
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (5): 511-520.DOI: 10.15541/jim20220684
Special Issue: 【能源环境】燃料电池(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
ZHANG Xiangsong( ), LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi(
), LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi( ), YANG Juan(
), YANG Juan( )
)
Received:2022-11-16
Revised:2023-01-04
Published:2023-01-11
Online:2023-01-11
Contact:
LI Yi, lecturer. E-mail: liyi5482@ujs.edu.cn;About author:ZHANG Xiangsong (1996-), male, Master candidate. E-mail: jsdaujszxs1996@163.com
Supported by:CLC Number:
ZHANG Xiangsong, LIU Yetong, WANG Yongying, WU Zirui, LIU Zhenzhong, LI Yi, YANG Juan. Self-assembled Platinum-iridium Alloy Aerogels and Their Efficient Electrocatalytic Ammonia Oxidation Performance[J]. Journal of Inorganic Materials, 2023, 38(5): 511-520.
| Sample | Pt/% | Ir/% | Pt/Ir |
|---|---|---|---|
| Pt55Ir45 | 54.33 | 45.67 | 1.19 |
| Pt70Ir30 | 67.24 | 32.76 | 2.05 |
| Pt80Ir20 | 79.34 | 20.66 | 3.84 |
| Pt87Ir13 | 88.36 | 11.64 | 7.59 |
Table S1 Elemental quantification (%, in atom) determined by XPS for different Pt100-xIrx aerogel catalysts
| Sample | Pt/% | Ir/% | Pt/Ir |
|---|---|---|---|
| Pt55Ir45 | 54.33 | 45.67 | 1.19 |
