
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (5): 547-554.DOI: 10.15541/jim20150516
• Orginal Article • Previous Articles Next Articles
LIU Jing-Jing1, HEULENS Jeroen2, GUO Mu-Xing1, MOELANS Nele1
Received:2015-10-21
Published:2016-05-20
Online:2016-04-25
About author:LIU Jing-Jing (1983–), female, candidate of PhD. E-mail: jingjing.liu@mtm.kuleuven.be
Supported by:CLC Number:
LIU Jing-Jing, HEULENS Jeroen, GUO Mu-Xing, MOELANS Nele. Isothermal Crystal Growth Behavior of CaSiO3 in Ternary Oxide Melts[J]. Journal of Inorganic Materials, 2016, 31(5): 547-554.
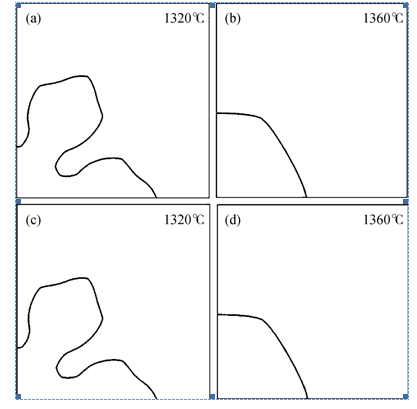
Fig.1 Simulated morphologies of wollastonite at t=1 s for slag A at (a) 1320℃; (b) 1360℃ with anisotropy in the interface energy only (c) 1320℃ and (d) 1360℃ with anisotropy in interface energy and interface kinetics
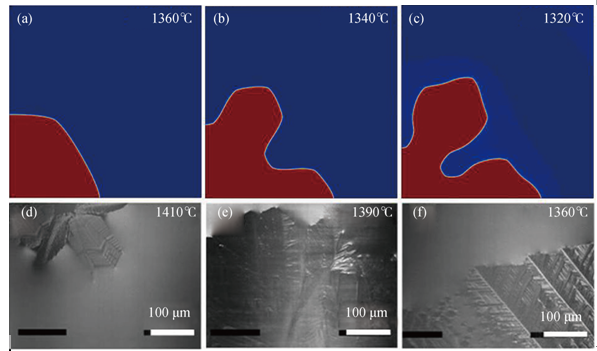
Fig. 2 Simulated and experimental morphologies of wollastonite for different undercoolings for slag A: (a) ∆=65℃; (b) ∆=85℃; (c) ∆=105℃ after a simulation time of 1 s and (d) ∆=15℃; (e) ∆=35℃; (f) ∆=65℃ observed in the experiments
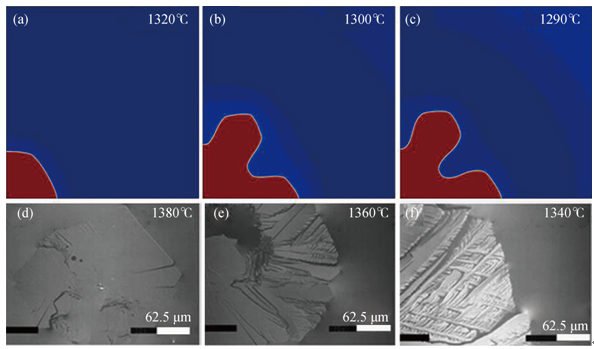
Fig. 3 Simulated and experimental morphologies of wollastonite for different undercoolings for slag B: (a) ∆=60℃; (b) ∆=80℃; (c) ∆=90℃ after a simulation time of 1 s and (d) ∆=0℃; (e) ∆=20℃; (f) ∆=40℃ observed in the experiments
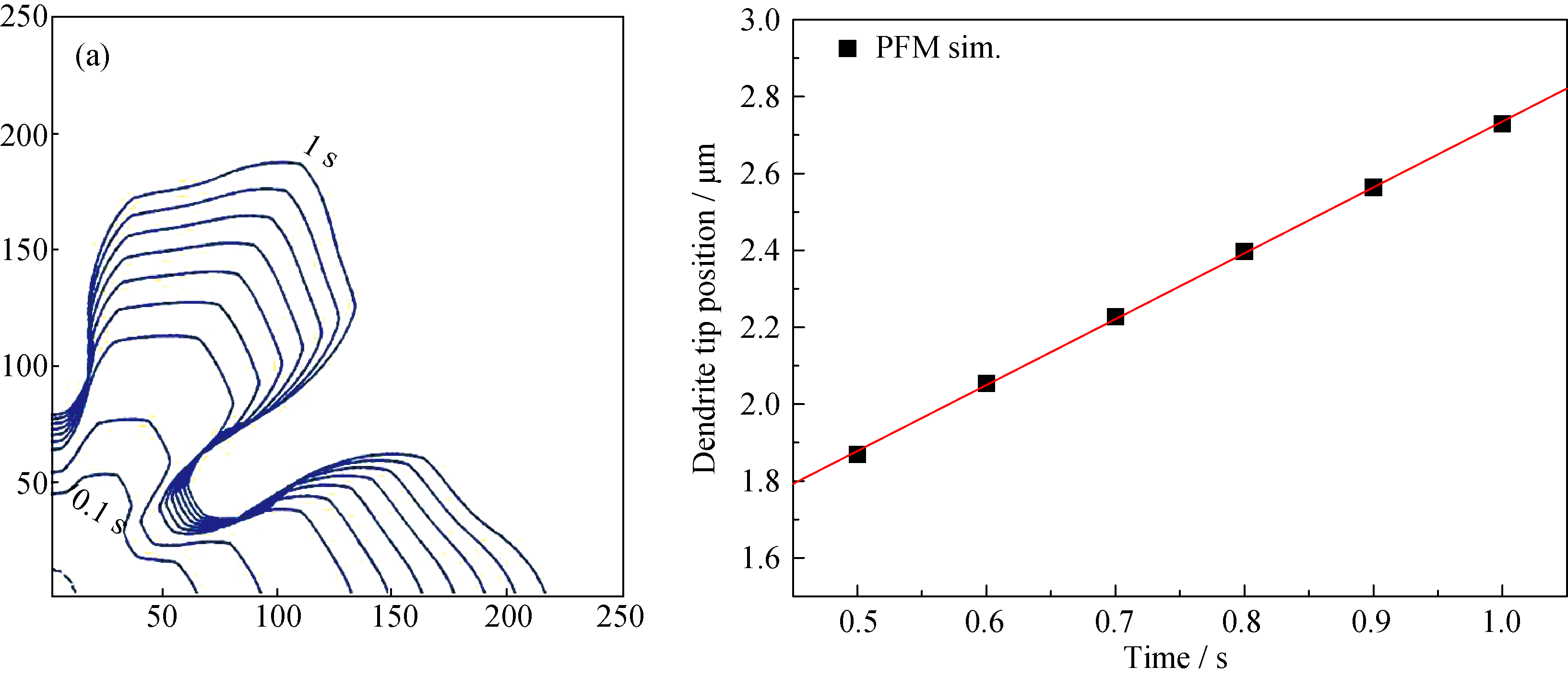
Fig. 4 (a) The simulated dendrite in slag A at 1320℃ is presented by means of the 0.5 contour of ηliquid for different time steps, (b) dendrite tip position as a function of time. The dendrite tip velocity is determined as the slope of this line
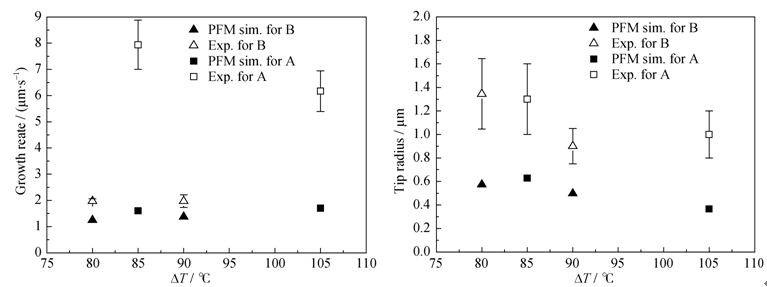
Fig. 5 (a) Dendrite tip velocity and (b) radius of wollastonite dendrite as a function of the undercoolings. The data of phase field simulations (filled mark) are shown in comparison with experimental data (open mark)
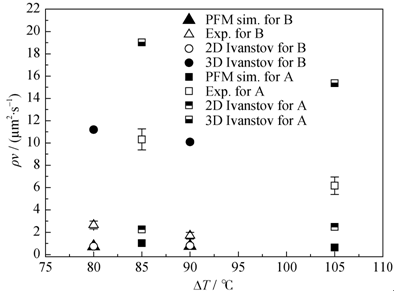
