
Journal of Inorganic Materials ›› 2017, Vol. 32 ›› Issue (6): 596-602.DOI: 10.15541/jim20160472
• Orginal Article • Previous Articles Next Articles
WANG Shu-Wei1, 2, HU He-Feng1, WANG De-Yu2, SHEN Cai2
Received:2016-08-17
Revised:2016-11-13
Published:2017-06-20
Online:2017-05-27
About author:WANG Shu-Wei.
Supported by:CLC Number:
WANG Shu-Wei, HU He-Feng, WANG De-Yu, SHEN Cai. AFM Investigation of Solid Electrolyte Interphase on HOPG Anode in Sodium Ion Battery[J]. Journal of Inorganic Materials, 2017, 32(6): 596-602.
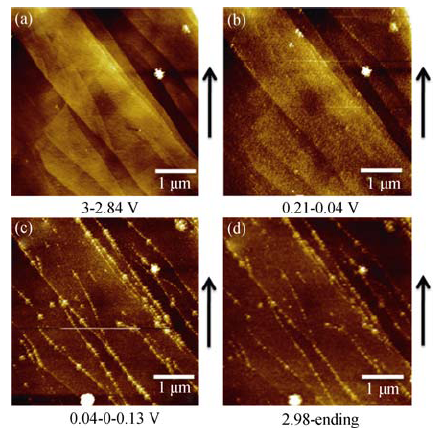
Fig. 2 In situ AFM images of HOPG electrode cycled at a scanning rate of 0.5 mV/s between 3.0 and 0 V in 1 mol/L NaClO4/EC/DMC(a) During discharging, potential range of 3.0-2.84 V; (b) During discharging, potential range of 0.24-0.04 V; (c) The potential is sweeped from 0.04 V to 0 V then rised to 0.13 V; (d) During charging, potential range of 2.98-3.0 V. The arrow indicates AFM scanning direction
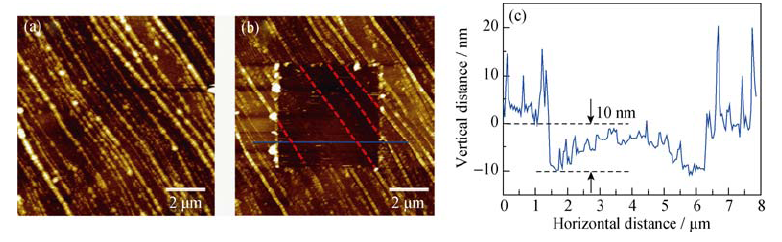
Fig. 3 (a) AFM image of HOPG electrode after first CV cycle in the 1 mol/L NaClO4/EC/DMC; (b) AFM image of the HOPG anode after the SEI in the middle of the image being scraped off and (c) cross-section analysis of the location marked by blue line
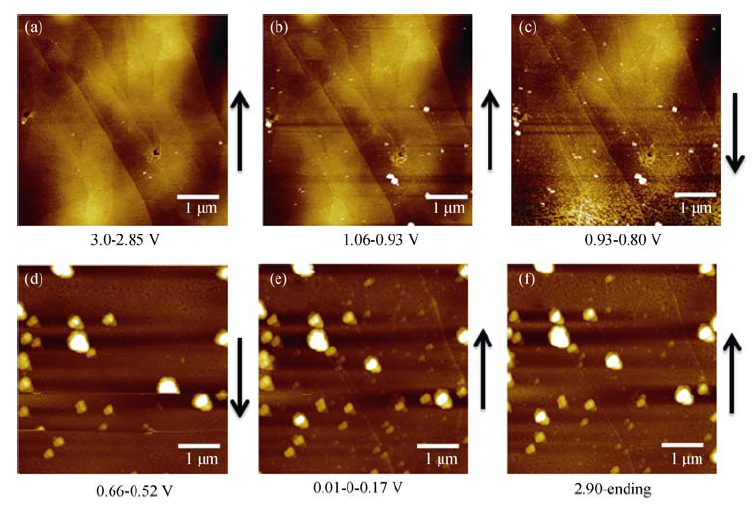
Fig. 4 In situ AFM images of HOPG electrode cycled at a scanning rate of 0.5 mV/s between 3.0 and 0.0 V in 1 mol/L NaClO4/FEC/DMC(a) During discharging, potential range of 3.0-2.85 V; (b) During discharging, potential range of 1.06-0.93 V; (c) During discharging, potential range: of 0.93-0.80 V; (d) During discharging, the potential range of 0.66-0.52 V; (e) The potential is sweeped from 0.01 V to 0 V then rised to 0.17 V; (f) During charging, the potential range of 2.90-3.0 V; The arrow indicates AFM scanning direction
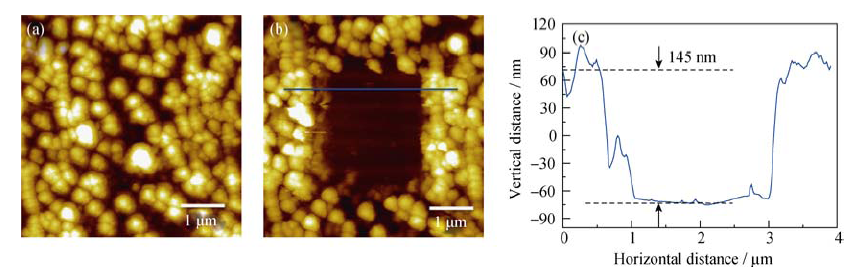
Fig. 5 (a) AFM image of HOPG electrode after first CV cycle in the 1 mol/L NaClO4/FEC/DMC; (b) AFM image of the HOPG anode after the SEI in the middle of the image being scraped off; and (c) cross-section analysis of the location marked by blue line
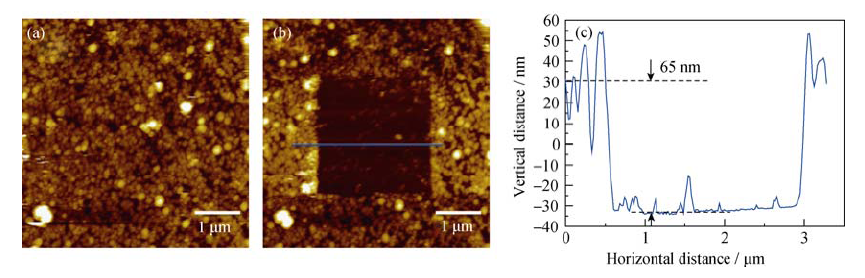
Fig. 6 (a) AFM image of under SEI layer on HOPG electrode after first CV cycle in the 1 mol/L NaClO4/FEC/DMC; (b) AFM image of the HOPG anode after the SEI in the middle of the image being scraped off; (c) cross-section analysis of the location marked by blue line
| HOPG | Atomic concentration /% | |||||
|---|---|---|---|---|---|---|
| C | O | Na | Cl | F | ||
| 1 mol/L NaClO4 /EC/DMC | Before etching | 80.22 | 16.63 | 2.56 | 0.59 | - |
| After etching | 83.16 | 11.62 | 3.42 | 1.80 | - | |