| Pt70Ir30 | 67.24 | 32.76 | 2.05 |
| Pt80Ir20 | 79.34 | 20.66 | 3.84 |
| Pt87Ir13 | 88.36 | 11.64 | 7.59 |
| Sample | Pt4f5/2/eV | ∆1/eV | Pt4f7/2/eV | ∆2/eV |
|---|---|---|---|---|
| Commercial Pt/C | 75.10 | - | 71.70 | - |
| Pt87Ir13 aerogel | 74.85 | -0.25 | 71.53 | -0.17 |
| Pt80Ir20 aerogel | 74.88 | -0.22 | 71.50 | -0.20 |
| Pt70Ir30 aerogel | 74.92 | -0.18 | 71.53 | -0.17 |
| Pt55Ir45 aerogel | 74.93 | -0.17 | 71.57 | -0.13 |
Table S2 Comparison of binding energy between Pt4f with different Pt-based catalysts
| Sample | Pt4f5/2/eV | ∆1/eV | Pt4f7/2/eV | ∆2/eV |
|---|---|---|---|---|
| Commercial Pt/C | 75.10 | - | 71.70 | - |
| Pt87Ir13 aerogel | 74.85 | -0.25 | 71.53 | -0.17 |
| Pt80Ir20 aerogel | 74.88 | -0.22 | 71.50 | -0.20 |
| Pt70Ir30 aerogel | 74.92 | -0.18 | 71.53 | -0.17 |
| Pt55Ir45 aerogel | 74.93 | -0.17 | 71.57 | -0.13 |
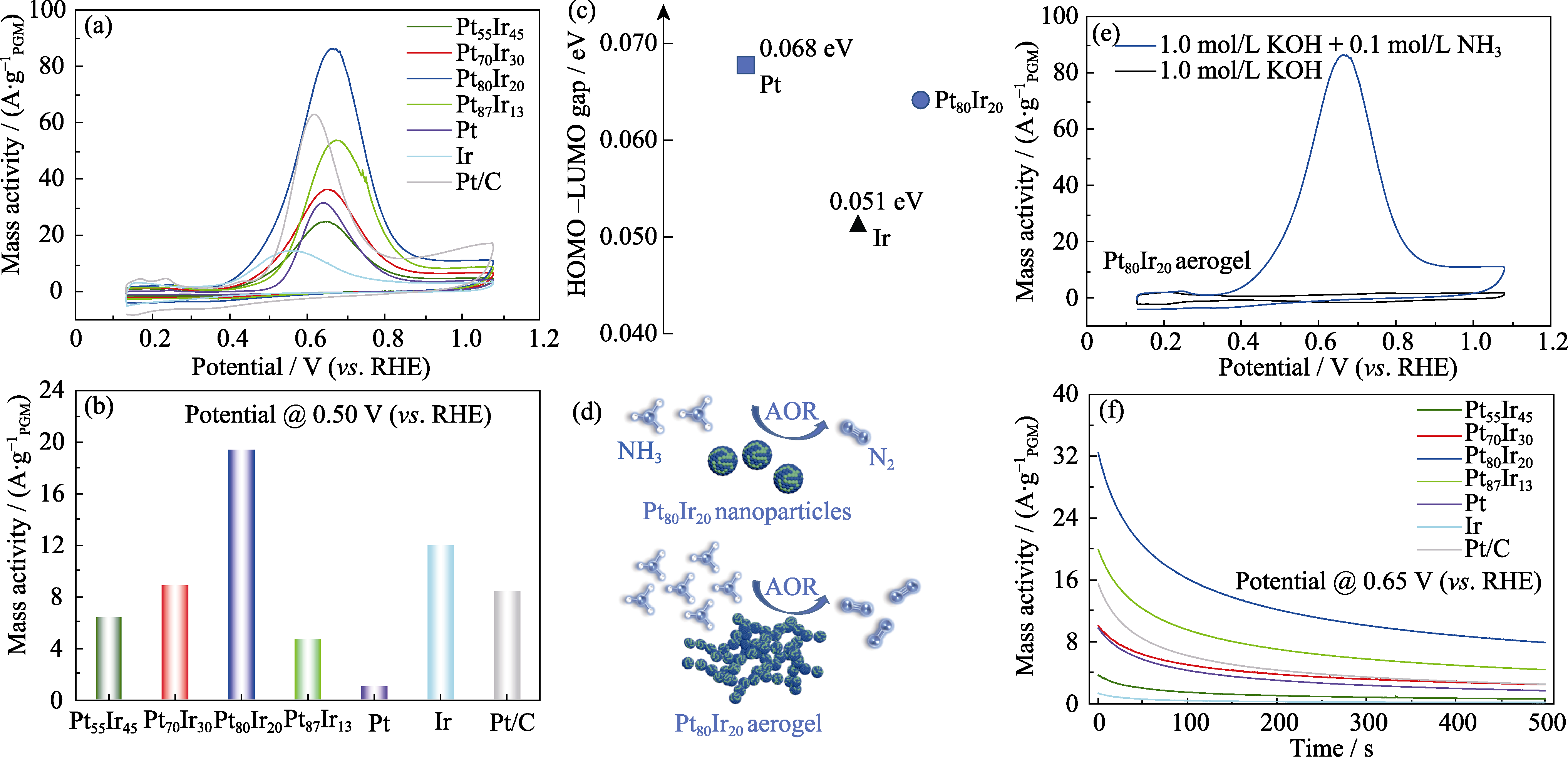
Fig. 5 (a) CV curves of Pt100-xIrx aerogels and commercial Pt/C catalysts under room temperature, (b) AOR activity comparison for Pt100-xIrx aerogels and commercial Pt/C catalysts at 0.5 V(vs. RHE), (c) energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Pt, Ir and Pt80Ir20 nanoparticles, (d) schematic diagram of electrocatalytic ammonia oxidation of