无机材料学报 ›› 2017, Vol. 32 ›› Issue (12): 1233-1242.DOI: 10.15541/jim20170066 CSTR: 32189.14.10.15541/jim20170066
• • 下一篇
田晓, 段如霞, 赵丽娟, 那仁格日乐
收稿日期:2017-02-08
修回日期:2017-04-17
出版日期:2017-12-20
网络出版日期:2017-11-21
作者简介:田 晓(1972-), 女, 教授. E-mail: nsdtx@126.com
基金资助:TIAN Xiao, DUAN Ru-Xia, ZHAO Li-Juan, NAREN Ge-Ri-Le
Received:2017-02-08
Revised:2017-04-17
Published:2017-12-20
Online:2017-11-21
摘要:
直接硼氢化物燃料电池(DBFC)具有理论电池电压高和能量密度大等特点, 而其阳极催化剂是决定电池性能的关键因素之一。因此, 研究者们在提高阳极催化剂催化活性和降低催化剂成本方面开展了大量的研究工作。本文在简要介绍DBFC工作原理和阳极反应机理的基础上, 从催化剂种类和性能角度综述了近年来DBFC中贵金属、过渡金属以及储氢合金阳极催化剂的主要研究进展, 指出了阳极催化剂研究所面临的问题, 同时提出了今后的发展方向。
中图分类号:
田晓, 段如霞, 赵丽娟, 那仁格日乐. 直接硼氢化物燃料电池(DBFC)阳极催化剂的研究进展[J]. 无机材料学报, 2017, 32(12): 1233-1242.
TIAN Xiao, DUAN Ru-Xia, ZHAO Li-Juan, NAREN Ge-Ri-Le. Anode Catalyst for the Direct Borohydride Fuel Cell[J]. Journal of Inorganic Materials, 2017, 32(12): 1233-1242.
| Type | Electrocatalyst | Fuel | Oxidant | Theoretical data of open circuit voltage | Main existence question | Possible application fields |
|---|---|---|---|---|---|---|
| PEMFC | Pt | H2/Reformer hydrogen | Air/Pure oxygen | 1.23 V | Hydrogen supply system, catalyst cost | Hybrid electric vehicles, portable power source |
| DMFC | Pt, Pt-Ru | Methanol | Air | 1.183 V | Catalyst poisoning, catalyst cost | A small mobile power source |
| DBFC | Non-noble metal | Borohydride | Air/O2/ H2O2 | 1.64 V | Hydrolysis reaction, catalyst cost | Portable electronics, mobile power |
表1 常见低温燃料电池的技术状态
Table 1 Technical state of common low temperature fuel cells
| Type | Electrocatalyst | Fuel | Oxidant | Theoretical data of open circuit voltage | Main existence question | Possible application fields |
|---|---|---|---|---|---|---|
| PEMFC | Pt | H2/Reformer hydrogen | Air/Pure oxygen | 1.23 V | Hydrogen supply system, catalyst cost | Hybrid electric vehicles, portable power source |
| DMFC | Pt, Pt-Ru | Methanol | Air | 1.183 V | Catalyst poisoning, catalyst cost | A small mobile power source |
| DBFC | Non-noble metal | Borohydride | Air/O2/ H2O2 | 1.64 V | Hydrolysis reaction, catalyst cost | Portable electronics, mobile power |
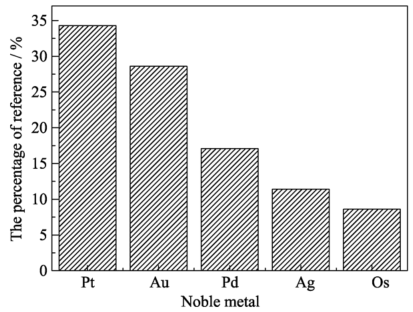
图2 贵金属单质用作DBFC阳极催化剂研究文献所占比例
Fig. 2 Percentage of reference on variously noble metal as DBFC anode catalyst According to the Web of Science (2000-2016)
| Anode catalyst | Cathode catalyst | Oxidant | T/℃ | Power density/(mW•cm-2) | Ref. |
|---|---|---|---|---|---|
| Au-Pt | MnO2 | Air | 25 | 20.0 | [25] |
| Pt3-Au2 | Pt/C | Humidified O2 | 65 | 161.0 | [26] |
| Pt1-Au1 | Pt-based | O2 | 60 | 47.0 | [18] |
| Au1-Pd1 | Pt-based | O2 | 60 | 31.0 | [11] |
| Pd | Pt/C | Humidified O2 | NA | ~185.0 | [27] |
| Au | Pt/C | Humidified O2 | NA | ~82.0 | [27] |
