANZA F, MACCHI G, et al. Evaluation of the wear resistance of ZrN coatings using thin layer activation. Surface and Coatings Technology, 1998,100:23-26. |
| [4] |
TAKEYAMA M B, ITOI T, AOYAGI E, et al. High performance of thin nano-crystalline ZrN diffusion barriers in Cu/Si contact systems. Applied Surface Science, 2002,190(1-4):450-454.
DOI URL |
| [5] |
TANG Y, ZHANG G J, XUE J X, et al. Densification and mechanical properties of hot-pressed ZrN ceramics doped with Zr or Ti. Journal of the European Ceramic Society, 2013,33(7):1363-1371.
DOI URL |
| [6] |
LI Y, HUANG Z, XU Y, et al. Synthesis of ZrN-Sialon composites from zircon and alumina by carbothermal reduction-nitridation. Materials Research Bulletin, 2012,47(11):3273-3276.
DOI URL |
| [7] |
SUN W Z, CHENG J G, HUANG Z K, et al. ZrC formation and the phase relations in the Si-Zr-Mg-O-C system. Journal of materials Science, 2016,51(17):8139-8147.
DOI URL |
| [8] |
SUN W Z, CHENG J G, HUANG Z K, et al. ZrC formation and the phase relations in the SiC-SiO2-ZrC-ZrO2-CaO system. Ceramics International, 2016,42(8):10165-10170.
DOI URL |
| [9] |
WEISS J, GAUCKLER L J, TIEN T Y. The System Si3N4-SiO2-ZrN-ZrO2. Journal of the American Ceramic Society, 1979,62(11/12):632-634.
DOI URL |
| [10] |
WEISS J, GAUCKLER L J, LUKAS H L, et al. Determination of phase equilibria in the system Si-Al-Zr-N-O by experiment and thermodynamic calculation. Journal of Materials Science, 1981,16(11):2997-3005.
DOI URL |
| [11] | JIANG G, WANG S. Study on mechanical properties and wear resistance of Si3N4-ZrO2 composites. Journal of the Chinese Ceramic Society, 1993(3):215-220. |
| [12] | RAO P, YE J, MAO J, et al. Chemical incompatibility of ZrO2-Si3N4 ceramic composites. Journal of Inorganic Materials, 1999,14(5):711-716. |
| [13] | JIANG G. Formation of ZrN in Si3N4-ZrO2 composite and its influence on mechanical properties. Journal of the Chinese Ceramic Society, 1995(5):580-583. |
| [14] |
MITOMO M, IZUMI F, HORIUCHI S, et al. Phase relationships in the system Si3N4-SiO2-La2O3. Journal of Materials Science, 1982,17(8):2359-2364.
DOI URL |
| [15] |
WU L, SUN W, CHEN Y, et al. Phase relations in Si-C-N-O-R (R= La, Gd, Y) systems. Journal of the American Ceramic Society, 2011,94(12):4453-4458.
DOI URL |
| [16] | TIAN Z L, WANG J Y. Research progress of rare earth silicate ceramics. Advanced Ceramics, 2018,39(5):3-28. |
| [17] | LU Y, YUAN Z, LI Y, et al. Effect of SrO on synthesis of ZrN in Si3N4-ZrO2-SrO system. Journal of the Chinese Ceramic Society, 2018,46(6):823-828. |
| [18] |
DOERNER P, GAUCKLER L J, KRIEG H, et al. On the calculation and representation of multicomponent systems. Calphad, 1979,3(4):241-257.
DOI URL |
| [19] |
KAMAEV D N, ARCHUGOV S A, MIKHAILOV G G. Study and thermodynamic analysis of the ZrO2-SiO2 system. Russian Journal of Applied Chemistry, 2005,78(2):200-203.
