无机材料学报 ›› 2015, Vol. 30 ›› Issue (7): 683-693.DOI: 10.15541/jim20140648 CSTR: 32189.14.10.15541/jim20140648
王丹军1, 2, 张 洁1, 郭 莉1, 申会东1, 付 峰1, 薛岗林2, 方轶凡1
收稿日期:2014-12-16
修回日期:2015-01-17
出版日期:2015-07-20
网络出版日期:2015-06-25
基金资助:WANG Dan-Jun1, 2, ZHANG Jie1, GUO Li1, SHEN Hui-Dong1, FU Feng1, XUE Gang-Lin2, FANG Yi-Fan1
Received:2014-12-16
Revised:2015-01-17
Published:2015-07-20
Online:2015-06-25
Supported by:摘要:
作为一种新型的环境净化技术, 半导体光催化技术已引起全世界范围的广泛关注。然而, 传统光催化剂对太阳能利用率低、且光生电子-空穴对易于复合, 极大限制了该技术的实际应用。因此, 通过不同的改性手段合成具有可见光响应活性的光催化材料成为光催化领域研究的热点课题。提高光催化剂的活性, 除了合成方法的优选(调控尺寸、形貌、结晶度、微结构)外, 改性也是提高催化剂活性的主要手段。本文从半导体光催化的基本原理出发, 概述了半导体光催化剂改性的基本思想: 即拓宽太阳光利用范围和提高光生电子-空穴的寿命。围绕这一思想, 常用的改性策略有化学结构调控(能带调控), 以拓宽光谱响应范围; 表面修饰(表面敏化、半导体耦合和贵金属沉积)以提高电荷的分离效率。合适的能带结构是拓展催化剂的可见光响应范围和提高电荷分离效率的关键。
中图分类号:
王丹军, 张 洁, 郭 莉, 申会东, 付 峰, 薛岗林, 方轶凡. 基于能带结构理论的半导体光催化材料改性策略[J]. 无机材料学报, 2015, 30(7): 683-693.
WANG Dan-Jun, ZHANG Jie, GUO Li, SHEN Hui-Dong, FU Feng, XUE Gang-Lin, FANG Yi-Fan. Modification Strategies for Semiconductor Photocatalyst Based on Energy Band Structure Theory[J]. Journal of Inorganic Materials, 2015, 30(7): 683-693.
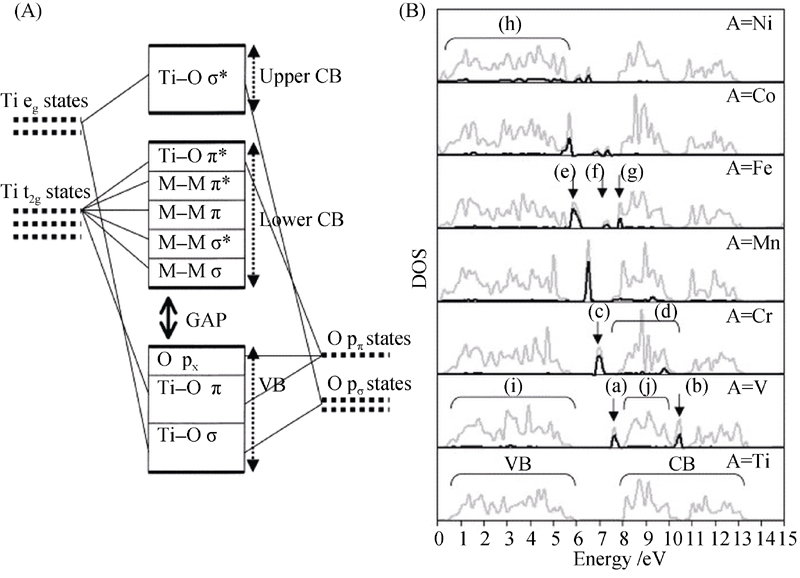
图5 (A)TiO2的成键图和(B)金属离子掺杂TiO2-xAxO2 (A=V、Cr、Mn、Fe, Ni)的态密度分布[9]
Fig. 5 (A) Bonding diagram of TiO2 and (B) DOS of metal-doped TiO2-xAxO2 (A=V、Cr、Mn、Fe、Co, or Ni)[9]
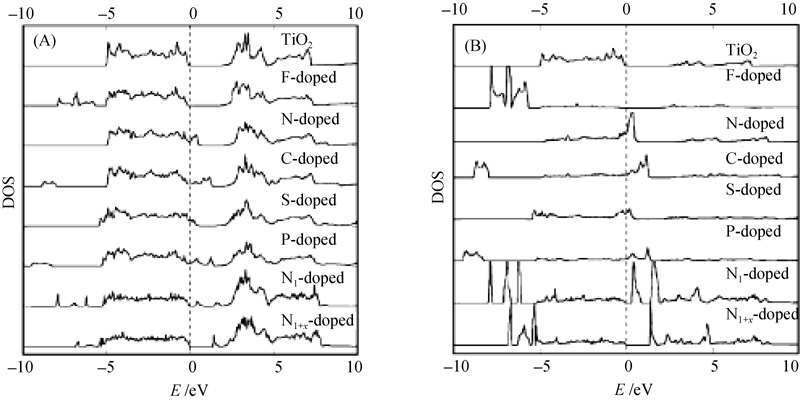
图6 (A)掺杂TiO2的总态密度分布和(B)阴离子取代锐钛矿型TiO2的氧原子态密度分布[10]
Fig. 6 (A) Total DOSs of doped TiO2 and (B) DOSs of the dopants anion located at a substitutional site for O atom in the anatase TiO2 crystal[10] Ni-doped stands for N doping at an interstitial sites, and Ni-s-doped stands for doping at both substitutional and interstitial sites
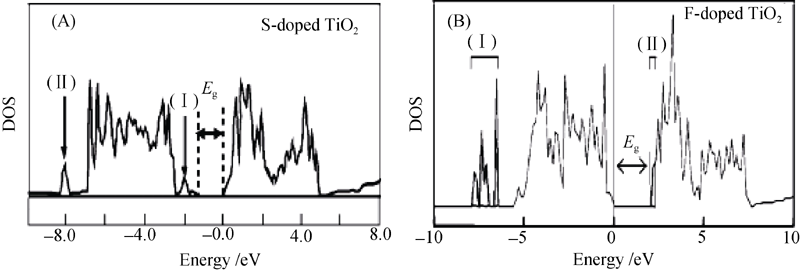
图 7 (A)S掺杂TiO2的总态密度分布和(B)F掺杂TiO2的总态密度分布[18]
Fig. 7 (A) Total DOS of S-doped TiO2 and (B) total DOS of F-doped TiO2[18] Eg indicates the band gap energy. The impurity states are labeled (I) and (II)
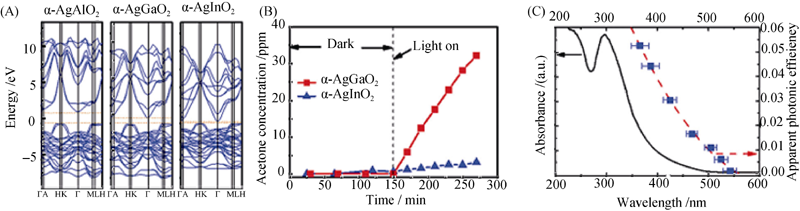
图8 系列α-AgMO2 (M=Al, Ga, In)催化剂的电子结构和光催化活性比较[28]
