|
|
Strategic Study on the Development of Inorganic Non-metallic Biomaterials
CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng
2025 Vol. 40 (5): 449–456
 Abstract
Abstract(
1150 )
 HTML
HTML(
64)
 PDF
PDF(910KB)(
2307
)
Inorganic non-metallic biomaterial is one of main types of biomaterials, which is widely used in biomedical fields such as tissue repair, tumor therapy, and drug delivery., making an important contribution to national life and health. Research on inorganic non-metallic biomaterials in China is flourishing, but their production and application are still in the stage of overcoming difficulties. To realize the high-quality development of China's inorganic non-metallic biomaterials and improve their hard power to protect national life and health, this paper analyzes hotspots and difficult problems in research and application of China's inorganic non-metallic biomaterials by means of strategic study. Based on current development opportunities and challenges, some suggestions are proposed for the development of inorganic non-metallic biomaterials, such as material design for unique performance, research on materiobiology, exploration of new principles and mechanisms mediated by materials, customization by intelligent personalization, design through big data screening and artificial intelligence, and standardization based evaluation/regulation. This aims to provide guidance for development of inorganic non-metallic biomedical products and push forward scientific research while accumulating talent resources.

|
|
|
Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance
AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao
2025 Vol. 40 (5): 457–465
 Abstract
Abstract(
694 )
 HTML
HTML(
42)
 PDF
PDF(9370KB)(
1380
)
Nano-hydroxyapatite (nHAP) possesses both biocompatibility and environmental friendliness, and holds the potential to become a novel ultraviolet (UV) absorbent material following iron doping modification. This study employed co-precipitation and hydrothermal methods to prepare iron-doped nano-hydroxyapatite (Fe-nHAP). Influence of the preparation process on UV absorption performance was investigated by adjusting reaction time, temperature, and iron doping ratio. The results indicate that with the increase of temperature from 37 ℃ to 150 ℃ or the extension of reaction time from 0.5 h to 3 h, both the crystallinity and the peak of UV absorption improve. It can be inferred that there is a certain positive correlation between the UV absorption performance and crystallinity of Fe-nHAP. Additionally, the UV absorption capacity is closely correlated to the iron doping ratio. As the iron doping molar ratio increases from 0 to 10%, the UV absorption capacity is gradually enhanced, with the maximum absorption value rising from 0.03 to 1.35. This phenomenon is attributed to the reduction in optical bandgap caused by iron doping. However, a high iron doping ratio leads to excessive reduction in material crystallinity, resulting in weakened enhancement of UV absorption performance. The safety assessment indicates that Fe-nHAP with an iron doping molar ratio of 7% does not demonstrate cytotoxicity, phototoxicity and skin irritation. In summary, Fe-nHAP has a suitable UV absorption performance. With its favorable biosecurity, it is anticipated to emerge as a new type of UV absorption material.