Fig. 6 Products of dendrite tip velocity and radius as a function of the undercoolings. The data of phase field simulations are shown in comparison with experimental data and the solutions from the 2D and 3D Ivantsov relations. The ρv values obtained from the simulations for slag B overlap with those obtained from the 2D Ivanstov equation
| [1] | SARASWAT R, MAIJER D M, LEE P D, et al.The effect of mould flux properties on thermo-mechanical behaviour during billet continuous casting.ISIJ Int., 2007, 47: 95-104. |
| [2] | KAJITANI T, OKAZAWA K, YAMADA W, et al.Cold model experiment on infiltration of mould flux in continuous casting of steel: simple analysis neglecting mould oscillation.ISIJ Int., 2006, 46: 250-256. |
| [3] | STONE D T, THOMAS B G.Measurement and modelling of the heat transfer across interfacial mold flux layers. Can. Metall. Q., 1999, 38(5): 363-375. |
| [4] | ZHANG Z T, LI J, LIU P.Crystallization behavior in fluoride-free mold fluxes containing TiO2/ZrO2.J. Iron Steel Res. Int. , 2011, 18: 31-37. |
| [5] | CHO J, SHIBATA H.Effect of solidification of mold fluxes on the heat transfer in casting mold.J. Non-Cryst. Solids, 2001, 282: 110-117. |
| [6] | CAO J W, WANG Z.Effect of Na2O and heat-treatment on crystallization of glass-ceramics from phosphorus slag.J. Alloys Compd., 2013, 557:190-195. |
| [7] | KASHIWAYA Y, CICUTTI C E, CRAMB A W, et al.An investigation of the crystallization of a continuous casting mold slag using the single hot thermocouple technique. ISIJ Int., 1998, 38: 348-356. |
| [8] | JONES P, DESMET D, GUO M, et al.Using confocal scanning laser microscopy for the in situ study of high-temperature behaviour of complex ceramic materials.J. Eur. Ceram. Soc., 2007, 27(12): 3497-3507. |
| [9] | ORRLING C, SRIDHAR S, CRAMB A W.In situ observation of the role of alumina particles on the crystallization behavior of slags.ISIJ Int., 2000, 40: 877-885. |
| [10] | HEULENS J, BLANPAIN B, MOELANS N.Analysis of the isothermal crystallization of CaSiO3 in a CaO-Al2O3-SiO2 melt through in situ observations.J. Eur. Ceram. Soc., 2011, 31(10): 1873-1879. |