Pt80Ir20 alloy nanoparticles and Pt80Ir20 alloy aerogel, (e) CV curves of the Pt80Ir20 catalyst in the presence and absence of NH3; (f) CA curves of Pt100-xIrx aerogels and commercial Pt/C catalysts Colorful figures are available on website
| Sample | Onset potential/V | Mass activity at 0.5 V (vs. RHE)/(mA·mgPGM-1) | Peak mass activity/ (mA·mgPGM-1) | Ref. |
|---|---|---|---|---|
| Pt87Ir13 aerogel | 0.411 | 4.7 | 53.7 | This work |
| Pt80Ir20 aerogel | 0.368 | 19.4 | 86.3 | This work |
| Pt70Ir30 aerogel | 0.361 | 8.9 | 31.5 | This work |
| Pt55Ir45 aerogel | 0.358 | 6.4 | 24.8 | This work |
| Pt | 0.511 | 1.1 | 31.5 | This work |
| Ir | 0.354 | 12.0 | 14.6 | This work |
| Commercial Pt/C | 0.495 | 8.4 | 62.9 | This work |
| Commercial PtIr/C | 0.428 | 10.4 | 25.1 | [S1] |
| Ir-decorate Pt NCs/C | ~ 0.43 | - | 100 | [S2] |
| Polycrystalline PtIr | ~ 0.41 | - | - | [S3] |
| PtRh/C(Pt:Rh = 9:1) | 0.44 | 9.0 | 93.8 | [S4] |
| Pt-decorated Ni particles | ~0.50 | - | 75.3 | [S5] |
Table S3 Comparison of AOR activity between different catalysts
| Sample | Onset potential/V | Mass activity at 0.5 V (vs. RHE)/(mA·mgPGM-1) | Peak mass activity/ (mA·mgPGM-1) | Ref. |
|---|---|---|---|---|
| Pt87Ir13 aerogel | 0.411 | 4.7 | 53.7 | This work |