|
|
|
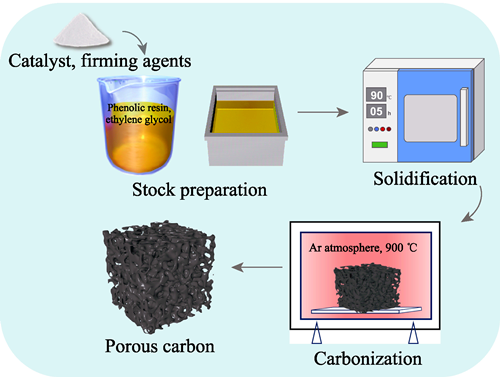
Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol
GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren
2025 Vol. 40 (5): 466–472
 Abstract
Abstract(
578 )
 HTML
HTML(
33)
 PDF
PDF(1503KB)(
1223
)
Microporous structure is crucial to the properties and applications of porous carbon materials, but how to modulate it by an ion catalyst faces a complex situation. Here, a uniform porous carbon was obtained from phenolic resin/ethylene glycol through polymerization-induced phase separation (PIPS) method. Meanwhile, the influences of Zn2+ content and curing temperature on the microporous structure of porous carbon were studied. Regarding curing temperature, it was observed that the stability of porous carbon decreased with increasing temperature, adversely affecting the uniformity of microporous structure. At a curing temperature of 90 ℃, porosity, mean pore size, and median pore size of the porous carbon varied from 40.22% to 70.38%, 49.8 nm to 279.4 nm, and 107.2 nm to 343.0 nm, respectively. Concerning Zn2+ content, an initial increase was noted in porosity, median pore size and average pore size of the porous carbon with rising Zn2+ content, followed by a decrease. Specifically, with 1.5% (in mass) Zn2+, the maximum pore size and porosity reached 343.0 nm and (70.38±0.37)%, respectively. These findings show that addition of Zn2+ increases the curing degree and backbone polymerization, which may be attributed to a reduction in the reaction barrier for interstitial substitution of phenol structures. However, excessive Zn2+ content leads to high polymerization levels in the resin mixture, impeding volatilization of the alcohol-rich phase and thus degrading the pore structure. In addition, introduction of Zn2+ promotes graphitization, resulting in a more pronounced carbon skeleton than that of non-introduced sample. This research provides a theoretical basis for modulating the microstructure of porous carbon materials and preparation of structural carbide ceramics.
|
|
|
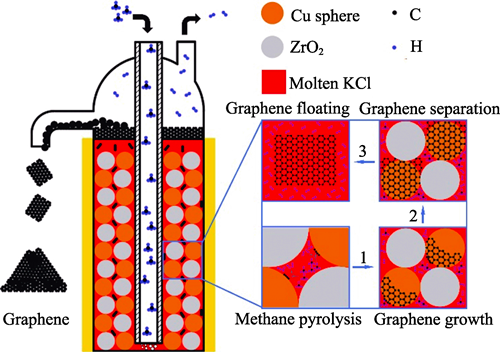
Co-production of Few-layer Graphene and Hydrogen from Methane Pyrolysis Based on Cu and Metal Oxide-KCl Molten Medium
YANG Mingkai, HUANG Zeai, ZHOU Yunxiao, LIU Tong, ZHANG Kuikui, TAN Hao, LIU Mengying, ZHAN Junjie, CHEN Guoxing, ZHOU Ying
2025 Vol. 40 (5): 473–480
 Abstract
Abstract(
821 )
 HTML
HTML(
41)
 PDF
PDF(19319KB)(
547
)
Methane pyrolysis is a technology that utilizes fossil energy to produce high added value carbon materials and hydrogen. However, traditional methods, such as chemical vapor deposition (CVD) and molten metal catalysis, face challenges in the production of graphene, including catalyst deactivation, difficulty in separating graphene from the catalyst, and high reaction temperatures (≥1100 ℃), which limit their industrial applications. This study proposes an innovative approach to produce graphene by catalyzing methane pyrolysis using Cu and metal oxides-KCl molten medium. By adding metal oxides (Al2O3, TiO2, ZrO2, MgO, SiO2) as dispersants, the dispersion of active Cu sites is enhanced. Notably, Cu/ZrO2 with a Cu content of 50% (in volume) and Cu/MgO with a Cu content of 75% (in volume) catalysts enable the efficient production of few-layer graphene. Cu/ZrO2 catalyst with a Cu content of 50% (in volume) exhibits the highest activity, achieving a methane conversion rate of 22%, a hydrogen production yield of 21.5 mmol/h, and formation of large-area and smooth few-layer graphene. This study provides a new technical route for co-production of graphene and hydrogen via methane pyrolysis, offering potential for large-scale graphene production in the future.