| [11] | LIU J, CHEN G, YAN P, et al.In-situ observation of isothermal CaSiO3 crystallization in CaO-Al2O3-SiO2 melts: a study of the effects of temperature and composition.J. Cryst. Growth, 2014, 402: 1-8. |
| [12] | MOELANS N, BLANPAIN B, WOLLANTS P.An introduction to phase-field modeling of microstructure evolution.Calphad, 2008, 32(2): 268-294. |
| [13] | KOBAYASHI R.Modeling and numerical simulations of dendritic crystal growth.Physica D, 1993, 63(3/4): 410-423. |
| [14] | WARREN J A, BOETTINGER W J.Prediction of dendritic growth and microsegregation patterns in a binary alloy using the phase-field method.Acta Metall Mater, 1995, 43(2): 689-703. |
| [15] | STEINBACH I, PEZZOLLA F, NESTLER B, et al.A phase field concept for multiphase systems. Physcia D, 1996, 94: 135-147. |
| [16] | TIADEN J, NESTLER B, DIEPERS H J, et al.The multiphase-field model with an integrated concept for modelling solute diffusion.Physcia D, 1998, 115: 73-86. |
| [17] | HEULENS J, BLANPAIN B, MOELANS N.A phase field model for isothermal crystallization of oxide melts.Acta Mater., 2011, 59(5): 2156-2165. |
| [18] | MOELANS N.A quantitative and thermodynamically consistent phase-field interpolation function for multi-phase systems.Acta Mater., 2011, 59(3): 1077-1086. |
| [19] | KIM S G, KIM W T, SUZUKI T.Phase-field model for binary alloys.Phys. Rev. E, 1999, 60: 7186-7197. |
| [20] | KARMA A. Phase-field formulation for quantitative modeling of alloy solidification. Phys. Rev. Lett., 2001, 87: 115701/1-4. |
| [21] | CHOI J Y, LEE H G.Wetting of solid Al2O3 with molten CaO-Al2O3-SiO2.ISIJ Int., 2003, 43: 1348-1355. |
| [22] | COURTIAL P, DINGWELL D B.Nonlinear composition dependence of molar volume of melts in the CaO-Al2O3-SiO2 system.GEOCHIM COSMOCHIM AC, 1995, 59(18): 3685-3695. |
| [23] | HEULENS J, NAGATA K.Interdiffusivities matrix of CaO-Al2O3- SiO2 melt at 1723 K to 1823 K.Metall. Mater. Trans. B, 2011, 42(6): 1080. |
