
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (11): 1200-1204.DOI: 10.15541/jim20190009
• RESEARCH LETTERS • Previous Articles Next Articles
SU Kun1,2,3,ZHANG Ya-Ru3,LU Fei3,ZHANG Jun1( ),WANG Xi3(
),WANG Xi3( )
)
Received:2019-01-04
Published:2019-11-20
Online:2019-05-13
Supported by:CLC Number:
SU Kun, ZHANG Ya-Ru, LU Fei, ZHANG Jun, WANG Xi. Platinum Decorated Titanium Dioxide Nanosheets for Efficient Photoelectrocatalytic Hydrogeu Evolution Reaction[J]. Journal of Inorganic Materials, 2019, 34(11): 1200-1204.
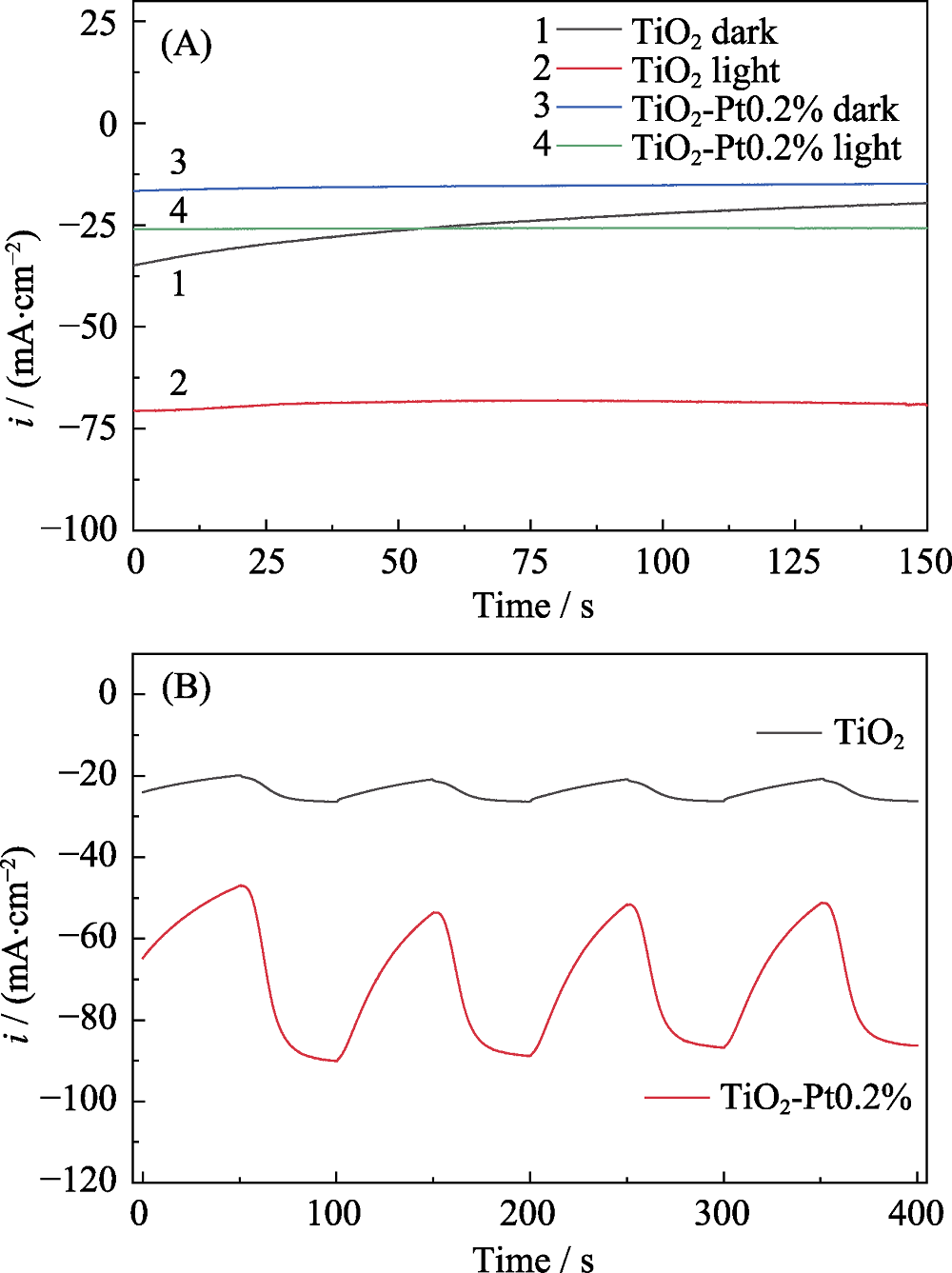
Fig. 3 (A)i-t curves of pure TNS and TiO2-Pt0.2% under continuous light irradiation or in darkness at -0.2 V external bias; (B) Periodic light irradiation induced i-t curves of pure TNS and TiO2-Pt0.2%
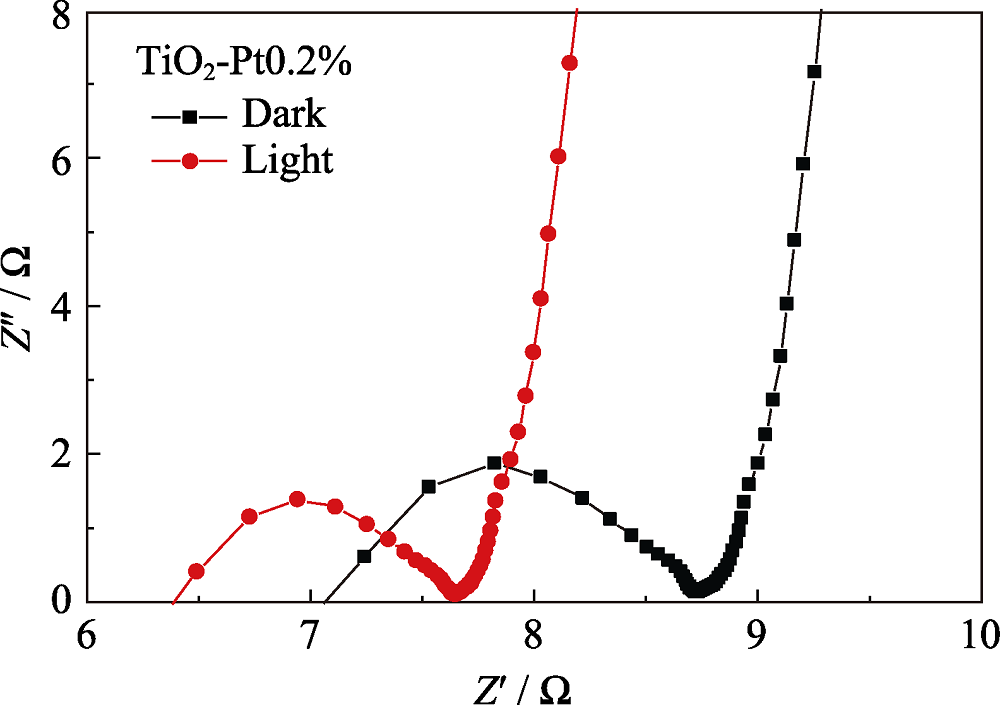
Fig. 4 Nyquist plots of glassy carbon electrode modified with TiO2-Pt0.2% under light irradiation (red curve) and darkness (black curve), mass loading of 0.2 mg/cm in 0.5 mol/L H2SO4
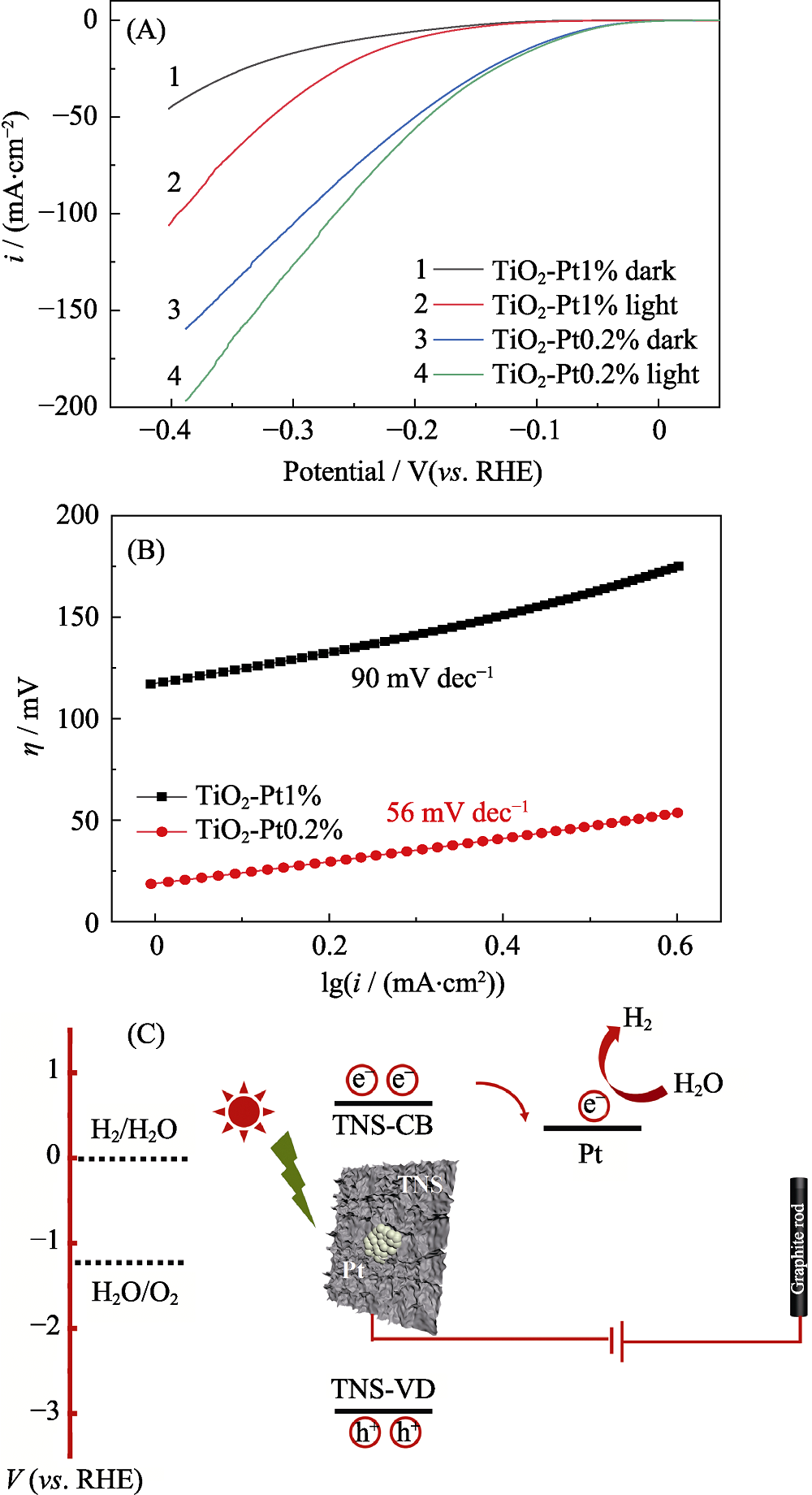
Fig. 5 (A) Linear sweep voltammetry (LSV) curves of glassy carbon electrode modified with pure TiO2-Pt0.2% or TiO2-Pt1% (mass loading of 0.2 mg/cm in 0.5 mol/L H2SO4); (B) corresponding Tafel plots; (C) Proposed schematic route of the photocatalytic process with Pt cluster decorated TiO2 nanosheets as the cathode and graphite rod as the anode
| [1] | KHAN M, NGO H H, GUO W S , et al. Biohydrogen production from anaerobic digestion and its potential as renewable energy. Renewable Energy, 2018,129:754-768. |
| [2] | GHOSH T, GHOSH P, MAAYAN G . A copper-peptoid as a highly stable, efficient, and reusable homogeneous water oxidation electrocatalyst. ACS Catalysis, 2018,8(11):10631-10640. |