| Pt80Ir20 aerogel | 0.368 | 19.4 | 86.3 | This work |
| Pt70Ir30 aerogel | 0.361 | 8.9 | 31.5 | This work |
| Pt55Ir45 aerogel | 0.358 | 6.4 | 24.8 | This work |
| Pt | 0.511 | 1.1 | 31.5 | This work |
| Ir | 0.354 | 12.0 | 14.6 | This work |
| Commercial Pt/C | 0.495 | 8.4 | 62.9 | This work |
| Commercial PtIr/C | 0.428 | 10.4 | 25.1 | [S1] |
| Ir-decorate Pt NCs/C | ~ 0.43 | - | 100 | [S2] |
| Polycrystalline PtIr | ~ 0.41 | - | - | [S3] |
| PtRh/C(Pt:Rh = 9:1) | 0.44 | 9.0 | 93.8 | [S4] |
| Pt-decorated Ni particles | ~0.50 | - | 75.3 | [S5] |
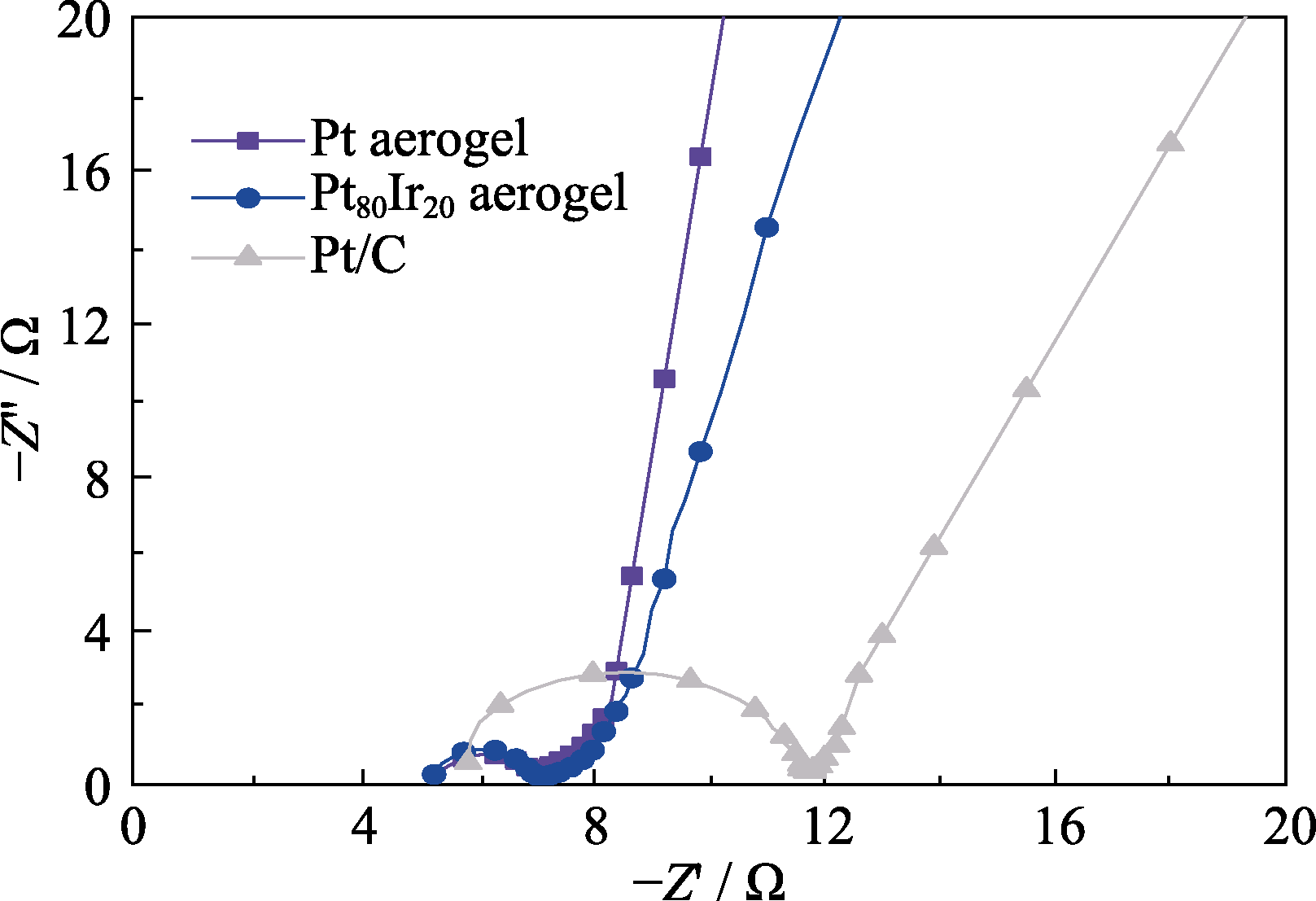
Fig. S6 Nyquist plots of EIS spectra measured for Pt (violet), Pt80Ir20 aerogel (blue) and commercial Pt/C (gray) in 1.0 mol/L KOH electrolyte at the open circuit potential
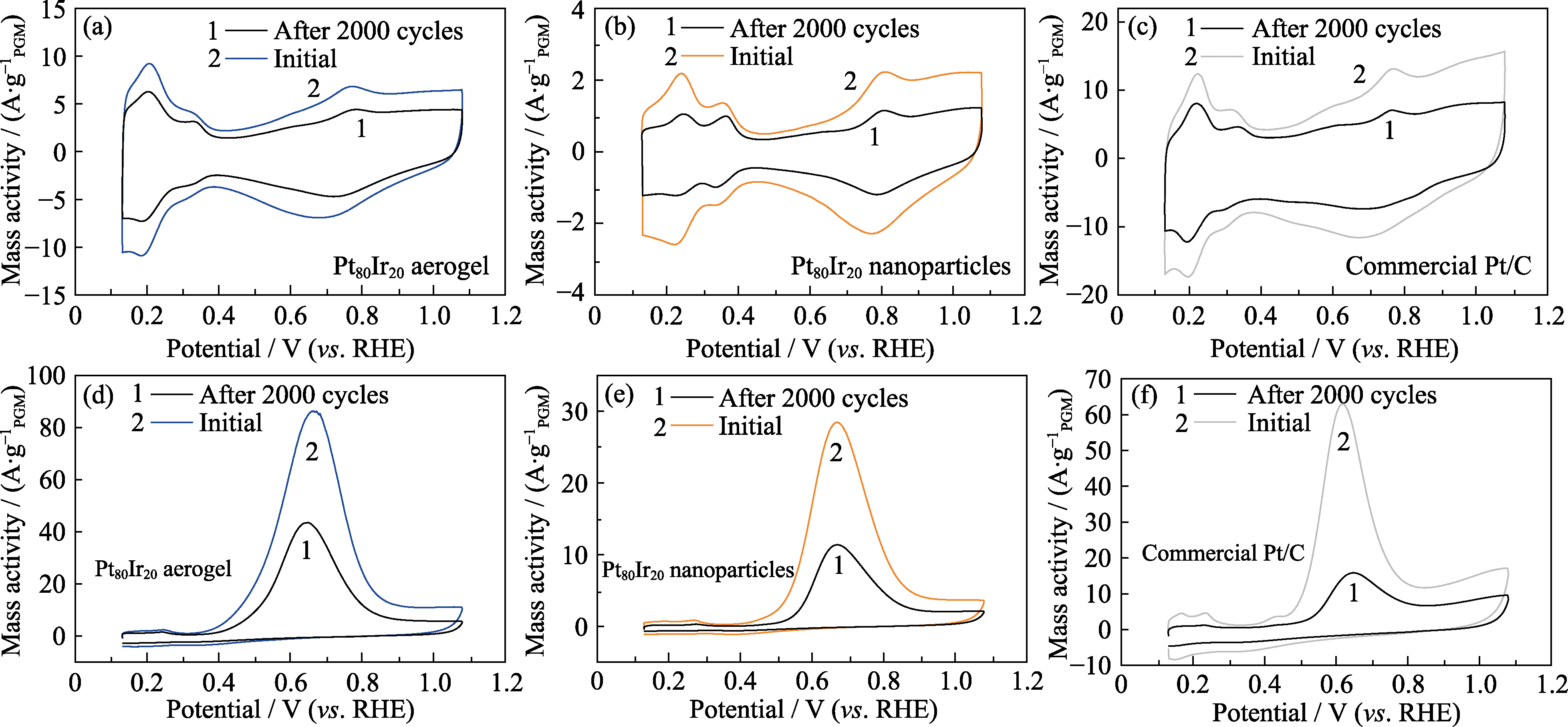
Fig. 6 Electrochemical active areas of catalysts and AOR performance before and after 2000 CV cycles (a, d) Pt80Ir20 aerogel; (b, e) Pt80Ir20 nanoparticles; (c, f) Commercial Pt/C
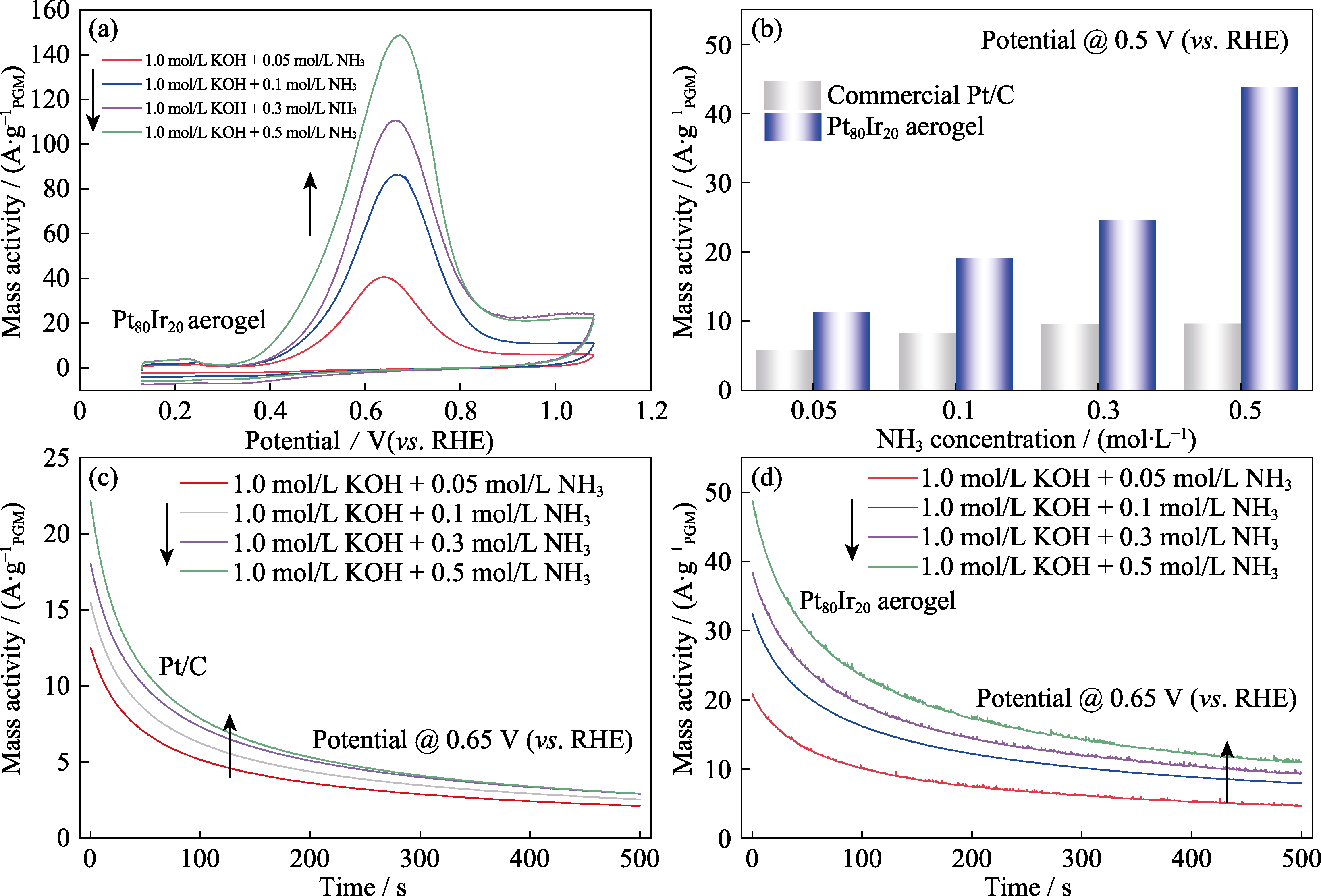
Fig. 7 (a) CV curves of the Pt80Ir20 aerogel tested in different NH3 concentrations, (b) AOR activity comparison for Pt80Ir20 aerogel and commercial Pt/C in different NH3 concentrations at 0.5 V(vs. RHE), and (c, d) CA curves of the Pt80Ir20 aerogel (c) and commercial Pt/C (d) at 0.65 V(vs. RHE)