| [24] | RENER E A.Effects of surface energy and kinetics on the growth of needle-like dendrites.J. Cryst. Growth, 1990, 99(1-4): 165-170. |
| [25] | KIRKPATRICK R J.Crystal growth from the melt: a review.Am. Mineral., 1975, 60: 798-814. |
| [26] | NAKAMOTO M, LEE J, TANAKA T.A model for estimation of viscosity of molten silicate slag.ISIJ Int., 2005, 45(5): 651-656. |
| [27] | EDWARD J T.Molecular volumes and the Stokes-Einstein equation.J. Chem. Educ., 1970, 47(4): 261. |
| [1] | LI Xianke, ZHANG Chaoyi, HUANG Lin, SUN Peng, LIU Bo, XU Jun, TANG Huili. High-quality Indium-doped Gallium Oxide Single Crystal Growth by Floating Zone Method [J]. Journal of Inorganic Materials, 2024, 39(12): 1384-1390. |
| [2] | CAI Hao, WANG Qihang, ZOU Zhaoyong. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion [J]. Journal of Inorganic Materials, 2024, 39(11): 1275-1282. |
| [3] | HAO Yongxin, QIN Juan, SUN Jun, YANG Jinfeng, LI Qinglian, HUANG Guijun, XU Jingjun. Impact of Crucible Bottom Shape on the Growth of Congruent Lithium Niobate Crystals by Czochralski Method [J]. Journal of Inorganic Materials, 2024, 39(10): 1167-1174. |
| [4] | QIN Juan, LIANG Dandan, SUN Jun, YANG Jinfeng, HAO Yongxin, LI Qinglian, ZHANG Ling, XU Jingjun. Flat Shoulder Congruent Lithium Niobate Crystals Grown by the Czochralski Method [J]. Journal of Inorganic Materials, 2023, 38(8): 978-986. |
| [5] | LIN Siqi, LI Airan, FU Chenguang, LI Rongbing, JIN Min. Crystal Growth and Thermoelectric Properties of Zintl Phase Mg3X2 (X=Sb, Bi) Based Materials: a Review [J]. Journal of Inorganic Materials, 2023, 38(3): 270-279. |
| [6] | YANG Jiaxue, LI Wen, WANG Yan, ZHU Zhaojie, YOU Zhenyu, LI Jianfu, TU Chaoyang. Spectroscopic and Yellow Laser Features of Dy3+: Y3Al5O12 Single Crystals [J]. Journal of Inorganic Materials, 2023, 38(3): 350-356. |
| [7] | WU Zhen, LI Huifang, ZHANG Zhonghan, ZHANG Zhen, LI Yang, LAN Jianghe, SU Liangbi, WU Anhua. Growth and Characterization of CeF3 Crystals for Magneto-optical Application [J]. Journal of Inorganic Materials, 2023, 38(3): 296-302. |