Fig. 8 Serial electronic structures of α-AgMO2(M=A1, Ga, In) and comparation of their photo catalytic activity (A) Electronic structures of α-AgMO2 (M=Al, Ga, In), (B) Photocatalytic degradation of isopropanol using α-AgGaO2 and α-AgInO2 under visible light irradiation (400 nm<λ<520 nm) and (C) Apparent photonic efficiency of acetone evolution using α-AgGaO2 for various wavelength ranges within the UV-visible absorption spectrum[28]
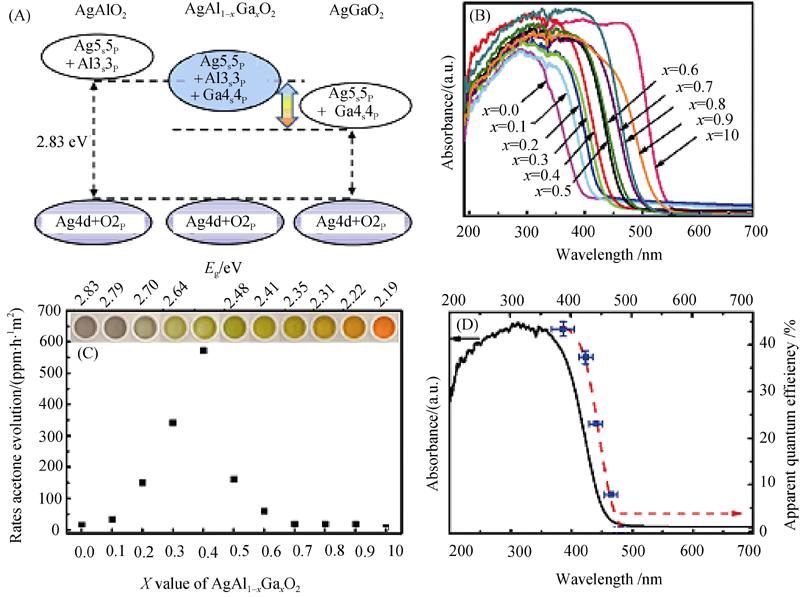
图11 固溶体AgAlO2、AgGaO2和AgAl1-xGaxO2的能带结构示意图(A), 固溶体β-AgAl1-xGaxO2吸收光谱(B), 丙酮产生速率、带隙以及β-AgAl1-xGaxO2 的颜色随组成的变化(C)和异丙醇在β-AgAl1-xGaxO2 上降解的表观量子产率(D)[32]
Fig. 11 (A)Schematic electronic structures of AgAlO2, AgGaO2 and AgAl1-xGaxO2 solid solutions; (B) UV-visible absorption spectra of β-AgAl1-xGaxO2 solid solutions; (C) Rate of acetone evolution, band-gap, and color of β-AgAl1-xGaxO2 as a function of x; (D) Apparent quantum efficiency of IPA photodegradation over β-AgAl0.6Ga0.4O2 in various wavelength ranges within the V-visible absorption spectrum[32]
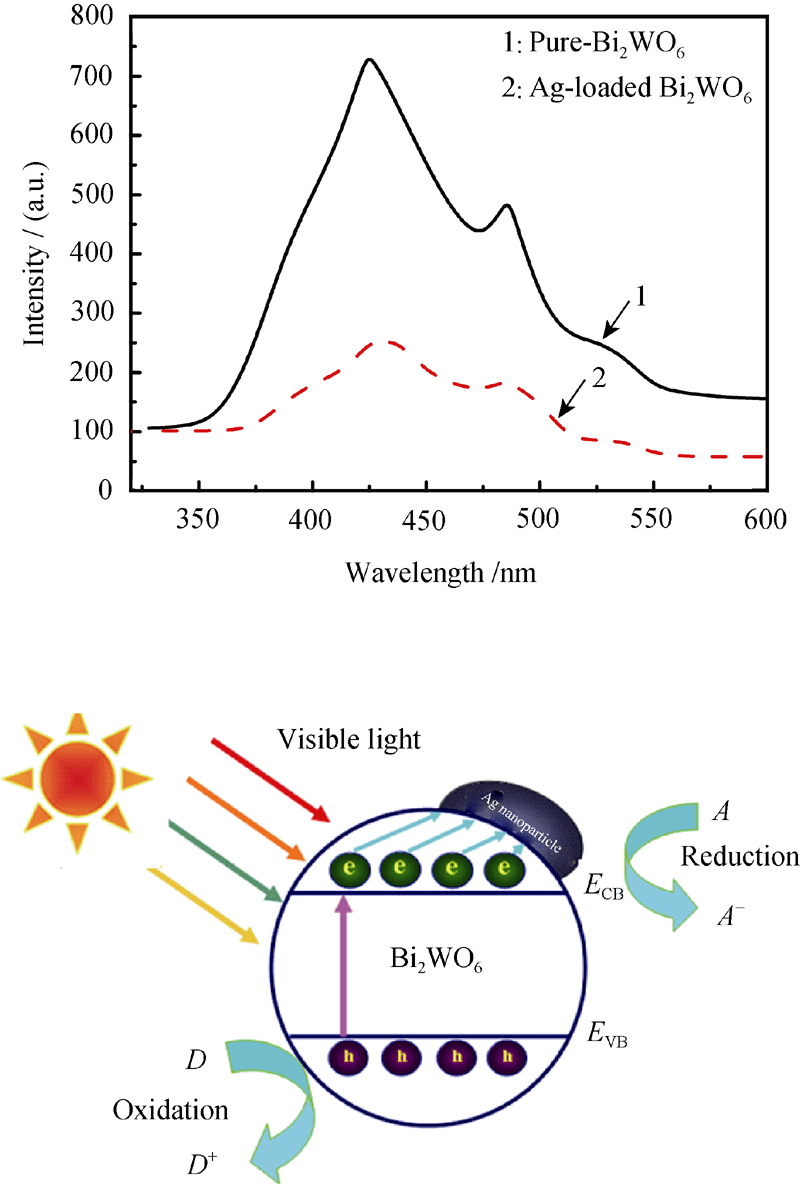
图14 Ag负载Bi2WO6光催化剂的光催化活性增强机理[48]
Fig. 14 Photocatalytic activity enhancement of Ag-loaded Bi2WO6[48] (Left: room temperature photoluminescence (PL) spectra of Bi2WO6 (a), and Ag-loaded Bi2WO6 nanoarchitecture, λexcitation=300 nm; Right: Energy band diagram and photocatalytic scheme of the Ag-loaded Bi2WO6)
| [1] | FUJISHIMA A, HONDA K.Electrochemical photocatalysis of water at a semiconductor electrode.Nature, 1972, 238(5358): 37-38. |
| [2] | CAREY J, LAWRENCE J, TOSINE H B.Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspension.Bull. Envirn. Contamin.Toxi., 1976, 16(6): 697-701. |
| [3] | FRANK S, BARD A.Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder.J. Am. Chem. Soc., 1977, 99(1): 303-304. |
| [4] | CHEN X B, SHEN S H, GUO L J, et al.Semiconductor-based photocatalytic hydrogen generation.Chem. Rev., 2010, 110(11): 6503-6570. |
| [5] | CHOI W, TERMIN A, HOFFMANN M R.Effect of metal-ion dopants on the photocatalytic reactivity of quantum-sized TiO2 particles.Angew. Chem. Int. Ed., 1994, 33(10): 1091-1092. |
| [6] | LI F B, LI X Z, HOU M F.Photocatalytic degradation of 2-mercaptobenzothiazole in aqueous La3+-TiO2 suspension for odor control.Appl. Catal. B 2004, 48(3): 185-194. |