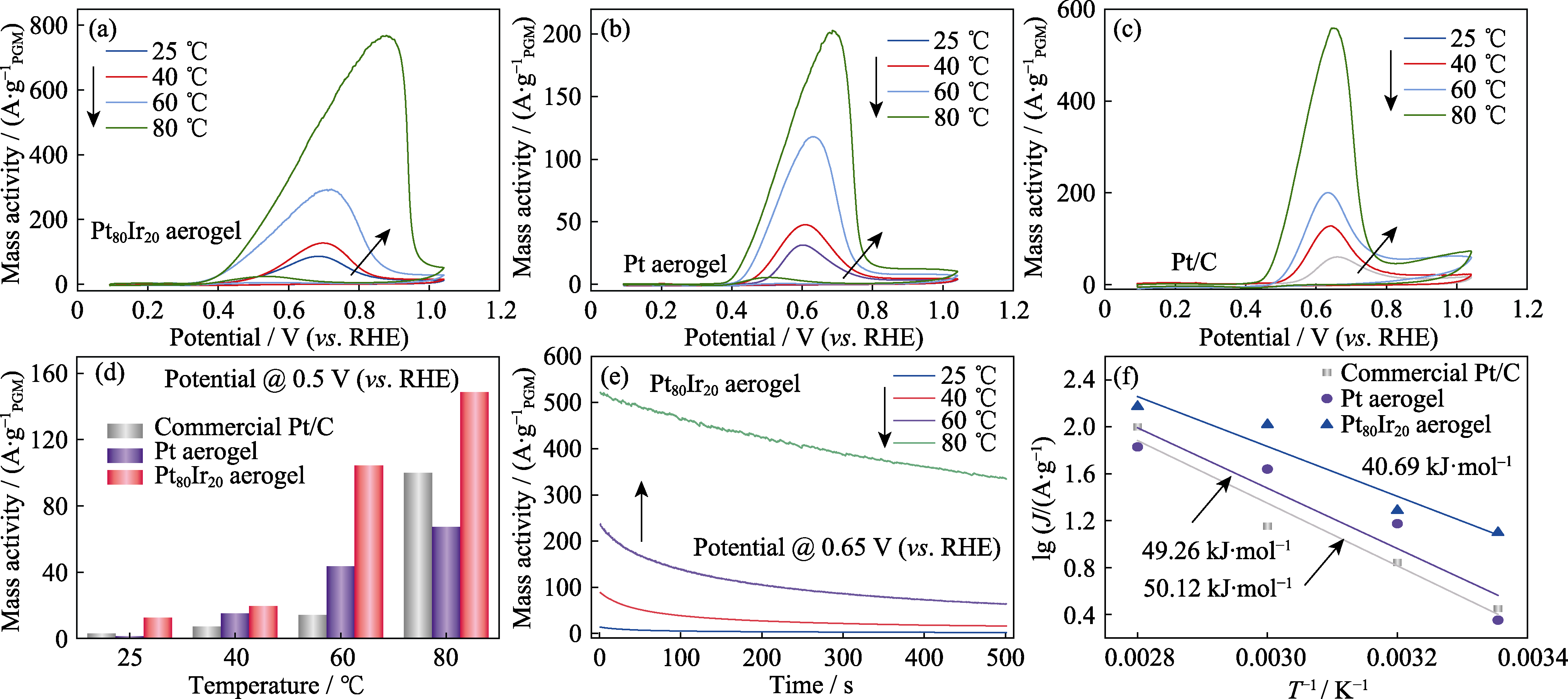
Fig. 8 (a-c) CV curves of catalysts at different temperatures, (d) AOR activity comparison for commercial Pt/C, Pt aerogel and Pt80Ir20 aerogel catalysts at different temperatures at 0.5 V(vs. RHE), (e) CA curves of the Pt80Ir20 aerogel at different temperatures at 0.65 V (vs. RHE), (f) Arrhenius plots for NH3 oxidation on commercial Pt/C, Pt aerogel and Pt80Ir20 aerogel catalysts at 0.5 V(vs. RHE)
| [1] | LI Y, WANG H H, PRIEST C, et al. Advanced electrocatalysis for energy and environmental sustainability via water and nitrogen reactions. Adv. Mater., 2020, 33(6): 2000381. |
| [2] |
JIN H, LEE S, SOHN Y. Capping agent-free synthesis of surface engineered Pt nanocube for direct ammonia fuel cell. Int. J. Energy Res., 2021, 45(12): 18281.
DOI URL |
| [3] |
LI Y, PILLAI H S, WANG T, et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci., 2021, 14(3): 1449.
DOI URL |
| [4] |
LI Y, LI X, PILLAI H S, et al. Ternary PtIrNi catalysts for efficient electrochemical ammonia oxidation. ACS Catal., 2020, 10(7): 3945.
DOI URL |
| [5] |
ZHAO Y, SETZLER B P, WANG J H, et al. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule, 2019, 3(10): 2472.