| [8] | QI Xuejun, ZHANG Jian, CHEN Lei, WANG Shaohan, LI Xiang, DU Yong, CHEN Junfeng. Macroscopic Defects of Large Bi12GeO20 Crystals Grown Using Vertical Bridgman Method [J]. Journal of Inorganic Materials, 2023, 38(3): 280-287. |
| [9] | QI Zhanguo, LIU Lei, WANG Shouzhi, WANG Guogong, YU Jiaoxian, WANG Zhongxin, DUAN Xiulan, XU Xiangang, ZHANG Lei. Progress in GaN Single Crystals: HVPE Growth and Doping [J]. Journal of Inorganic Materials, 2023, 38(3): 243-255. |
| [10] | ZHANG Chaoyi, TANG Huili, LI Xianke, WANG Qingguo, LUO Ping, WU Feng, ZHANG Chenbo, XUE Yanyan, XU Jun, HAN Jianfeng, LU Zhanwen. Research Progress of ScAlMgO4 Crystal: a Novel GaN and ZnO Substrate [J]. Journal of Inorganic Materials, 2023, 38(3): 228-242. |
| [11] | CHEN Kunfeng, HU Qianyu, LIU Feng, XUE Dongfeng. Multi-scale Crystallization Materials: Advances in in-situ Characterization Techniques and Computational Simulations [J]. Journal of Inorganic Materials, 2023, 38(3): 256-269. |
| [12] | WANG Haidong, WANG Yan, ZHU Zhaojie, LI Jianfu, LAKSHMINARAYANA Gandham, TU Chaoyang. Crystal Growth and Structural, Optical, and Visible Fluorescence Traits of Dy3+-doped SrGdGa3O7 Crystal [J]. Journal of Inorganic Materials, 2023, 38(12): 1475-1482. |
| [13] | LI Qiaolei, GU Yue, YU Xuehua, ZHANG Chaowei, ZOU Mingke, LIANG Jingjing, LI Jinguo. Effect of Sintering Temperature on Surface Morphology and Roughness of 3D-printed Silicon Ceramic Cores [J]. Journal of Inorganic Materials, 2022, 37(3): 325-332. |
| [14] | MING Yue, HU Yue, MEI Anyi, RONG Yaoguang, HAN Hongwei. Application of Lead Acetate Additive for Printable Perovskite Solar Cell [J]. Journal of Inorganic Materials, 2022, 37(2): 197-203. |
| [15] | SUN Peng, ZHANG Shaoning, BI Hui, DONG Wujie, HUANG Fuqiang. Tuning Nitrogen Species and Content in Carbon Materials through Constructing Variable Structures for Supercapacitors [J]. Journal of Inorganic Materials, 2021, 36(7): 766-772. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||