| [7] | NAGAVENI K, HEGDE M S, MADRAS G.Structure and photocatalytic activity of Ti1-xMxO2+δ (M=W, V, Ce, Zr, Fe and Cu) synthesized by solution combustion method.J. Phys. Chem. B., 2004, 108(40): 20204-24212. |
| [8] | SORANTIN P I, SCHWARZ K.Chemical bonding in rutile-type compounds.Inorg. Chem., 1992, 31(4): 567-576. |
| [9] | UMEBAYASHI T, YAMAKI T, ITOH H, et al.Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations.J. Phys. Chem. Solids, 2002, 63(10): 1909-1920. |
| [10] | ASAHI R, MORIKAWA T, OHWAKL T, et al.Visible-light photocatalysis in nitrogen-doped titanium oxides.Science, 2001, 293(5528): 269-271. |
| [11] | YIN S, ZHANG Q, SAITO F, et al.Preparation of visible- activated titania photocatalyst by mechanochemical method.Mater. Letters, 2003, 32(4): 358-359. |
| [12] | CHEN X, WANG X, HOU Y, et al.The effect of post-nigridation annealing on the surface property and photocatalytic performance of N-doped TiO2 under visible-light irradation.J. Catal., 2008, 255(1): 59-67. |
| [13] | OHNO T, MITSUI T, MATSUMURA M.Photocatalytic activity of S-doped TiO2 photocatalyst under visible light.Chem. Lett., 2003, 32(4): 364-365. |
| [14] | NAKANO Y, MORIKAWA T, OHWAKI T, et al. Electrical characterization of band gap states in C-doped TiO2 films. Appl. Phys. Lett., 2005, 87(5): 052111-1-3. |
| [15] | LEE J Y, PARK J, CHO J H. Electronid properties of N- and C-doped TiO2. Appl. Phys. Lett., 2005, 87(1): 011904-1-3. |
| [16] | DI V C, Pacchion G, Selloni A, et al.Characterization of paramagnetic species in N-doped TiO2 powders by ERR spectroscopy and DFT calculations.J. Phys. Chem. B, 2005, 109(23): 11414-11419. |
| [17] | UMEBAYASHI T, YAMAKI T, YAMAMOTO S, et al.Sulf-doping of rutile-titanium dioxide by ion implantation: photocurent specctroscopy and firsr-principles band clculation studies.J. Appl. Phys., 2003, 93(9): 5156-5160. |
| [18] | YAMAKI T, UMEBAYASHI T, SUMITA T, et al.Fluorine-doping in titanium dioxide by ion implantation technology.Nucl. Instrum.Methods Phys. Res.Sect. B: Beam Interact. Mater., 2003, 206: 254-258. |
| [19] | YU J C, LI G S, WANG X C, et al.An ordered cubic Im3m mesoporous Cr-TiO2 visible light photocatalyst.Chem. Commun., 2006, 25: 2717-2719. |