| [3] | WENG G M, LI C Y V, CHAN K Y . Hydrogen battery using neutralization energy. Nano Energy, 2018,53:240-244. |
| [4] | KUMAR G, CHO S K, SIVAGURUNATHAN P , et al. Insights into evolutionary trends in molecular biology tools in microbial screening for biohydrogen production through dark fermentation. Int. J. Hydrog. Energy, 2018,43(43):19885-198901. |
| [5] | DING C, SHI J, WANG Z , et al. Photoelectrocatalytic water splitting: significance of cocatalysts. electrolyte, and Interfaces. ACS Catalysis, 2017,7(1):675-688. |
| [6] | YANAGIDA S . Nano/microsized TiO2 composite photocatalysts for environmental purification. Journal of the Ceramic Society of Japan, 2018,126(8):625-631. |
| [7] | TAN T L, LAI C W HONG S L , et al. New insights into the photocatalytic endocrine disruptors dimethyl phathalate esters degradation by UV/MWCNTs-TiO2 nanocomposites. Journal of Photochemistry and Photobiology A-Chemistry, 2018,364:177-189. |
| [8] | YI S S, ZHANG X B, WULAN B R , et al. Non-noble metals applied to solar water splitting. Energy & Environmental Science, 2018,11(11):3128-3156. |
| [9] | TAYEL A, RAMADAN A R, El SEOUD O A . Titanium dioxide/ graphene and titanium dioxide/graphene oxide nanocomposites: synthesis, characterization and photocatalytic applications for water decontamination. Catalysts, 2018, 8(11): 491-1-45. |
| [10] | EFTEKHARI A . Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy, 2017,42(16):11053-11077. |
| [11] | TAN H L, DU A, AMAL R , et al. Decorating platinum on nitrogen- doped graphene sheets: control of the platinum particle size distribution for improved photocatalytic H2 generation. Chemical Engineering Science, 2019,194:85-93. |