|
|
|
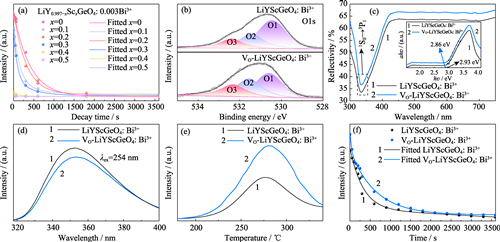
Organic Pollutant Fenton Degradation Driven by Self-activated Afterglow from Oxygen-vacancy-rich LiYScGeO4: Bi3+ Long Afterglow Phosphor
FAN Xiaoxuan, ZHENG Yonggui, XU Lirong, YAO Zimin, CAO Shuo, WANG Kexin, WANG Jiwei
2025 Vol. 40 (5): 481–488
 Abstract
Abstract(
463 )
 HTML
HTML(
28)
 PDF
PDF(3715KB)(
2260
)
Self-activated long afterglow photocatalysts show great potential for all-weather wastewater treatment, with sustained photocatalytic activity even under dark conditions. However, the radiative combination of afterglow luminescence and photocatalytic degradation reaction has competitive utilization for photogenerated carriers, reducing afterglow duration and generating excessive hole accumulation, which significantly limits the efficiency of long afterglow driven photocatalytic degradation. Here, a long afterglow photocatalyst LiYGeO4: Bi3 based on oxygen vacancy (VO) was prepared, which released ultraviolet afterglow after activation by ultraviolet light irradiation and degraded organic pollutants via photocatalytic degradation driven by its own afterglow in dark condition. The trap concentration was improved by engineering oxygen vacancies and crystal fields, significantly enhancing the afterglow duration and intensity of VO-LiYScGeO4: Bi3+. A Fenton reaction system was constructed to further increase the concentration of active species, which maximized the photocatalytic degradation efficiency of VO-LiYScGeO4: Bi3+ during the afterglow duration. After 10 min UV irradiation to activate VO-LiYScGeO4: Bi3+ continuously released ultraviolet afterglow for photocatalytic degradation of Rhodamine B (RhB), reaching a degradation efficiency of 63% within 1 h in Fenton environment, which increased by 3.5 folds when compared to that of LiYScGeO4: Bi3+ in the initial environment. This work provides a new approach for the design of afterglow photocatalysts and their application in wastewater treatment.
|
|
|
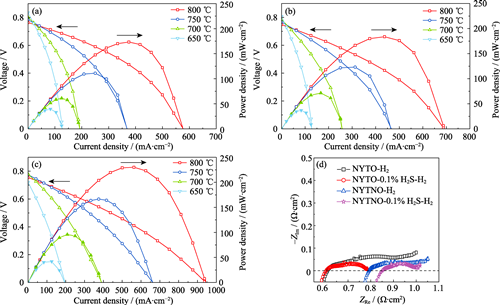
Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4
QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi
2025 Vol. 40 (5): 489–496
 Abstract
Abstract(
527 )
 HTML
HTML(
28)
 PDF
PDF(8059KB)(
1413
)
Solid oxide fuel cells (SOFCs) are highly efficient energy conversion devices. However, the sulfur poison, which deteriorates traditional Ni-based anodes, restricts commercialization of the technology. Here, layered perovskite oxide NaYTiO4 was prepared using solid-state method and modified by partial substitution with different valence ions. Properties of NaYTiO4 before and after doping were systematically studied. Ni was doped into the perovskite layer and contributed to forming NaYTi0.95Ni0.05O4, which regulated growth characteristics of the crystal and in-situ exsoluted in reduction conditions. Two-dimensional distribution of alkali metals and polar structures in the material provides advantages, including excellent chemical water absorption capacity and good sulfur-resistance. With an increased chemisorbed oxygen species of 64.5%, Ni-doped material becomes more outstanding. SOFC with NaYTi0.95Ni0.05O4 as composite anode showed superior electrocatalytic activity. The peak power density reached 183.8 mW·cm-2 at 800 ℃ with H2 as fuel. Furthermore, the power density increased by 25.2% with an addition of 0.1% H2S in H2. The modified cell could work stably even at 700 ℃, a more toxic condition, for 40 h without significant poisoning effect. These results indicate that the modified layered perovskite oxides are excellent sulfur-resistance anodes.