| [20] | BURDA C, LUO Y B, CHEN X B, et al.Enhanced nitrogen doping TiO2 nanoparticles.Nano Letters, 2003, 3(8): 1049-1051. |
| [21] | LIVRAGHI S, PAGANINI M C, GIAMELLO E, et al.Origin of photoactivity of nitrogen-doped titanium dioxide under visible light.J. Am. Chem. Soc., 2006, 128(46): 15666-15671. |
| [22] | KUMAR S, BARUAH A, TONDA S, et al.Cost-effective eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis.Nanoscale, 2014, 6(9): 4830-4842. |
| [23] | TANG J W, ZOU Z G, YE J H.Effect of substituting Sr2+ and Ba2+ for Ca2+ on the structural properties and photocatlytic behaviors of CaIn2O4.Chem. Mater., 2004, 16(9): 1644-1649. |
| [24] | YIN J, ZOU Z G, YE J H.A novel series of the new visible-light driven photocatalysts MCo1/3Nb2/3O3 (M=Ca, Sr, Ba) with special electronic structures.J. Phys. Chem. B., 2003, 107(21): 4936-4941. |
| [25] | ZHANG W F, TANG J W, YE J H.Structural, photocatalytic, and photophysical properties of perovskite MSnO3 (M=Ca, Sr, and Ba ).J. Mater. Res., 2007, 22(7): 1859-1871. |
| [26] | SATO J, KOBAYASHI H, SAITO N, et al.Photocatalytic activities for water decomposition of RuO2-loaded AinO2 (A=Li, Na) with d10 configuration.J. Photochem. Photobio. A, 2003, 158(2/3): 139-144. |
| [27] | ZOU Z G., YE J H, ARAKAWA H.Subsitution effects of In3+ by Al3+ and Ga3+ on the photocatalytic and structureal properties of the Bi2InNbO7 photocatalyst.Chem. Mater., 2001, 13(5): 1765-1769. |
| [28] | OUYANG S X, KIKUGAWA N, CHEN D, et al.A systematical study on photocatalytic properties of AgMO2 (M=Al, Ga, In): effects of chemical compositions, crystal structures, and electronic structures.J. Phys. Chem. C, 2009, 113(4): 1560-1566. |
| [29] | BI Y P, OUYANG S X, UMEZAWA N, et al.Facet effect of single- crystalline Ag3PO4 sub-microcrystals on photocatalytic properties.J. Am. Chem. Soc., 2011, 133(17): 6490-6492. |
| [30] | BI Y P, HU H Y, JIAO Z B, et al.Two-dimensional dendritic Ag3PO4 nanotructures and their photocatalytic properties.Phys. Chem. Chem. Phys., 2012, 14: 14486-14488. |
| [31] | MAEDA K, DOMEN K.Solid Solution of GaN and ZnO as a stable photocatalyst for overall water splitting under visible light.Chem. Mater., 2010, 22(3): 612-623. |