DOI URL |
| [6] |
PILLAI H S, XIN H. New insights into electrochemical ammonia oxidation on Pt(100) from first principles. Ind. Eng. Chem. Res., 2019, 58(25): 10819.
DOI URL |
| [7] |
KANG Y M, WANG W, LI J M, et al. High performance PtxEu alloys as effective electrocatalysts for ammonia electro-oxidation. Int. J. Hydrogen. Energy, 2017, 42(30): 18959.
DOI URL |
| [8] |
XUE Q, ZHAO Y, ZHU J Y, et al. PtRu nanocubes as bifunctional electrocatalysts for ammonia electrolysis. J. Mater. Chem. A, 2021, 9(13): 8444.
DOI URL |
| [9] | CHEN R Y, ZHENG S S, YAO Y D, et al. Performance of direct ammonia fuel cell with PtIr/C, PtRu/C, and Pt/C as anode electrocatalysts under mild conditions. Int. J. Hydrogen. Energy, 2021 (46): 27749. |
| [10] |
GOTTESFELD S. The direct ammonia fuel cell and a common pattern of electrocatalytic processes. J. Electrochem. Soc., 2018, 165(15): J3405.
DOI URL |
| [11] | SONG L, LIANG Z X, MA Z, et al. Temperature-dependent kinetics and reaction mechanism of ammonia oxidation on Pt, Ir, and PtIr alloy catalysts. J. Electrochem. Soc., 2018, 165: 3095. |
| [12] |
SACRE N, DUCA M, GARBARION S, et al. Tuning Pt-Ir interactions for NH3 electrocatalysis. ACS Catal., 2018, 8(3): 2508.