|
|
|
Structural Evolution and Electrochemical Performance of Na4FexP4O12+x/C Cathode Materials for Sodium-ion Batteries
WAN Junchi, DU Lulu, ZHANG Yongshang, LI Lin, LIU Jiande, ZHANG Linsen
2025 Vol. 40 (5): 497–503
 Abstract
Abstract(
668 )
 HTML
HTML(
27)
 PDF
PDF(6787KB)(
2251
)
The development of low-cost and long-lifespan sodium-ion battery (SIB) cathode materials is crucial for large-scale energy storage. Iron-based phosphate cathode materials have attracted significant attention in recent years for their high theoretical capacity, excellent structural stability and rich resources. Here, a series of Na4FexP4O12+x/C (x=2.6-3.3) electrode materials are prepared using Sol-Gel technique and thermal treatment process. Effect of the phase structure on electrochemical performance of Na4FexP4O12+x/C electrode materials is investigated. It is found that three phases, including Na2FeP2O7 (NFPO), Na4Fe3(PO4)2P2O7 (NFPP) and NaFePO4 (NFP), mainly exist in the Na4FexP4O12+x/C system. Among Na4FexP4O12+x/C electrode materials, Na4Fe3.1P4O15.1/C electrode material with the highest content of NFPP phase possesses rapid electronic and sodium-ion conduction characteristics, thereby exhibiting the optimal electrochemical performance. As a result, the SIB equipped with Na4Fe3.1P4O15.1/C electrode material shows high reversible capacity, with a discharge specific capacity of 102.8 mAh·g-1 at a current density of 0.1C (1C=129 mAh·g-1), as well as capacity retention of 88.7% after 700 cycles. Furthermore, the as-assembled battery exhibits excellent rate performance with a discharge specific capacity of 61.5 mAh·g-1 at a current density of 5C.

|
|
|
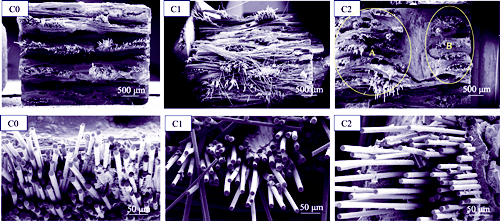
SiC/SiC Composite: Matrix Boron Modification and Mechanical Properties
CHEN Yi, QIU Haipeng, CHEN Mingwei, XU Hao, CUI Heng
2025 Vol. 40 (5): 504–510
 Abstract
Abstract(
736 )
 HTML
HTML(
66)
 PDF
PDF(5856KB)(
1337
)
SiC/SiC composites have emerged as essential thermal structure materials for development of hypersonic vehicles and high thrust-to-weight ratio aero-engines. Design and utilization of boron-containing ceramic precursors as impregnation agents for precursor infiltration and pyrolysis (PIP) to introduce self-healing components into matrix represent a key strategy for enhancing the antioxidant properties of SiC/SiC composites. Here, borane pyridine or borane triethylamine were utilized as boron sources and subsequently mixed with a solid polycarbosilane (PCS) xylene solution to prepare different boron-modified PCS solutions. These solutions were used as PIP impregnation agents to fabricate various boron-modified SiC/PyC (pyrolytic carbon)/SiC composites. The physicochemical properties of boron-modified PCS-derived ceramics, along with the physical and mechanical properties of SiC/PyC/SiC composites before and after matrix boron modification, were investigated. Results demonstrated that addition of appropriate amounts of borane pyridine and borane triethylamine as boron sources in solid PCS solutions effectively introduced boron as a heterogeneous element into the derived SiC ceramics. Compared to PCS, the boron-modified PCS solutions (BP-1 and BP-2) exhibited increased ceramic yields. The derived ceramics exhibited a semi-crystalline β-SiC structure, with boron element contents of 1.7% and 2.2% (in mass), respectively. In contrast to unmodified composite, the boron-modified SiC/SiC composites exhibited negligible changes in density, apparent porosity, and fracture toughness. However, the flexural modulus increased from 116 GPa to 132 GPa. Furthermore, the flexural strength of the modified composite using borane pyridine alone as boron source was 658 MPa, comparable to the unmodified composite's strength of 643 MPa, but with a reduced dispersion coefficient. All above data demonstrate that borane pyridine can be used as boron source for preparation of boron-modified SiC/SiC composites, providing valuable insights for developing high-performance SiC/SiC composite hot-end components.