| [32] | OUANG S X, YE J H.β-AgAl1-xGaxO2 solid-solution photocatalysts: continuous modulation of electronic structure toward high-performance visible-light photoactivity. J. Am. Chem. Soc., 2011, 133(20): 7757-7763. |
| [33] | HARA K, SATO T, KATOH R, et al.Novel conjugated organic dyes for efficient dye-sensitized solar cell.Adv. Funct. Mater., 2005, 15(2): 246-252. |
| [34] | ABE R, SAYAMA K, SUGIHARA H.Development of new photocatalytic water splitting into H2 and O2 using two different semiconductor photocatalysts and a shuttle redox mediator IO3-/I-.J. Phys. Chem. B, 2005, 109(33): 16052-16061. |
| [35] | MATSUMOTO Y, UNAL U, TANAKA N, et al.Electrochemical approach to evaluate the mechanism of photocatalytic water splitting on oxide photocatalysts.J. Solid State Chem., 2004, 177(110): 4205-4212. |
| [36] | MAEDA K, KURIKI R, ZHANG M W, et al.The effect of the pore-wall structure of carbon nitride on photocatalytic CO2 reduction under visible.J. Mater. Chem. A, 2014, 2(36): 15416-16151. |
| [37] | SANT P A, KAMAT P V.Inter-particle electron transfer between size quantized CdS and TiO2 semiconductor nanoclusters.Phys. Chem. Chem. Phys., 2002, 4(2): 198-203. |
| [38] | ROBEL I, SUBRAMANIAN V, KUNO M, et al.Quantum dot solar cells. Havrvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films.J. Am. Chem. Soc., 2006, 128(7): 2385-2393. |
| [39] | WANG D J, GUO L, ZHEN Y Z, et al.AgBr quantum dots decorated mesoporous Bi2WO6 architectures with enhanced photocatalytic activities for methylene blue. J. Mater. Chem. A, 2014, 2(30): 11716-11727. |
| [40] | YU J G, WANG S H, LOW J X, et al.Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air.Phys. Chem. Chem. Phys., 2013, 15(39): 16883-16890. |
| [41] | LI Y J, CAO T P, SHAO C L, et al.Preparation and photocatalytic properties of γ-Bi2O3/TiO2 composite fibers. J. Inorg. Mater., 2012, 27(7): 687-692. |
| [42] | MEI Z W, OUYANG S X, TANG D M, et al.An ion-exchange route for the synthesis of hierarchical In2S3/ZnIn2S4 bulk composite and its photocatalytic activity under visible-light irradiation.Dalton Trans., 2013, 42(8): 2687-2690. |