| [12] | WANG C, SHI H, LIU H , et al. Quasi-atomic-scale platinum anchored on porous titanium nitride nanorod arrays for highly efficient hydrogen evolution. Electrochimica Acta, 2018,292:727-735. |
| [13] | HAN X, KUANG Q, JIN M , et al. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. Journal of the American Chemical Society, 2009,131(9):3152-3153. |
| [14] | JIA L, SHU D J, WANG M . Tuning the area percentage of reactive surface of TiO2 by strain engineering. Physical Review Letters, 2012,109(15):156104 |
| [15] | MINGYAEV M E, KORCHAGINA SYA, TAVTORKIN A N , et al. Crystal structures of mono- and binuclear neodymium diarylphosphate complexes and their catalytic activity in 1,3-diene polymerization. Structural Chemistry, 2018,29(5):1475-1487. |
| [16] | NOGUEIRA L S, NEVES P, GOMES A C , et al. Molybdenum(0) tricarbonyl and tetracarbonyl complexes with a cationic pyrazolylpyridine ligand: synthesis, crystal structures and catalytic performance in olefin epoxidation. RSC Advances, 2018,8(29):16294-16302. |
| [17] | RAMADAN A E M M, IBRAHIM M M E L, MEHASSEBY I M . New mononuclear copper(I) and copper(II) complexes containing N-4 donors: crystal structure and catechol oxidase biomimetic catalytic activity. Journal of Coordination Chemistry, 2012,65(13):2256-2279. |
| [18] | DI J, YAN C, HANDOKO A D, SEH Z W , et al. Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Materials Today, 2018,21(7):749-770. |
| [19] | MOMENI M M, GHAYEB Y, GHONCHEGI Z . Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceramics International, 2015,41(7):8735-8741. |
| [20] | GUO R, XU X, XIA Y , et al. Insights into electrocatalytic hydrogen evolution reaction in acidic medium at in-situ dispersed Pt atoms on nanoporous gold films. Journal of Catalysis, 2018,368:379-388. |