DOI URL |
| [13] | ESTEJAB A, BOTTE G. Ammonia oxidation kinetics on bimetallic clusters of platinum and iridium: a theoretical approach. Mol. Catal., 2018, 445: 279. |
| [14] |
DOUK A S, SARAVANI H. Porous 3D inorganic superstructure of Pd-Ir aerogel as advanced support-less anode electrocatalyst toward ethanol oxidation. ACS Omega, 2020, 5: 22031.
DOI PMID |
| [15] |
ZHU C, DU D, EYCHMULLER A. Engineering ordered and nonordered porous noble metal nanostructures: synthesis, assembly, and their applications in electrochemistry. Chem. Rev., 2015, 115: 8896.
DOI PMID |
| [16] |
ZHU C Z, GUO S J, DONG S J. PdM (M= Pt, Au) bimetallic alloy nanowires with enhanced electrocatalytic activity for electro- oxidation of small molecules. Adv. Mater., 2012, 24: 2326.
DOI URL |
| [17] | CAI B, DIANAT A, HUBNER R, et al. Multimetallic hierarchical aerogels: shape engineering of the building blocks for efficient electrocatalysis. Adv. Mater., 2017, 29: 1605254. |
| [18] |
HE FEI, LI YA, LUO JIN, et al. Development of SiO2/C and SiC/C composites featuring aerogel structures. J. Inorg. Mater., 2017, 32(5): 449.
DOI URL |
| [19] |
LIU W, HERRMAANN A K, BIGALL N C, et al. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res., 2015, 48: 154.
DOI URL |
| [20] |
RAJIB S, AHMED A F, INDIKA U A. Oxidative self-assembly of Au/Ag/Pt alloy nanoparticles into high-surface area, mesoporous, and conductive aerogels for methanol electro-oxidation. Chem. Mater., 2022, 34(13): 5874.
DOI URL |
| [21] |
LIU W, HAUBOLD D, RUTKOWSKI B, et al. Self-supporting hierarchical porous PtAg alloy nanotubular aerogels as highly active and durable electrocatalysts. Chem. Mater., 2016, 28: 6477.
DOI URL |
| [22] |
DOUK A S, SARAVANI H, NOROOZIFAR M. Three-dimensional assembly of building blocks for the fabrication of Pd aerogel as a high performance electrocatalyst toward ethanol oxidation. Electrochim. Acta, 2018, 275: 182.
DOI URL |
| [23] |
LYU ZI YE, TANG YI PING, CAO HUA ZHEN, et al. Effect of V doping on electrocatalytic performance of Ni-Co-S on bacterial cellulose-derived carbon aerogel. J. Inorg. Mater., 2020, 35(10): 1142.
DOI |
| [24] | CAI B, SAYEVICH V, GAPONIK N, et al. Emerging hierarchical aerogels: self-assembly of metal and semiconductor nanocrystals. Adv. Mater., 2018, 30(33): 1707518. |
| [25] |
BURPO F J, NAGELLI E A, MORRIS L A, et al. Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J. Mater. Res., 2017, 32(22): 4153.
DOI URL |
| [26] | MIAO B Q, LIU Y C, DING Y, et al. Rhodium nanodendrites catalyzed alkaline methanol oxidation reaction in direct methanol fuel cells. SM&T, 2022, ( 31):e00379. |
| [27] |
MIAO J, ZHAO X J, HU H Y, et al. Porous palladium phosphide nanotubes for formic acid electrooxidation. Carbon Energy, 2022, 4: 283.
DOI URL |
| [28] |
QIAO Z, WANG S, LI X, et al. 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: a balance between graphitization and hierarchical porosity. Energy Environ. Sci., 2019, 12: 2830.
DOI URL |
| [29] |
LIU Q T, LI Y C, ZHENG L R, et al. Sequential synthesis and active-site coordination principle of precious metal single-atom catalysts for oxygen reduction reaction and PEM fuel cells. Adv. Energy Mater., 2020, 10(20): 2000689.
DOI URL |
| [30] |
ALMANA N, PHIVILAY S P, LAVEILLE P, et al. Design of a core-shell Pt-SiO2 catalyst in a reverse microemulsion system: distinctive kinetics on CO oxidation at low temperature. J. Catal., 2016, 340: 368.
DOI URL |
| [31] |
SIDDHARTH K, HONG Y M, QIN X P, et al. Surface engineering in improving activity of Pt nanocubes for ammonia electrooxidation reaction. Appl. Catal. B, 2020, 269: 118821.
DOI URL |
| [32] |
ESTEJAB A, G. BOTTE G. DFT calculations of ammonia oxidation reactions on bimetallic clusters of platinum and iridium. Comput. Theor. Chem., 2016, 1091: 31.
DOI URL |
| [33] |
CHAN Y T, SIDDHARTH K, SHAO M H. Investigation of cubic Pt alloys for ammonia oxidation reaction. Nano Res., 2020, 13(7): 1920.