|
|
|
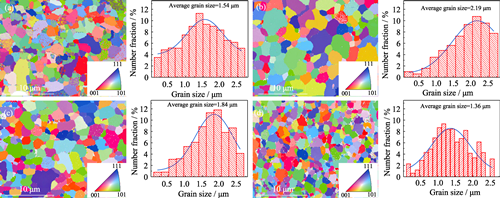
Single-phase Formation Process and Carbon Vacancy Regulation of (TiVNbMoW)Cx High-entropy Ceramics
CUI Ning, ZHANG Yuxin, WANG Lujie, LI Tongyang, YU Yuan, TANG Huaguo, QIAO Zhuhui
2025 Vol. 40 (5): 511–520
 Abstract
Abstract(
593 )
 HTML
HTML(
77)
 PDF
PDF(9005KB)(
1479
)
High-entropy transition metal carbides (HETMCs) have emerged as promising candidate materials in advanced structural application due to their superior physical and chemical properties compared to traditional carbides. Among these materials, (TiVNbMoW)C has garnered attention due to its outstanding mechanical properties and wear resistance. However, previous studies on the single-phase formation process of (TiVNbMoW)C and the effect of carbon vacancy concentration on its mechanical properties remain inadequate. In this study, TiC, VC, NbC, Mo2C, WC, elemental W powder, and graphite powder were innovatively selected as raw materials, and (TiVNbMoW)Cx with different carbon vacancy concentrations was successfully prepared by spark plasma hot pressing sintering technology. Effects of carbon vacancies on phase composition, phase evolution, microstructure, and mechanical property of the material were systematically studied. The results show that in the Ti-V-Nb-Mo-W-C system, the carbides corresponding to Mo, Ti, Nb and V elements began to dissolve into each other at 1500 ℃, forming (MoTiNbV)C phase. With increase of temperature, W element gradually participates in solid solution, resulting in densification of the material at 1700 ℃, and formation of (TiVNbMoW)C as a high entropy single phase occurs at 1800 ℃. The mass ratio of carbon to transition metals (C/TM) has a great influence on phase structure and microstructure of the material. When the C/TM is 0.7, the W element cannot dissolve sufficiently to form a single-phase structure, resulting in a composition consisting of (MoTiNbV)C and W2C phases. At a C/TM of 0.8, the sample exhibits a single-phase (TiVNbMoW)C structure characterized by a large number of carbon vacancies. At a C/TM of 0.9, carbon vacancies reach saturation. At a C/TM of 1.0, excessive carbon enrichment within the material results in a decreased degree of densification. An optimal concentration of carbon vacancies is beneficial for grain refinement and enhancement of the mechanical properties of materials. The sintered sample with a C/TM of 0.8 exhibits the highest hardness, elastic modulus and fracture toughness, demonstrating the most favorable integral mechanical properties. Therefore, this study provides an important basis for a comprehensive understanding of (TiVNbMoW)Cx high-entropy carbides. Future research may introduce additional elements to optimize material properties and broaden its application in high-end manufacturing and related fields.