| 1 mol/L NaClO4 /FEC/DMC | Before etching | 77.90 | 12.36 | 4.18 | 0.87 | 4.69 |
| After etching | 78.02 | 5.21 | 6.95 | 2.03 | 7.79 | |
Table 1 Atomic concentrations of C, O, Na, Cl and F obtained from XPS
| HOPG | Atomic concentration /% | |||||
|---|---|---|---|---|---|---|
| C | O | Na | Cl | F | ||
| 1 mol/L NaClO4 /EC/DMC | Before etching | 80.22 | 16.63 | 2.56 | 0.59 | - |
| After etching | 83.16 | 11.62 | 3.42 | 1.80 | - | |
| 1 mol/L NaClO4 /FEC/DMC | Before etching | 77.90 | 12.36 | 4.18 | 0.87 | 4.69 |
| After etching | 78.02 | 5.21 | 6.95 | 2.03 | 7.79 | |
| [1] | XU K.Electrolytes and interphases in Li-ion batteries and beyond.Chemical Reviews, 2014, 114(23): 11503-11618. |
| [2] | SHEN CBUCK M.Nanoscale patterning of a self-assembled monolayer by modification of the molecule-substrate bond.Beilstein journal of nanotechnology, 2014, 5: 258-267. |
| [3] | SHEN C, CEBULA I, BROWN C, et al.Structure of isophthalic acid based monolayers and its relation to the initial stages of growth of metal-organic coordination layers.Chemical Science, 2012, 3(6): 1858-1865. |
| [4] | CEBULA I, SHEN CBUCK M.Isophthalic acid: a basis for highly ordered monolayers.Angewandte Chemie International Edition, 2010, 49(35): 6220-6223. |
| [5] | CRAMER J R, NING Y, SHEN C, et al.Oligo (naphthylene- ethynylene) Molecular Rods.European Journal of Organic Chemistry, 2013, 2013(14): 2813-2822. |
| [6] | SHEN C, HARYONO M, Grohmann A, et al.Self-assembled monolayers of a bis(pyrazol-1-yl)pyridine-substituted thiol on Au(111).Langmuir, 2008, 24(22): 12883-12891. |
| [7] | SHEN C, CRAMER J R, JACOBSEN M F, et al.Steering supramolecular patterns by nucleobase-terminated molecules.Chemical communications, 2013, 49(5): 508-510. |
| [8] | ZHU J, FENG J, LU L, et al.In situ study of topography, phase and volume changes of titanium dioxide anode in all-solid-state thin film lithium-ion battery by biased scanning probe microscopy.Journal of Power Sources, 2012, 197: 224-230. |
| [9] | ZHANG J, WANG R, YANG X, et al.Direct observation of inhomogeneous solid electrolyte interphase on MnO anode with atomic force microscopy and spectroscopy.Nano letters, 2012, 12(4): 2153-2157. |
| [10] | LIU R R, DENG X, LIU X R, et al.Facet dependent SEI formation on the LiNi(0.5)Mn(1.5)O4 cathode identified by in situ single particle atomic force microscopy.Chemical communications, 2014, 50(99): 15756-15759. |
| [11] | ZHENG J, ZHENG H, WANG R, et al.3D visualization of inhomogeneous multi-layered structure and Young's modulus of the solid electrolyte interphase (SEI) on silicon anodes for lithium ion batteries.Physical Chemistry Chemical Physics, 2014, 16(26): 13229-13238. |