| 5wt%Au-15wt%Pd | Pt/C | Humidified O2 | NA | ~120.0 | [27] |
| 10wt%Au-10wt%Pd | Pt/C | Humidified O2 | 50 | ~90.0 | [27] |
| 15wt%Au-5wt%Pd | Pt/C | Humidified O2 | 50 | ~75.0 | [27] |
| Pt black | Pt black | NA | 60 | 31.6 | [28] |
| Pt1-Ru1 black | Pt black | NA | 60 | 35.1 | [28] |
| Au | Au/C | NA | 20 | ~28.0 | [30] |
| Pd | Au/C | NA | 20 | ~41.0 | [30] |
| Au2-Pd1 | Au/C | NA | 20 | ~46.0 | [30] |
| Au1-Pd1 | Au/C | NA | 20 | ~49.0 | [30] |
| Au1-Pd2 | Au/C | NA | 20 | 56.8 | [30] |
表2 二元贵金属作为阳极催化剂的DBFC的性能参数
Table 2 Performance data for DBFCs employing binary noble metal anodes
| Anode catalyst | Cathode catalyst | Oxidant | T/℃ | Power density/(mW•cm-2) | Ref. |
|---|---|---|---|---|---|
| Au-Pt | MnO2 | Air | 25 | 20.0 | [25] |
| Pt3-Au2 | Pt/C | Humidified O2 | 65 | 161.0 | [26] |
| Pt1-Au1 | Pt-based | O2 | 60 | 47.0 | [18] |
| Au1-Pd1 | Pt-based | O2 | 60 | 31.0 | [11] |
| Pd | Pt/C | Humidified O2 | NA | ~185.0 | [27] |
| Au | Pt/C | Humidified O2 | NA | ~82.0 | [27] |
| 5wt%Au-15wt%Pd | Pt/C | Humidified O2 | NA | ~120.0 | [27] |
| 10wt%Au-10wt%Pd | Pt/C | Humidified O2 | 50 | ~90.0 | [27] |
| 15wt%Au-5wt%Pd | Pt/C | Humidified O2 | 50 | ~75.0 | [27] |
| Pt black | Pt black | NA | 60 | 31.6 | [28] |
| Pt1-Ru1 black | Pt black | NA | 60 | 35.1 | [28] |
| Au | Au/C | NA | 20 | ~28.0 | [30] |
| Pd | Au/C | NA | 20 | ~41.0 | [30] |
| Au2-Pd1 | Au/C | NA | 20 | ~46.0 | [30] |
| Au1-Pd1 | Au/C | NA | 20 | ~49.0 | [30] |
| Au1-Pd2 | Au/C | NA | 20 | 56.8 | [30] |
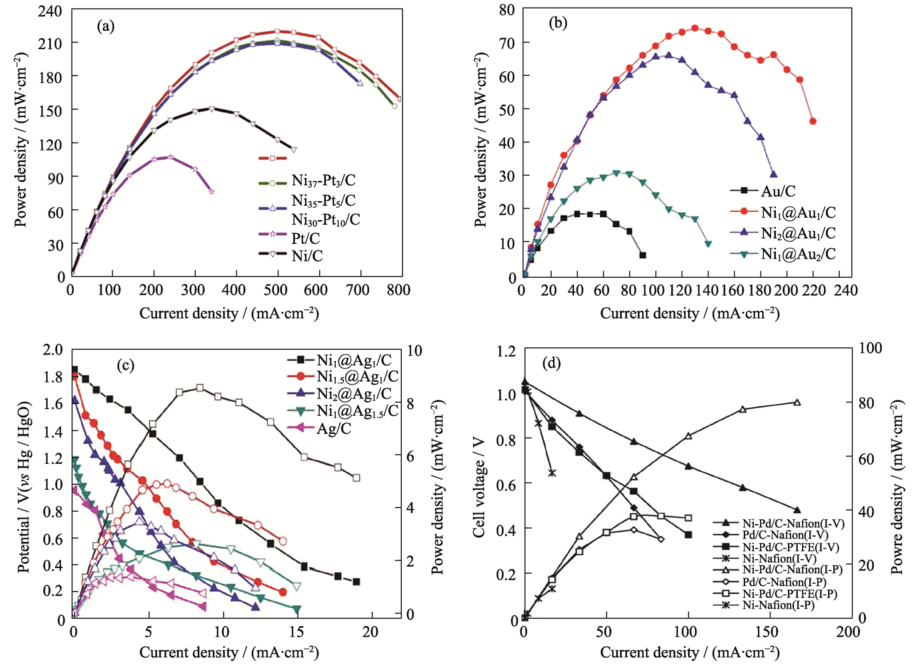
图3 不同阳极催化剂下DBFC的功率密度曲线[37-38,40,42]
Fig. 3 Power density curves of the DBFC using different anode catalysts[37-38,40,42]^(a) 60℃; (b) 20℃; (c) 25℃; (d) 25℃
| Anode catalyst | Cathode catalyst | Oxidant | T/℃ | Power density/ (mW•cm-2) | Ref. |
|---|---|---|---|---|---|
| LmNi4.78Mn0.22 | Nickel foam | NA | NA | NA | [65] |
| MmNi3.55Al0.3Mn0.4Co0.75 | FeTMPP/C | H2O2+H2SO4 | 70 | 82 | [66] |
| MmNi3.55Al0.3Mn0.4Co0.75 | PbSO4/C | H2O2+H2SO4 | 70 | 120 | [66] |
| MmNi3.35Co0.75Mn0.4Al0.3 | MnO2/C | O2 | 25 | 70 | [67] |
| MmNi3.6Al0.4Mn0.3Co0.7 | Au/SS mesh | H2O2+H2SO4 | 25 | 50 | [68] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Iron phthalocyanin/C | Air | RT | 92 | [69] |
| MmNi3.6Al0.4Mn0.3Co0.7 | PbSO4/C | H2O2+H2SO4 | 25 | 10 | [70] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Cobalt phthalocyanin | Air | RT | 90 | [71] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Prussian blue | H2O2+H2SO4+KCl | 30 | 68 | [72] |
| MmNi3.55Co0.75Mn0.4Al0.3 | LaNiO3/C | Air | 65 | 127 | [73] |
| La10.5Ce4.3Pr0.5Nd1.4Ni60.0Co12.7Mn5.9Al4.7 | Pd/C | Air | NA | 81 | [74] |
| MmNi3.55Co0.75Mn0.4Al0.3 | LaCoO3/C/Ni-foam | Air | RT | 65 | [62] |
| LaMnNi3.55Al0.30Mn0.40Co0.75 | Nickel foam | Air | NA | NA | [61] |