| [21] | LIU L L, DU C J, WANG S L , et al. Three new bifunctional additive for safer nickel-cobalt-aluminum based lithium ion batteries. Chinese Chemical Letters, 2018,29:1781-1784. |
| [1] | WANG Zhaoyang, QIN Peng, JIANG Yin, FENG Xiaobo, YANG Peizhi, HUANG Fuqiang. Sandwich Structured Ru@TiO2 Composite for Efficient Photocatalytic Tetracycline Degradation [J]. Journal of Inorganic Materials, 2024, 39(4): 383-389. |
| [2] | HU Yue, AN Lin, HAN Xin, HOU Chengyi, WANG Hongzhi, LI Yaogang, ZHANG Qinghong. RhO2 Modified BiVO4 Thin Film Photoanodes: Preparation and Photoelectrocatalytic Water Splitting Performance [J]. Journal of Inorganic Materials, 2022, 37(8): 873-882. |
| [3] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [4] | CHI Congcong, QU Panpan, REN Chaonan, XU Xin, BAI Feifei, ZHANG Danjie. Preparation of SiO2@Ag@SiO2@TiO2 Core-shell Structure and Its Photocatalytic Degradation Property [J]. Journal of Inorganic Materials, 2022, 37(7): 750-756. |
| [5] | PAN Bichen,REN Penghe,ZHOU Tejun,CAI Zhenyang,ZHAO Xiaojun,ZHOU Hongming,XIAO Lairong. Microstructure and Property of Thermal Insulation Coating on the Carbon Fiber Reinforced Epoxy Resin Composites [J]. Journal of Inorganic Materials, 2020, 35(8): 947-952. |
| [6] | ZHU Ben-Bi,ZHANG Wang,ZHANG Zhi-Jian,ZHANG Jian-Zhong,IMRAN Zada,ZHANG Di. Photothermal Enhanced Photocatalytic Properties of Titanium Dioxide (B)/Glass Fiber Cloth [J]. Journal of Inorganic Materials, 2019, 34(9): 961-966. |
| [7] | FAN Wen, WU Li-Min. Controllable Preparation of Nano-TiO2 Lens by Silicon Oil Two-step Dehydration Method [J]. Journal of Inorganic Materials, 2018, 33(12): 1337-1342. |
| [8] | LIN Jing-Cheng, TANG Xiao, CHU Wan-Yi. Synthesis and Photocatalysis Property of Ultra-small TiO2 Nanoclusters in Aqueous Media [J]. Journal of Inorganic Materials, 2017, 32(8): 863-869. |