| [12] | INABA M, KAWATATE Y, FUNABIKI A, et al.STM study on graphite/electrolyte interface in lithium-ion batteries: solid electrolyte interface formation in trifluoropropylene carbonate solution.Electrochimica Acta, 1999, 45(1/2): 99-105. |
| [13] | V CRESCE A, RUSSELL S M, BAKER D R, et al. In situ and quantitative characterization of solid electrolyte interphases.Nano letters, 2014, 14(3): 1405-1412. |
| [14] | LIU X R, WANG L, WAN L J, et al.In situ observation of electrolyte-concentration-dependent solid electrolyte interphase on graphite in dimethyl sulfoxide.ACS Applied Materials & Interfaces, 2015, 7(18): 9573-9580. |
| [15] | LUCAS I T, POLLAK EKOSTECKI R.In situ AFM studies of SEI formation at a Sn electrode.Electrochemistry Communications, 2009, 11(11): 2157-2160. |
| [16] | DOMI Y, OCHIDA M, TSUBOUCHI S, et al.In Situ AFM study of surface film formation on the edge plane of HOPG for lithium-ion batteries.The Journal of Physical Chemistry C, 2011, 115(51): 25484-25489. |
| [17] | DENG X, LIU X, YAN H, et al.Morphology and modulus evolution of graphite anode in lithium ion battery: an in situ AFM investigation.Science China-Chemistry, 2014, 57(1): 178-183. |
| [18] | HERRERA S E, TESIO A Y, CLARENC R, et al.AFM study of oxygen reduction products on HOPG in the LiPF6-DMSO electrolyte.Physical Chemistry Chemical Physics, 2014, 16(21): 9925-9929. |
| [19] | WANG L, DENG D, LEV L C, et al.In-situ investigation of solid-electrolyte interphase formation on the anode of Li-ion batteries with atomic force microscopy.Journal of Power Sources, 2014, 265: 140-148. |
| [20] | LACEY S D, WAN J, VON WALD CRESCE A, et al. Atomic force microscopy studies on molybdenum disulfide flakes as sodium-ion anodes.Nano letters, 2015, 15(2): 1018-1024. |
| [21] | SHEN C, WANG S, JIN Y, et al.In situ AFM imaging of solid electrolyte interfaces on HOPG with ethylene carbonate and fluoroethylene carbonate-based electrolytes.ACS applied materials & interfaces, 2015, 7(45): 25441-25447. |
| [22] | JIAN Z, HAN W, LU X, et al.Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries.Advanced Energy Materials, 2013, 3(2): 156-160. |
| [23] | PAN H, LU X, YU X, et al.Sodium storage and transport properties in layered Na2Ti3O7 for room-temperature sodium-ion batteries.Advanced Energy Materials, 2013, 3(9): 1186-1194. |
| [24] | XU KVON CRESCE A. Interfacing electrolytes with electrodes in Li ion batteries.Journal of Materials Chemistry, 2011, 21(27): 9849-9864. |
| [25] | AMINE K, BELHAROUAK I, CHEN Z, et al.Nanostructured anode material for high-power battery system in electric vehicles.Advanced materials, 2010, 22(28): 3052-3057. |