| Zr0.9Ti0.1Mn0.6V0.2Co0.1Ni1.1 | Pt/C | O2 | 85 | 190 | [75] |
| Zr0.9Ti0.1Mn0.6V0.2Co0.1Ni1.1 | Pt/C | O2 | 60 | NA | [76] |
| ZrCr0.8Ni1.2 | Pt/C | O2 | 25 | NA | [57] |
| Zr0.9Ti0.1V0.2Mn0.6Cr0.05Co0.05Ni1.2 | Pt/C | H2O2 | 70 | 70 | [77] |
表3 储氢合金作为阳极催化剂的DBFC的性能参数
Table 3 Performance data for DBFCs employing hydrogen storage alloy anodes
| Anode catalyst | Cathode catalyst | Oxidant | T/℃ | Power density/ (mW•cm-2) | Ref. |
|---|---|---|---|---|---|
| LmNi4.78Mn0.22 | Nickel foam | NA | NA | NA | [65] |
| MmNi3.55Al0.3Mn0.4Co0.75 | FeTMPP/C | H2O2+H2SO4 | 70 | 82 | [66] |
| MmNi3.55Al0.3Mn0.4Co0.75 | PbSO4/C | H2O2+H2SO4 | 70 | 120 | [66] |
| MmNi3.35Co0.75Mn0.4Al0.3 | MnO2/C | O2 | 25 | 70 | [67] |
| MmNi3.6Al0.4Mn0.3Co0.7 | Au/SS mesh | H2O2+H2SO4 | 25 | 50 | [68] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Iron phthalocyanin/C | Air | RT | 92 | [69] |
| MmNi3.6Al0.4Mn0.3Co0.7 | PbSO4/C | H2O2+H2SO4 | 25 | 10 | [70] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Cobalt phthalocyanin | Air | RT | 90 | [71] |
| MmNi3.55Co0.75Mn0.4Al0.3 | Prussian blue | H2O2+H2SO4+KCl | 30 | 68 | [72] |
| MmNi3.55Co0.75Mn0.4Al0.3 | LaNiO3/C | Air | 65 | 127 | [73] |
| La10.5Ce4.3Pr0.5Nd1.4Ni60.0Co12.7Mn5.9Al4.7 | Pd/C | Air | NA | 81 | [74] |
| MmNi3.55Co0.75Mn0.4Al0.3 | LaCoO3/C/Ni-foam | Air | RT | 65 | [62] |
| LaMnNi3.55Al0.30Mn0.40Co0.75 | Nickel foam | Air | NA | NA | [61] |
| Zr0.9Ti0.1Mn0.6V0.2Co0.1Ni1.1 | Pt/C | O2 | 85 | 190 | [75] |
| Zr0.9Ti0.1Mn0.6V0.2Co0.1Ni1.1 | Pt/C | O2 | 60 | NA | [76] |
| ZrCr0.8Ni1.2 | Pt/C | O2 | 25 | NA | [57] |
| Zr0.9Ti0.1V0.2Mn0.6Cr0.05Co0.05Ni1.2 | Pt/C | H2O2 | 70 | 70 | [77] |
| [1] | ROSEN M A, KOOHI-FAYEGH S.The prospects for hydrogen as an energy carrier: an overview of hydrogen energy and hydrogen energy systems.Energy, Ecology and Environment, 2016, 1(1): 10-29. |
| [2] | SONG M Y, PARK H R, KWON S N.Evaluation of the metal-added Mg hydrogen storage material and comparison with the oxide-added Mg.Journal of Industrial and Engineering Chemistry, 2015, 21: 378-386. |
| [3] | DE LEON C P, WALSH F C, PLETCHER D,et al.Direct borohydride fuel cells. Journal of Power Sources, 2006, 155(2): 172-181. |
| [4] | MA J, CHOUDHURY N A, SAHAI Y.A comprehensive review of direct borohydride fuel cells.Renewable and Sustainable Energy Reviews, 2010, 14(1): 183-199. |
| [5] | MERINO-JIMENEZ I, DE LEON C P, SHAH A A,et al Developments in direct borohydride fuel cells and remaining challenges.Journal of Power Sources, 2012, 219: 339-357. |
| [6] | OLU P Y, JOB N, CHATENET M,et al.Evaluation of anode (electro)catalytic materials for the direct borohydride fuel cell: methods and benchmarks. Journal of Power Sources, 2016, 327: 235-257. |