|
|
|
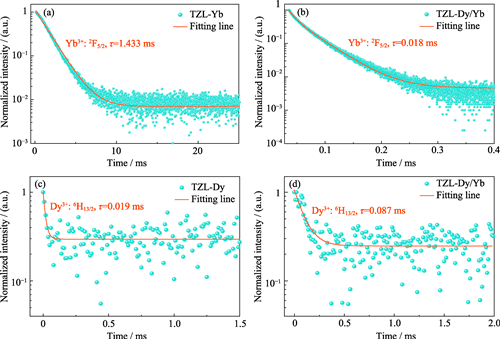
Broadband 3 μm Mid-infrared Emission in Dy3+/Yb3+ Co-doped Tellurite Glass under 980 nm LD Excitation
PAN Yuzhou, HE Fajian, XU Lulu, DAI Shixun
2025 Vol. 40 (5): 521–528
 Abstract
Abstract(
411 )
 HTML
HTML(
19)
 PDF
PDF(1102KB)(
991
)
Three to five μm mid-infrared laser has broad applications in atmospheric communication, environmental monitoring, medical treatment, and defense. Here, a series of glasses with compositions of 70TeO2-25ZnO-5La2O3, doped with Dy3+ or Yb3+, and co-doped with Dy3+/Yb3+, were prepared using the melt-quenching method in an inert atmosphere-protected glovebox. Thermal properties, structural characteristics, hydroxyl content, and mid-infrared luminescence of the glasses were characterized through measurements such as differential scanning calorimetry (DSC), X-ray diffraction (XRD), Raman spectra, transmission spectra, and 3 μm band fluorescence spectra. The results indicate that 70TeO2-25ZnO-5La2O3 glass possesses high resistance to crystallization (ΔT=101 ℃) and low phonon energy (760 cm-1). Under 980 nm laser diode (LD) excitation, Dy3+/Yb3+ co-doped tellurite glass produces a broadband fluorescence emission around 3 μm region, with a full width at half maximum (FWHM) of 326 nm. This is attributed to the high energy transfer efficiency from Yb3+ to Dy3+ (98.74%) and the low hydroxyl absorption coefficient near 3 μm (0.32 cm-1). Based on Judd-Ofelt and Dexter theories, spontaneous radiative transition probability, fluorescence branching ratio, and other spectroscopic parameters of Dy3+ ions, as well as microscopic parameters of Yb3+→Dy3+ energy transfer, were calculated. The primary energy transfer pathway is analyzed and identified as Yb3+: 2F5/2→Dy3+: 6H7/2, 6F9/2. This study demonstrates that the low-hydroxyl Dy3+/Yb3+ co-doped TeO2-ZnO-La2O3 glass can serve as an excellent 3 μm mid-infrared gain medium.
|
|
|
Nd:YLF Crystal Growth: Raw Materials Preparation by Melting Method and Property
ZHAO Kaixuan, LIU Wenpeng, DING Shoujun, DOU Renqin, LUO Jianqiao, GAO Jinyun, SUN Guihua, REN Hao, ZHANG Qingli
2025 Vol. 40 (5): 529–535
 Abstract
Abstract(
625 )
 HTML
HTML(
15)
 PDF
PDF(901KB)(
706
)
Nd3+-doped LiYF4 (Nd:YLF) crystal is a laser crystal with excellent performance, which is widely used in scientific research, industrial and medical fields. But its existing crystal growth method using binary fluoride mixtures is problematic due to fluoride oxides being formed in fluoride raw materials, and process of preparing raw materials by using HF gas fluorination is relatively complicated. Therefore, preparation of high-purity fluoride raw materials is one of the important factors to realize the growth of high-quality fluoride crystals. Meanwhile, crystal growth atmosphere usually contains CF4 or HF, which is highly corrosive to growth system and increases cost of crystal growth. In this work, to obtain high-quality Nd:YLF crystal, the polycrystalline growth raw material with high-purity YLF crystalline phase was first prepared using a nearly closed melting material device, which was a novel design facilitating the melting process and floating salvage process at the temperature above the crystal melting point. Intact Nd:YLF crystal was obtained after growth under a high-purity Ar atmosphere. X-ray diffraction (XRD) patterns of polycrystalline raw materials and growing crystals were tested, while lattice parameters, atomic coordinates, atomic occupancy and temperature factor were obtained by Rietveld refinement. By measuring X-ray rocking curve (XRC) of (100) crystallographic plane of the as-obtained Nd:YLF crystal, the full width at half maximum (FWHM) of diffraction peak is 0.007°. The segregation coefficient of Nd3+ in YLF calculated by measuring the content of each element is 0.3. The strongest absorption peaks of Nd:YLF crystal locate at 797.4 nm (a direction) and 792.3 nm (c direction) with absorption cross sections of 3.37×10-20 and 5.49×10-20 cm2, respectively. The strongest emission peak of Nd:YLF crystal locates at 1047 nm with stimulated emission cross section of 1.598×10-19 cm2 and fluorescence lifetime of 483 μs. Based on above data, Nd:YLF polycrystalline raw materials prepared by melting method achieve high phase purity. Combined with Czochralski method of crystal growth with vacuum extraction and heating process, the present growth method can greatly reduce the formation of fluoride oxide during the growth process, which proves a successful growth under Ar atmosphere.