DOI URL |
| [20] | ROTH R S. Pyrochlore-type compounds containing double oxides of trivalent and tetravalent ions. J. Res. Natl. Bur. Stand.(U.S.), 1956,56(8):17-25. |
| [1] | 穆浩洁, 张源江, 喻彬, 付秀梅, 周世斌, 李晓东. ZrO2掺杂Y2O3-MgO纳米复相陶瓷的制备及性能研究[J]. 无机材料学报, 2025, 40(3): 281-289. |
| [2] | 王博, 蔡德龙, 朱启帅, 李达鑫, 杨治华, 段小明, 李雅楠, 王轩, 贾德昌, 周玉. SrAl2Si2O8增强BN陶瓷的力学性能及抗热震性能[J]. 无机材料学报, 2024, 39(10): 1182-1188. |
| [3] | 吴东江, 赵紫渊, 于学鑫, 马广义, 由竹琳, 任冠辉, 牛方勇. Al2O3-TiCp复相陶瓷激光定向能量沉积直接增材制造[J]. 无机材料学报, 2023, 38(10): 1183-1192. |
| [4] | 琚印超, 刘小勇, 王琴, 张伟刚, 魏玺. 超高温复相陶瓷基复合材料烧蚀行为研究[J]. 无机材料学报, 2022, 37(1): 86-92. |
| [5] | 刘洋, 陆有军, 李彦瑞, 林立群, 袁振侠, 黄振坤. Hf-Si-La-O-N体系中HfN的形成及相关系[J]. 无机材料学报, 2021, 36(4): 443-448. |
| [6] | 王义良, 艾云龙, 杨书伟, 梁炳亮, 郑振环, 欧阳晟, 何文, 陈卫华, 刘长虹, 张建军, 刘智勇. M3O4(M=FeCoCrMnMg)高熵氧化物粉体的简易制备及超电容性能研究[J]. 无机材料学报, 2021, 36(4): 425-430. |
| [7] | 徐晓虹, 田江洲, 吴建锋, 张乾坤, 金昊, 杜怿鑫. Fe2O3对原位制备SiCw/SiC太阳能储热陶瓷的结构与性能的影响[J]. 无机材料学报, 2019, 34(10): 1103-1108. |
| [8] | 孙川, 万春磊, 潘伟, 宗鹏安, 李云凯, 周士猛. 反应烧结B4C/Al2O3复合陶瓷的装甲防护性能研究[J]. 无机材料学报, 2018, 33(5): 545-549. |
| [9] | 彭 旭, 朱德贵, 李杨绪, 周加敏, 吕 振, 郭鹏超. AlN-BN复相陶瓷的热等静压制备与性能研究[J]. 无机材料学报, 2016, 31(5): 535-541. |
| [10] | 陈春霞, 李浩然, 郑仁奎. PrBi4Fe0.5Co0.5Ti3O15多铁陶瓷的性能研究[J]. 无机材料学报, 2015, 30(5): 511-515. |
| [11] | 张德兴, 孙云蕾, 阿布都外力阿布力米提, 曹光旱. LaFeAsO纳米晶的中低温合成与表征[J]. 无机材料学报, 2015, 30(12): 1273-1277. |
| [12] | 马西飞, 康 庄, 黄 晓, 张国军. 尿素法制备纳米氮化锆粉体[J]. 无机材料学报, 2015, 30(1): 77-80. |
| [13] | 胡海龙, 姚冬旭, 夏咏锋, 左开慧, 曾宇平. 反应烧结制备Si3N4/SiC复相陶瓷及其力学性能研究[J]. 无机材料学报, 2014, 29(6): 594-598. |
| [14] | 李 荐, 姚书恒, 周宏明, 耿文俊. 用固相和水热结合法制备LiMn0.4Fe0.6PO4/C复合材料[J]. 无机材料学报, 2014, 29(4): 443-448. |
| [15] | 谢 慧, 袁淑娟, 康保娟, 鲁 波, 曹世勋, 张金仓 . Ho0.5Pr0.5FeO3的磁性与巨磁介电效应[J]. 无机材料学报, 2014, 29(1): 77-80. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||