| [7] | YI L H, SONG Y F, YI W,et al Carbon supported Pt hollow nanospheres as anode catalysts for direct borohydride-hydrogen peroxide fuel cells.International Journal of Hydrogen Energy, 2011, 36(18): 11512-11518. |
| [8] | WEI J L, WANG X Y, WANG Y,et al Investigation of carbon- supported Au hollow nanospheres as electrocatalyst for electrooxidation of sodium borohydride.International Journal of Hydrogen Energy, 2009, 34(8): 3360-3366. |
| [9] | CHATENET M, MICOUD F, ROCHE I,et al. Kinetics of sodium borohydride direct oxidation and oxygen reduction in sodium hydroxide electrolyte-Part I. BH4- electro-oxidation on Au and Ag catalysts. Electrochimica Acta, 2006, 51(25): 5459-5467. |
| [10] | CHENG H, SCOTT K.Determination of kinetic parameters for borohydride oxidation on a rotating Au disk electrode.Electrochimica Acta, 2006, 51(17): 3429-3433. |
| [11] | ATWAN M H, MACDONALD C L B, NORTHWOOD D O,et al.Colloidal Au and Au-alloy catalysts for direct borohydride fuel cells: electrocatalysis and fuel cell performance. Journal of Power Sources, 2006, 158(1): 36-44. |
| [12] | CELIKKAN H, SAHIN M, AKSU M L,et al The investigation of the electrooxidation of sodium borohydride on variousmetal electrodes in aqueous basic solutions.International Journal of Hydrogen Energy, 2007, 32(5): 588-593. |
| [13] | PONCE-DE-LEON C, BAVYKIN D V, WALSH F C. The oxidation of borohydride ion at titanate nanotube supported gold electrodes.Electrochemistry Communications, 2006, 8(10): 1655-1660. |
| [14] | CONCHA B M, CHATENET M.Direct oxidation of sodium borohydride on Pt, Ag and alloyed Pt-Ag electrodes in basic media Part II. carbon-supported nanopaticles.Electrochimica Acta, 2009, 54(26): 6130-6139. |
| [15] | LIU B H, LI Z P.A review: hydrogen generation from borohydride hydrolysis reaction.Journal of Power Sources, 2009, 187(2): 527-534. |
| [16] | QIN H Y, CHEN K J, ZHU C,et al.High electrocatalytic activity for borohydride oxidation on palladium nanocubes enclosed by {200} facets. Journal of Power Sources, 2015, 299: 241-245. |
| [17] | LIU B H, LI Z P, SUDA S.Electrocatalysts for the anodic oxidation of borohydrides.Electrochimica Acta, 2004, 49(19): 3097-3105. |
| [18] | GYENGE E, ATWAN M, NORTHWOOD D.Electrocatalysis of borohydride oxidation on colloidal Pt and Pt-alloys (Pt-Ir, Pt-Ni, and Pt-Au) and application fordirect borohydride fuel cell anodes.Journal of the Electrochemical Society, 2006, 153(1): A150-A158. |
| [19] | CHENG H, SCOTT K, LOVELL K.Material aspects of the design and operation of direct borohydride fuel cells.Fuel Cells, 2006, 6(5): 367-375. |
| [20] | LAM V W S, KANNANGARA D C W, ALFANTAZI A,et al Electrodeposited osmium three-dimensional anodes for direct borohydride fuel cells.Journal of Power Sources, 2012, 212: 57-65. |