| [26] | LEE S W, GALLANT B M, BYON H R, et al.Nanostructured carbon-based electrodes: bridging the gap between thin-film lithium-ion batteries and electrochemical capacitors.Energy & Environmental Science, 2011, 4(6): 1972-1985. |
| [27] | PALOMARES V, CASAS-CABANAS M, CASTILLO-MARTÍNEZ E, et al. Update on Na-based battery materials. A growing research path.Energy & Environmental Science, 2013, 6(8): 2312-2337. |
| [28] | SLATER M D, KIM D, LEE E, et al.Sodium-ion batteries.Advanced Functional Materials, 2013, 23(8): 947-958. |
| [29] | DARWICHE A, MARINO C, SOUGRATI M T, et al.Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: an unexpected electrochemical mechanism.Journal of the American Chemical Society, 2012, 134(51): 20805-20811. |
| [30] | XIA XDAHN J R. Study of the reactivity of Na/hard carbon with different solvents and electrolytes.Journal of the Electrochemical Society, 2011, 159(5): A515-A519. |
| [31] | ELLIS L D, HATCHARD T DOBROVAC M N. Reversible insertion of sodium in tin.Journal of the Electrochemical Society, 2012, 159(11): 32-42. |
| [32] | JI L W, GU M, SHAO Y Y, et al.Controlling SEI formation on SnSb-porous carbon nanofibers for improved Na ion storage.Advanced materials, 2014, 26(18): 2901-2908. |
| [33] | MU L, XU S, LI Y, et al.Prototype sodium-ion batteries using an air-stable and Co/Ni-Free O3-layered metal oxide cathode.Advanced materials, 2015, 27(43): 6928-6933. |
| [34] | LI Y, HU Y-S, QI X, et al.Advanced sodium-ion batteries using superior low cost pyrolyzed anthracite anode: towards practical applications.Energy Storage Materials, 2016, 5: 191-197. |
| [35] | KOMABA S, MURATA W, ISHIKAWA T, et al.Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries.Advanced Functional Materials, 2011, 21(20): 3859-3867. |
| [36] | BREITUNG B, BAUMANN P, SOMMER H, et al.In situ and operando atomic force microscopy of high-capacity nano-silicon based electrodes for lithium-ion batteries.Nanoscale, 2016, 8(29): 14048-14056. |
| [1] | ZHANG Jinghui, LU Xiaotong, MAO Haiyan, TIAN Yazhou, ZHANG Shanlin. Effect of Sintering Additives on Sintering Behavior and Conductivity of BaZr0.1Ce0.7Y0.2O3-δ Electrolytes [J]. Journal of Inorganic Materials, 2025, 40(1): 84-90. |
| [2] | KONG Jianfeng, HUANG Jiecheng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on DPEPA-derived Gel Polymer Electrolyte [J]. Journal of Inorganic Materials, 2024, 39(12): 1331-1338. |
| [3] | WEN Zhipeng, WEI Yi, HOU Xianghua, GUO Jiawen, LI Qu, ZHU Manqing, ZHANG Jiahao, PAN Kai, WU Lian. Research Progress of Bentonite-based Functional Materials in Electrochemical Energy Storage [J]. Journal of Inorganic Materials, 2024, 39(12): 1301-1315. |