| [43] | JIANG D L, CHEN L L, XIE J M, et al.Ag2S/g-C3N4 composite photocatalysts for efficient Pt-free hydrogen production. The co-catalyst function of Ag/Ag2S formed by simultaneous photodeposition.Dalton. Trans., 2014, 43(12): 4878-4885. |
| [44] | LIN F, WANG D E, JIANG Z X, et al.Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalyst Pt and RuO2 under visible light irrdiation using molecular oxygen as oxidant.Energy Environ. Sci., 2012, 5: 6400-6406. |
| [45] | DENG D S, YANG Y, GONG Y T.Palladium nanoparticles supported on mpg-C3N4 as active catalyst for semihydrogenation of phenylacetylene under mild conditions.Green Chem., 2013, 15(9): 2525-2531. |
| [46] | KAWAHARA K, SUZUKI K, OHKO Y, et al.Electron transport in silver semiconductor nanocomposite films exhibiting multicolor photochromism.Phys. Chem. Chem. Phys., 2005, 7(22): 3851-3855. |
| [47] | TIAN Y, TATSUMA T.Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc., 2005, 127(20): 7632-7637. |
| [48] | WANG D J, XUE G L, ZHEN Y Z, et al.Monodispersed Ag nanoparticles loaded on the surface of spherical Bi2WO6 nanoarchitectures with enhanced photocatalytic activities.J. Mater. Chem., 2012, 22(11): 4751-4758. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 孙晶, 李翔, 毛小建, 章健, 王士维. 月桂酸改性剂对氮化铝粉体抗水解性能的影响[J]. 无机材料学报, 2025, 40(7): 826-832. |
| [3] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [4] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [5] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [6] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [7] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [8] | 陈莉波, 盛盈, 伍明, 宋季岭, 蹇建, 宋二红. Na和O元素共掺杂氮化碳高效光催化制氢[J]. 无机材料学报, 2025, 40(5): 552-562. |
| [9] | 陈义, 邱海鹏, 陈明伟, 徐昊, 崔恒. SiC/SiC复合材料基体硼改性方法及其力学性能研究[J]. 无机材料学报, 2025, 40(5): 504-510. |
| [10] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [11] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [12] | 李建军, 陈芳明, 张梨梨, 王磊, 张丽亭, 陈慧雯, 薛长国, 徐良骥. CoFe2O4/MgAl-LDH催化剂活化过氧一硫酸盐促进抗生素降解[J]. 无机材料学报, 2025, 40(4): 440-448. |
| [13] | 梁锐辉, 钟鑫, 洪督, 黄利平, 牛亚然, 郑学斌. Yb2O3改性硅黏结层的环境障涂层体系耐高温水氧腐蚀行为研究[J]. 无机材料学报, 2025, 40(4): 425-432. |
| [14] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [15] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||