| [21] | LIU J, WANG H, WU C,et al Preparation and characterization of nanoporous carbon-supported platinum as anode electrocatalyst for direct borohydride fuel cell.International Journal of Hydrogen Energy, 2014, 39(12): 6729-6736. |
| [22] | REZA O, JAHAN-BAKHSH R, ROUDABEH V.Pt nanoparticles/ graphene paste electrode for sodium borohydride electrooxidation.Journal of Solid State Electrochemistry, 2013, 17(1): 217-221. |
| [23] | MARTINS M, ŠLJUKIĆ B, SEQUEIRA C A C,et al.Biobased carbon-supported palladium electrocatalysts for borohydride fuel cells. International Journal of Hydrogen Energy, 2016, 41(25): 10914-10922. |
| [24] | CHENG K, JIANG J T, KONG S Y,et al. Pd nanoparticles support on rGO-C@TiC coaxial nanowires as a novel 3D electrode for NaBH4 electrooxidation. International Journal of Hydrogen Energy, 2017, 42(5): 2943-2951. |
| [25] | COOWAR F A, VITINS G, MEPSTED G O,et al Electrochemical oxidation of borohydride at nano-gold-based electrodes: application in direct borohydride fuel cells.Journal of Power Sources, 2008, 175(1): 317-324. |
| [26] | KARADAG C I, BEHMENYAR G, BOYACI SAN F,et al Investigation of carbon supported nanostructured PtAu alloy as electrocatalyst for direct borohydride fuel cell.Fuel Cells, 2015, 15(2): 262-269. |
| [27] | LEE H M, PARK S Y, PARK K T,et al Development of Au-Pd catalysts supported on carbon for a direct borohydride fuel cell.Research on Chemical Intermediates, 2008, 34(8/9): 787-792. |
| [28] | LAM V W S, ALFANTAZI A, GYENGE E L. The effect of catalyst support on the performance of PtRu in direct borohydride fuel cell anodes.Journal of Applied Electrochemistry, 2009, 39(10): 1763-1770. |
| [29] | MERINO-JIMENEZ I, JANIK M J, DE LEON C P,et al Pd-Ir alloy as an anode material for borohydride oxidation.Journal of Power Sources, 2014, 269: 498-508. |
| [30] | WANG H, WANG X Y, HE P Y,et al. Performance of AuPd/C as anode catalyst of direct NaBH4-H2O2 fuel cell. The Chinese Journal of Nonferrous Metals, 2011, 21(2): 405-410. |
| [31] | LI J, QIAN G C, ZHU Y X,et al Electrochemical behaviour of Pd-Ag/C towards sodium borohydride electrooxidation.Chemical Industry and Engineering, 2016, 33(5): 45-49. |
| [32] | BALCIUNAITE A, SUKACKIENE Z, TAMASAUSKAITE- TAMASIUNAITE L,et al.CoB/Cu and PtCoB/Cu catalysts for borohydride fuel cells. Electrochimica Acta, 2017, 225: 255-262. |
| [33] | LIU B H, LI Z P, SUDA S.Anodic oxidation of alkali borohydrides catalyzed by nickel.Journal of the Electrochemical Society, 2003, 150(3): A398-A402. |
| [34] | LIU B H, LI Z P, ARAI K,et al Performance improvement of a micro borohydride fuel cell operating at ambient conditions.Electrochimica Acta, 2005, 50(18): 3719-3725. |