| [4] | HU Mengfei, HUANG Liping, LI He, ZHANG Guojun, WU Houzheng. Research Progress on Hard Carbon Anode for Li/Na-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(1): 32-44. |
| [5] | GUO Yuxiang, HUANG Liqiang, WANG Gang, WANG Hongzhi. Dual-lithium-salt Gel Complex Electrolyte: Preparation and Application in Lithium-metal Battery [J]. Journal of Inorganic Materials, 2023, 38(7): 785-792. |
| [6] | FANG Renrui, REN Kuan, GUO Zeyu, XU Han, ZHANG Woyu, WANG Fei, ZHANG Peiwen, LI Yue, SHANG Dashan. Associative Learning with Oxide-based Electrolyte-gated Transistor Synapses [J]. Journal of Inorganic Materials, 2023, 38(4): 399-405. |
| [7] | QIU Haiyang, MIAO Guangtan, LI Hui, LUAN Qi, LIU Guoxia, SHAN Fukai. Effect of Plasma Treatment on the Long-term Plasticity of Synaptic Transistor [J]. Journal of Inorganic Materials, 2023, 38(4): 406-412. |
| [8] | CHEN Xinli, LI Yan, WANG Weisheng, SHI Zhiwen, ZHU Liqiang. Gelatin/Carboxylated Chitosan Gated Oxide Neuromorphic Transistor [J]. Journal of Inorganic Materials, 2023, 38(4): 421-428. |
| [9] | JIANG Yiyi, SHEN Min, SONG Banxia, LI Nan, DING Xianghuan, GUO Leyi, MA Guoqiang. Effect of Dual-functional Electrolyte Additive on High Temperature and High Voltage Performance of Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(7): 710-716. |
| [10] | SU Dongliang, CUI Jin, ZHAI Pengbo, GUO Xiangxin. Mechanism Study on Garnet-type Li6.4La3Zr1.4Ta0.6O12 Regulating the Solid Electrolyte Interphases of Si/C Anodes [J]. Journal of Inorganic Materials, 2022, 37(7): 802-808. |
| [11] | XIA Qiuying, SUN Shuo, ZAN Feng, XU Jing, XIA Hui. Amorphous LiSiON Thin Film Electrolyte for All-solid-state Thin Film Lithium Battery [J]. Journal of Inorganic Materials, 2022, 37(2): 230-236. |
| [12] | LI Wenkai, ZHAO Ning, BI Zhijie, GUO Xiangxin. Na3Zr2Si2PO12 Ceramic Electrolytes for Na-ion Battery: Preparation Using Spray-drying Method and Its Property [J]. Journal of Inorganic Materials, 2022, 37(2): 189-196. |
| [13] | ZHAO Wei, XU Yang, WAN Yingjie, CAI Tianxun, MU Jinxiao, HUANG Fuqiang. Metal Cyanamides/Carbodiimides: Structure, Synthesis and Electrochemical Energy Storage Performance [J]. Journal of Inorganic Materials, 2022, 37(2): 140-151. |
| [14] | FAN Shuai, JIN Tian, ZHANG Shanlin, LUO Xiaotao, LI Chengxin, LI Changjiu. Effect of Li2O Sintering Aid on Sintering Characteristics and Electrical Conductivity of LSGM Electrolyte for Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2022, 37(10): 1087-1092. |
| [15] | ZHANG Xiaojun, LI Jiale, QIU Wujie, YANG Miaosen, LIU Jianjun. Electrochemical Activity of Positive Electrode Material of P2-Nax[Mg0.33Mn0.67]O2 Sodium Ion Battery [J]. Journal of Inorganic Materials, 2021, 36(6): 623-628. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||