|
|
|
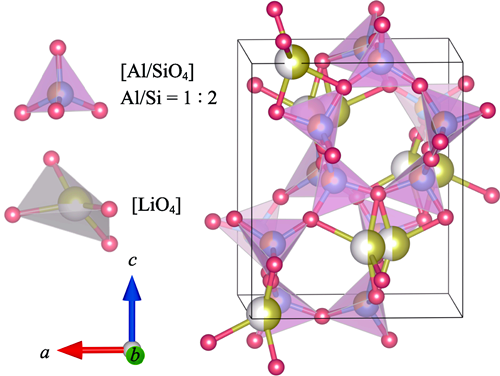
Low-temperature Sintering of LiBxAl1-xSi2O6 Microwave Dielectric Ceramics with Ultra-low Permittivity
XIONG Siyu, MO Chen, ZHU Xiaowei, ZHU Guobin, CHEN Deqin, LIU Laijun, SHI Xiaodong, LI Chunchun
2025 Vol. 40 (5): 536–544
 Abstract
Abstract(
693 )
 HTML
HTML(
9)
 PDF
PDF(2205KB)(
1180
)
The lithium-based silicate microwave dielectric ceramics with ultra-low permittivity show great potential as substrate materials in the fifth-generation wireless communication technology. However, the residual stress caused by higher sintering temperatures leads to increased dielectric loss, thereby deteriorating the microwave dielectric performance. In this work, B3+ was introduced into LiAlSi2O6 ceramics to reduce Al3+ content, aiming to improve their sintering temperature and microwave dielectric performance. LiBxAl1-xSi2O6 (0≤x≤0.20) microwave dielectric ceramics were prepared using a combination of solid-state reaction and cold isostatic pressing techniques. Effects of B3+ doping on the sintering characteristics, phase structure, microstructure, and microwave dielectric properties of the materials were characterized. The results show that with a gradual increase in the doping concentration, sintering temperature of the ceramics decreases significantly from 1400 to 1000 ℃. Meanwhile, the relative permittivity (εr) decreases from 3.95 to 3.69, the quality factor (Q×f) increases significantly from 24300 to 30560 GHz, and the temperature coefficient of resonant frequency (τf) increases from -45.9×10-6 to -20.9×10-6 ℃-1. Specifically, the change in εr is mainly influenced by intrinsic polarization, lattice vibrations, and covalent bond strength of the material; the improvement in Q×f is closely related to the increase in packing fraction (PF) and the decrease in damping coefficient; the increase in τf is strongly correlated with the bond valence of oxygen (VO). Furthermore, the composition with x = 0.20 exhibits the best microwave dielectric performance with εr = 3.69, Q×f = 30560 GHz, and τf = -20.9×10-6 ℃-1. Findings of this study on LiBxAl1-xSi2O6 provide important theoretical guidance and practical insights for development and application of high-performance microwave dielectric ceramics in the future.