| [35] | MALYALA R V, RODE C V, ARAI M,et al Activity, selectivity and stability of Ni and bimetallic Ni-Pt supported on zeolite Y catalysts for hydrogenation of acetophenone and its substituted derivatives.Applied Catalysis A General, 2000, 193(1/2): 71-86. |
| [36] | JAMARD R, LATOUR A, SALOMON J,et al Study of fuel efficiency in a direct borohydride fuel cell.Journal of Power Sources, 2008, 176(1): 287-292. |
| [37] | GENG X Y, ZHANG H M, YE W,et al Ni-Pt/C as anode electrocatalyst for a direct borohydride fuel cell.Journal of Power Sources, 2008, 185(2): 627-632. |
| [38] | DUAN D H, LIANG J W, LIU H H,et al.The effective carbon supported coreeshell structure of Ni@Au catalysts for electro- oxidation of borohydride. International Journal of Hydrogen Energy, 2015, 40(1): 488-500. |
| [39] | LIANG J W, LIU H H, WEI H K,et al. Studies of anode of sodium borohydride fuel cell. Chinese Journal of Power Sources, 2015, 39(10): 2119-2122. |
| [40] | DUAN D H, WANG Q, LIU H H,et al Investigation of carbon- supported Ni@Ag core-shell nanoparticles as electrocatalyst for electrooxidation of sodium borohydride.Journal of Solid State Electrochemistry, 2016, 20(10): 2699-2711. |
| [41] | FENG R X, CAO Y L, AI X P,et al AgNi alloy used as anodic catalyst for direct borohydride fuel cells.Acta Physico-Chimica Sinica, 2007, 23(6): 932-934. |
| [42] | LIU B H, LI Z P, SUDA S.Development of high-performance planar borohydride fuel cell modules for portable applications. Journal of Power Sources, 2008, 175(1): 226-231. |
| [43] | SANTOS D M F, SLJUKIC B, AMARAL L,et al Nickel and nickel-cerium alloy anodes for direct borohydride fuel cells.Journal of the Electrochemical society, 2014, 161(5): F594-F599. |
| [44] | SANTOS D M F, SLJUKIC B, AMARAL L,et al Nickel-rare earth electrodes for sodium borohydride electrooxidation.Electrochimica Acta, 2016, 190: 1050-1056. |
| [45] | YU D M, SHEN Y, YE Z,et al The preparation and performance of high activity Ni-Cr binary catalysts for direct borohydride fuel cells.Chinese Science Bulletin, 2013, 58(20): 2435-2439. |
| [46] | ZHIANI M, MOHAMMADI I.Performance study of passive and active direct borohydride fuel cell employing a commercial Pd decorated Ni-Co/C anode catalyst. Fuel, 2016, 166: 517-525. |
| [47] | HE P Y, WANG X Y, FU P,et al The studies of performance of the Au electrode modified by Zn as the anode electrocatalyst of direct borohydride fuel cell.International Journal of Hydrogen Energy, 2011, 36(15): 8857-8863. |
| [48] | YI L H, WEI W, ZHAO C X,et al Electrochemical oxidation of sodium borohydride on carbon supported Pt-Zn nanoparticle bimetallic catalyst and its implications to direct borohydride-hydrogen peroxide fuel cell.Electrochimica Acta, 2015, 158: 209-218. |