| [9] | LI Cui-Xia, JIN Hai-Ze, YANG Zhi-Zhong, YANG Xuan, DONG Qi-Zheng, LI Ting-Ting. Preparation and Photocatalytic Properties of Mesoporous RGO/TiO2 Composites [J]. Journal of Inorganic Materials, 2017, 32(4): 357-364. |
| [10] | MA Ya-Ting, LI Qiao-Ling. Preparation and Characterization of TiO2/Co3O4 Nanocomposites and Their Photocatalytic Activity for Hydrogen Evolution [J]. Journal of Inorganic Materials, 2016, 31(8): 841-844. |
| [11] | SHEN Zhong, ZHONG Jin-Yi, ZHAO Yuan-Zhong, CUI Yan, CHEN Li-Kun, ZHENG He. Degradation of Chemical Warfare Agents by Germanium-doped Nanosized TiO2 under Simulated Sunlight Irradiation [J]. Journal of Inorganic Materials, 2016, 31(4): 427-433. |
| [12] | MA Xin-Guo, YAN Jie, CHEN Zi-Meng, ZHU Lin, XU Guo-Wang, HUANG Chu-Yun, LV Hui. First-principles Calculation on Pt- and Au-modified Anatase TiO2(101) Surface [J]. Journal of Inorganic Materials, 2016, 31(3): 291-297. |
| [13] | WEI Jie, LI Xue-Dong, WANG Hong-Zhi, ZHANG Qing-Hong, LI Yao-Gang. Nitrogen Doped Carbon Quantum Dots/Titanium Dioxide Composites for Hydrogen Evolution under Sunlight [J]. Journal of Inorganic Materials, 2015, 30(9): 925-930. |
| [14] | LIU Yang-Long, ZHENG Yu-Ying, SHANG Peng-Bo. Preparation, Characterization and Photocatalytic Property of Eu-doped TiO2 Hollow Microspheres [J]. Journal of Inorganic Materials, 2015, 30(7): 699-705. |
| [15] | LI Yan-Chun, ZHANG Xiu-Ling, ZHAN Zhi-Bin, DI Lan-Bo. Influence of Ionic Liquids on Structure and Properties of Magnetically Separable TiO2 Photocatalytic Materials [J]. Journal of Inorganic Materials, 2015, 30(7): 706-712. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||