DOI |
| [34] |
LIU Z Z, LI Y, ZHANG X S, et al. Surface structure engineering of PtPd nanoparticles for boosting ammonia oxidation electrocatalysis. ACS Appl. Mater. Interfaces, 2022, 14: 28816.
DOI URL |
| [1] | ZHOU Houlin, SONG Zhiqing, TIAN Guo, GAO Xingsen. Effects of Growth Conditions on the Formation of Self-assembly Grown Topological Domain in BiFeO3 Nanoislands [J]. Journal of Inorganic Materials, 2025, 40(6): 667-674. |
| [2] | YUAN Liping, WU Yuanbo, YU Jiajing, ZHANG Shiyan, SUN Yi, HU Yunchu, FAN Youhua. CNFs Aerogel Composite with Phosphomolybdic Acid Intercalated Hydrotalcite: Preparation and Thermal Insulation Performance [J]. Journal of Inorganic Materials, 2025, 40(4): 415-424. |
| [3] | LUO Yi, XIA Shuhai, NIU Bo, ZHANG Yayun, LONG Donghui. Preparation and High Temperature Inorganic Transformation of Flexible Silicone Aerogels [J]. Journal of Inorganic Materials, 2022, 37(12): 1281-1288. |
| [4] | PENG Fei, JIANG Yonggang, FENG Jian, CAI Huafei, FENG Junzong, LI Liangjun. Research Progress on Alumina Aerogel Composites for High-temperature Thermal Insulation [J]. Journal of Inorganic Materials, 2021, 36(7): 673-684. |
| [5] | LI Huaxin, CHEN Junyong, XIAO Zhou, YUE Xian, YU Xianbo, XIANG Junhui. Research Progress of Biomimetic Self-assembly of Nanomaterials in Morphology and Performance Control [J]. Journal of Inorganic Materials, 2021, 36(7): 695-710. |
| [6] | ZHANG Xiaoshan, WANG Bing, WU Nan, HAN Cheng, WU Chunzhi, WANG Yingde. Micro-nano Ceramic Fibers for High Temperature Thermal Insulation [J]. Journal of Inorganic Materials, 2021, 36(3): 245-256. |
| [7] | ZHANG Ze,WANG Xiaodong,SHEN Jun. Effect of Organic-inorganic Crosslinking Degree on the Mechanical and Thermal Properties of Aerogels [J]. Journal of Inorganic Materials, 2020, 35(4): 454-460. |
| [8] | LUO Yi,FENG Junzong,FENG Jian,JIANG Yonggang,LI Liangjun. Research Progress on Advanced Carbon Materials as Pt Support for Proton Exchange Membrane Fuel Cells [J]. Journal of Inorganic Materials, 2020, 35(4): 407-415. |
| [9] | LIU Fengqi, FENG Jian, JIANG Yonggang, LI Liangjun. Preparation and Application of Boron Nitride Aerogels [J]. Journal of Inorganic Materials, 2020, 35(11): 1193-1202. |
| [10] | DING Zhuofeng, YANG Yongqiang, LI Zaijun. Synthesis and Supercapacitor Performance of Histidine-functionalized Carbon Dots/Graphene Aerogel [J]. Journal of Inorganic Materials, 2020, 35(10): 1130-1136. |
| [11] | LYU Ziye, TANG Yiping, CAO Huazhen, ZHENG Guoqu, HOU Guangya. Effect of V Doping on Electrocatalytic Performance of Ni-Co-S on Bacterial Cellulose-derived Carbon Aerogel [J]. Journal of Inorganic Materials, 2020, 35(10): 1142-1148. |
| [12] | PENG Xin-Cun, WANG Zhi-Dong, ZENG Meng-Si, LIU Yun, ZOU Ji-Jun, ZHU Zhi-Fu, DENG Wen-Juan. Improvement on Size Uniformity of SiO2 Nanospheres Applied in Si Optical Resonance Nanopillar-arrays [J]. Journal of Inorganic Materials, 2019, 34(7): 734-740. |
| [13] | ZHU Zhao-Xian,WANG Fei,YAO Hong-Jun,DONG Jin-Xin,LONG Dong-Hui. High-temperature Insulation Property of Opacifier-doped Al2O3-SiO2 Aerogel/Mullite Fiber Composites [J]. Journal of Inorganic Materials, 2018, 33(9): 969-975. |
| [14] | WANG Jing-Ping, CHENG Fang-Yuan, DU Xian-Feng, XU You-Long. Preparation of Al2O3/TiO2 Composite film with High Specific Capacitance by Surface Self-assembly Method [J]. Journal of Inorganic Materials, 2018, 33(6): 617-622. |
| [15] | WANG Yong, YU Yun, FENG Ai-Hu, JIANG Feng, HU Xue-Bing, SONG Li-Xin. Nafion Modified Graphene Aerogel with Hierarchical Porous Structures [J]. Journal of Inorganic Materials, 2018, 33(4): 469-474. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||