| [49] | DUAN D H, LIU H H, WANG Q,et al.Kinetics of sodium borohydride direct oxidation on carbon supported Cu-Ag bimetallic nanocatalysts. Electrochimica Acta, 2016, 198: 212-219. |
| [50] | BEHMENYAR G, AKIN A N.Investigation of carbon supported Pd-Cu nanoparticles as anode catalysts for direct borohydride fuel cell.Journal of Power Sources, 2014, 249: 239-246. |
| [51] | DUAN D H, YOU X, LIANG J W,et al Carbon supported Cu-Pd nanoparticles as anode catalyst for direct borohydride-hydrogen peroxide fuel cells.Electrochimica Acta, 2015, 176: 1126-1135. |
| [52] | YI L H, WEI W, ZHAO C X,et al Enhanced activity of Au-Fe/C anodic electrocatalyst for direct borohydride-hydrogen peroxide fuel cell.Journal of Power Sources, 2015, 285: 325-333. |
| [53] | CHENG H, SCOTT K.Investigation of Ti mesh-supported anodes for direct borohydride fuel cells. Journal of Applied Electrochemistry, 2006, 36(12): 1361-1366. |
| [54] | LI S, YANG X D, ZHU H Y,et al Investigation of amorphous CoB alloy as the anode catalyst for a direct borohydride fuel cell.Journal of Power Sources, 2011, 196(14): 5858-5862. |
| [55] | LI S, CHEN Y Z, YANG X D,et al Amorphous metal borides used as anode catalyst for DBFC.Battery Bimonthly, 2013, 43(6): 325-328. |
| [56] | LI S, WANG L N, CHU J,et al Investigation of Au@Co-B nanoparticles as anode catalyst for direct borohydride fuel cells.International Journal of Hydrogen Energy, 2016, 41(20): 8583-8588. |
| [57] | LEE S M, KIM J H, LEE H,et al The characterization of an alkaline fuel cell that uses hydrogen storage alloys.Journal of the Electrochemical Society, 2002, 149(5): A603-A606. |
| [58] | WANG L B, MA C A, MAO X B,et al Rare earth hydrogen storage alloy used in borohydride fuel cells.Electrochemistry Communications, 2005, 7(12): 1477-1481. |
| [59] | WANG L B, MA C A, MAO X B.LmNi4.78Mn0.22 alloy modified with Si used as anodic materials in borohydride fuel cells.Journal of Alloys and Compounds, 2005, 397(1/2): 313-316. |
| [60] | YANG Z Z, WANG L B, GAO Y F,et al. LaNi4.5Al0.5 alloy doped with Au used as anodic materials in a borohydride fuel cell. Journal of Power Sources, 2008, 184(1): 260-264. |
| [61] | GRAS M, SIERCZYNSKA A, LOTA K,et al The modification of anode material for direct borohydride fuel cell.Ionics, 2016, 22(12): 2539-2544. |
| [62] | LI S, YANG X D, ZHU H Y,et al Hydrogen storage alloy and carbon nanotubes mixed catalyst in a direct borohydride fuel cell.Journal of Materials Science & Technology, 2011, 27(12): 1089-1093. |
| [63] | WANG G L, CHENG Y H, ZHANG W C,et al. Electrocatalytic performances of MmNi3.2Al0.2Mn0.6Co1.0 modified with MnO2 for NaBH4 oxidation. Chemical Journal of Chinese Universities, 2010, 31(1): 153-156. |