|
|
|
Defect Dipole Thermal-stability to the Electro-mechanical Properties of Fe Doped PZT Ceramics
SUN Yuxuan, WANG Zheng, SHI Xue, SHI Ying, DU Wentong, MAN Zhenyong, ZHENG Liaoying, LI Guorong
2025 Vol. 40 (5): 545–551
 Abstract
Abstract(
705 )
 HTML
HTML(
13)
 PDF
PDF(5540KB)(
231
)
The accepted doping ion in Ti4+-site of PbZryTi1-yO3 (PZT)-based piezoelectric ceramics is a well-known method to increase mechanical quality factor (Qm), since the acceptor coupled by oxygen vacancy becomes defect dipole, which prevents the domain rotation. In this field, a serious problem is that generally, Qm decreases as the temperature (T) increases, since the oxygen vacancies are decoupled from the defect dipoles. In this work, Qm of Pb0.95Sr0.05(Zr0.53Ti0.47)O3 (PSZT) ceramics doped by 0.40% Fe2O3 (in mole) abnormally increases as T increases, of which the Qm and piezoelectric coefficient (d33) at room temperature and Curie temperature (TC) are 507, 292 pC/N, and 345 ℃, respectively. The maximum Qm of 824 was achieved in the range of 120-160 ℃, which is 62.52% higher than that at room temperature, while the dynamic piezoelectric constant (d31) was just slightly decreased by 3.85%. X-ray diffraction (XRD) and piezoresponse force microscopy results show that the interplanar spacing and the fine domains form as temperature increases, and the thermally stimulated depolarization current shows that the defect dipoles are stable even the temperature up to 240 ℃. It can be deduced that the aggregation of oxygen vacancies near the fine domains and defect dipole can be stable up to 240 ℃, which pins domain rotation, resulting in the enhanced Qm with the increasing temperature. These results give a potential path to design high Qm at high temperature.

|
|
|
Na and O Co-doped Carbon Nitride for Efficient Photocatalytic Hydrogen Evolution
CHEN Libo, SHENG Ying, WU Ming, SONG Jiling, JIAN Jian, SONG Erhong
2025 Vol. 40 (5): 552–562
 Abstract
Abstract(
542 )
 HTML
HTML(
15)
 PDF
PDF(5222KB)(
447
)
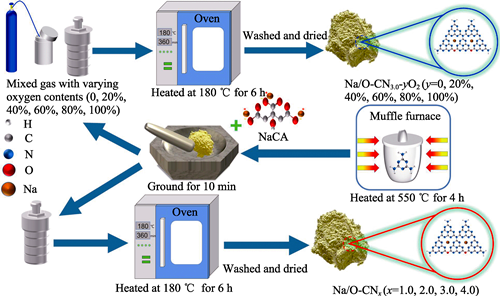
Elemental doping is an effective strategy for tuning the band structure of graphite carbon nitride (CN) to enhance its photocatalytic performance. In this study, sodium (Na) and oxygen (O) co-doped carbon nitride (Na/O-CNx, x=1.0, 2.0, 3.0, 4.0) was synthesized via solid-phase reaction of sodium citrate (NaCA) and pure CN powder in the Teflon-sealed autoclave under air conditions at 180 ℃. Surface area of Na/O-CN3.0 is measured to be 18.8 m2/g, increasing by 60.7% compared to that of pure CN (11.7 m2/g). Bandgap energy of Na/O-CN3.0 is determined to be 2.68 eV, marginally lower than that of pure CN (2.70 eV), thereby enhancing its capacity for sunlight absorption. Meanwhile, the incorporation of Na and O atoms into Na/O-CNx is found to effectively reduce recombination rates of photogenerated electron-hole pairs. As a result, Na/O-CNx samples exhibit markedly enhanced photocatalytic hydrogen evolution activity under visible light irradiation. Notably, the optimal Na/O-CN3.0 sample achieves a photocatalytic hydrogen production rate of 103.2 μmol∙g-1∙h-1, which is 8.2 times greater than that of pure CN (11.2 μmol∙g-1∙h-1). Furthermore, a series of Na/O-CNx-yO2 (y=0, 20%, 40%, 60%, 80%, 100%) samples were prepared by modulating the oxygen content within reaction atmosphere. The catalytic performance evaluations reveal that the incorporation of both Na and O atoms in Na/O-CN3.0 enhances photocatalytic activity. This study also introduces novel methodologies for synthesis of metal atom-doped CN materials at lower temperature, highlighting the synergistic effect of Na and O atoms in photocatalytic hydrogen production of Na/O-CNx samples.
|
|