| [64] | SAN F G B, KARADAG C L, OKUR O,et al.Optimization of the catalyst loading for the direct borohydride fuel cell. Energy, 2016, 114: 214-224. |
| [65] | WANG L B, MA C N, SUN Y M,et al. AB5-type hydrogen storage alloy used as anodic materials in borohydride fuel cell. Journal of Alloys Compounds, 2005, 391(1/2): 318-322. |
| [66] | RAMAN R K, SHUKLA A K.Electro-reduction of hydrogen peroxide on iron tetramethoxy phenyl porphyrin and lead sulfate electrodes with application in direct borohydride fuel cells.Journal of Applied Electrochemistry, 2005, 35(11): 1157-1161. |
| [67] | WANG Y G, XIA Y Y.A direct borohydride fuel cell using MnO2- catalyzed cathode and hydrogen storage alloy anode.Electrochemistry Communications, 2006, 8(11): 1775-1778. |
| [68] | RAMAN R K, PRASHANT S K, SHUKLA A K.A 28-W portable direct borohydride-hydrogen peroxide fuel-cell stack.Journal of Power Sources, 2006, 162(2): 1073-1076. |
| [69] | MA J F, WANG J, LIU Y N.Iron phthalocyanine as a cathode catalyst for a direct borohydride fuel cell.Journal of Power Sources, 2007, 172(1): 220-224. |
| [70] | RAMAN R K, SHUKLA A K.A direct borohydride/hydrogen peroxide fuel cell with reduced alkali crossover.Fuel Cells, 2007, 7(3): 225-231. |
| [71] | MA J F, LIU Y N, ZHANG P,et al A simple direct borohydride fuel cell with a cobalt phthalocyanine catalyzed cathode.Electrochemistry Communications, 2008, 10(1): 100-102. |
| [72] | SELVARANI G, PRASHANT S K, SAHU A K,et al.A direct borohydride fuel cell employing Prussian Blue as mediated electron- transfer hydrogen peroxide reduction catalyst. Journal of Power Sources, 2008, 178(1): 86-91. |
| [73] | MA J, LIU Y, LIU Y,et al. A membraneless direct borohydride fuel cell using LaNiO3-catalysed cathode. Fuel Cells, 2008, 8(6): 394-398. |
| [74] | CHOUDHURY N A, SAHAI Y, BUCHHEIT R G.Chitosan chemical hydrogel electrode binder for direct borohydride fuel cells.Electrochemistry Communications, 2011, 13(1): 1-4. |
| [75] | LI Z P, LIU B H, ARAI K,et al A fuel cell development for using borohydrides as the fuel.Journal of the Electrochemical Society, 2003, 150(7): A868-A872. |
| [76] | LI Z P, LIU B H, ARAI K,et al.Evaluation of alkaline borohydride solutions as the fuel for fuel cell. Journal of Power Sources, 2004, 126(1/2): 28-33. |
| [77] | CHOUDHURY N A, RAMAN R K, SAMPATH S,et al An alkaline direct borohydride fuel cell with hydrogen peroxide as oxidant.Journal of Power Sources, 2005, 143(1/2): 1-8. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 李娜, 曹锐霄, 魏进, 周晗, 肖红梅. 铁基正仲氢转化催化剂的影响因素[J]. 无机材料学报, 2025, 40(1): 47-52. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||