无机材料学报 ›› 2024, Vol. 39 ›› Issue (10): 1151-1158.DOI: 10.15541/jim20240143 CSTR: 32189.14.10.15541/jim20240143
所属专题: 【材料计算】材料模拟计算(202506); 【信息功能】MAX、MXene及其他二维材料(202506)
周云凯1,2( ), 刁亚琪1,3, 王明磊1,3, 张宴会1,3(
), 刁亚琪1,3, 王明磊1,3, 张宴会1,3( ), 王利民1,3
), 王利民1,3
收稿日期:2024-03-25
修回日期:2024-05-29
出版日期:2024-10-20
网络出版日期:2024-10-09
通讯作者:
张宴会, 讲师. E-mail: yhzhang@ysu.edu.cn作者简介:周云凯(1986-), 男, 助理研究员. E-mail: zhouyunkai1986@hotmail.com
基金资助:
ZHOU Yunkai1,2( ), DIAO Yaqi1,3, WANG Minglei1,3, ZHANG Yanhui1,3(
), DIAO Yaqi1,3, WANG Minglei1,3, ZHANG Yanhui1,3( ), WANG Limin1,3
), WANG Limin1,3
Received:2024-03-25
Revised:2024-05-29
Published:2024-10-20
Online:2024-10-09
Contact:
ZHANG Yanhui, lecturer. E-mail: yhzhang@ysu.edu.cnAbout author:ZHOU Yunkai (1986-), male, lecturer. E-mail: zhouyunkai1986@hotmail.com
Supported by:摘要:
Ti3C2(OH)2的抗氧化性和结构稳定性不佳极大地限制了其实际应用推广。本工作通过第一性原理计算方法, 对比研究了Ti3C2(OH)2、聚苯胺(PANI)和PANI/Ti3C2(OH)2复合材料的表面氧吸附行为。计算结果表明, Ti3C2基体上-OH官能团改变了活性吸附位点, 并一定程度上改善了Ti3C2基体的抗氧化性和结构稳定性。Ti3C2(OH)2与PANI复合后, 表面PANI的氧吸附活性较高, 而Ti3C2(OH)2的氧吸附能大幅度降低。经Bader电荷计算发现, 复合后从PANI端到Ti3C2(OH)2发生了电子转移, 从而保护了后者。因此, PANI改性Ti3C2(OH)2复合材料可以通过牺牲表面PANI来保护Ti3C2(OH)2基体, 进而提高其结构稳定性和抗氧化性能。本工作对于提高MXene体系抗氧化性能、结构和电化学稳定性具有一定的理论指导意义。
中图分类号:
周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158.
ZHOU Yunkai, DIAO Yaqi, WANG Minglei, ZHANG Yanhui, WANG Limin. First-principles Calculation Study of the Oxidation Resistance of PANI Modified Ti3C2(OH)2[J]. Journal of Inorganic Materials, 2024, 39(10): 1151-1158.
| System | a/Å | dTi1-C/Å | dTi2-C/Å |
|---|---|---|---|
| Ti3C2 | 3.10[TW], 3.06[ | 2.14[TW], 2.22[ | 2.09[TW], 2.05[ |
| Ti3C2(OH)2 | 3.08[TW], 3.09[ | 2.18[TW], 2.21[ | 2.08[TW], 2.08[ |
| Ti3C2O2 | 3.04[TW], 3.09[ | 2.23[TW], 2.20[ | 2.16[TW], 2.14[ |
表1 Ti3C2、Ti3C2(OH)2和Ti3C2O2单胞晶胞参数和Ti-C键长
Table 1 Lattice parameters and Ti-C bond lengths in Ti3C2, Ti3C2(OH)2 and Ti3C2O2
| System | a/Å | dTi1-C/Å | dTi2-C/Å |
|---|---|---|---|
| Ti3C2 | 3.10[TW], 3.06[ | 2.14[TW], 2.22[ | 2.09[TW], 2.05[ |
| Ti3C2(OH)2 | 3.08[TW], 3.09[ | 2.18[TW], 2.21[ | 2.08[TW], 2.08[ |
| Ti3C2O2 | 3.04[TW], 3.09[ | 2.23[TW], 2.20[ | 2.16[TW], 2.14[ |
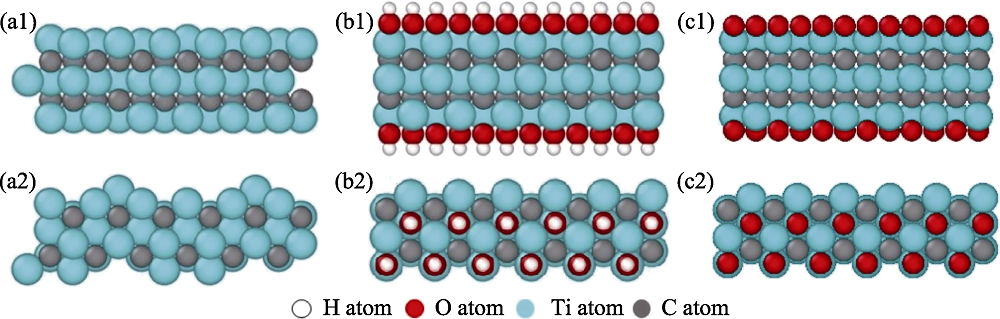
图1 MXene纳米片层表面构型的原子结构正视和俯视示意图
Fig. 1 Front and top views of the atomic structures of nano-slabs of MXene (a1, a2) Ti3C2; (b1, b2) Ti3C2(OH)2; (c1, c2) Ti3C2O2

图4 纯Ti3C2纳米片层表面的氧吸附位点正视图和俯视图及其吸附能(eV/O)
Fig. 4 Front and top views of the oxygen adsorption sites on Ti3C2 surface and their adsorption energies (a1, a2) TiA, the atop site, (b1, b2) TiB, the bridge site and (c1, c2) TiC, the hollow site of Ti atoms located on the Ti3C2 surface
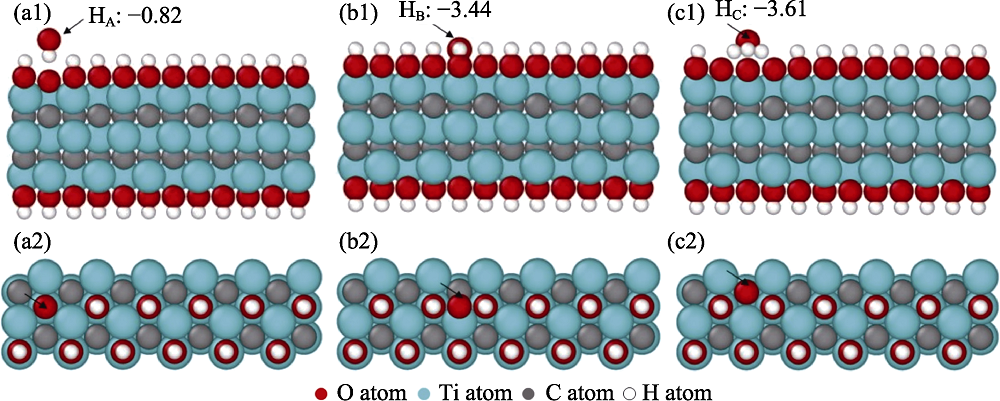
图5 Ti3C2(OH)2纳米片层表面的氧吸附位点正视图和俯视图及其吸附能(eV/O)
Fig. 5 Front and top views of the oxygen adsorption sites on Ti3C2(OH)2 surface and their adsorption energies (a1, a2) HA, the atop site, (b1, b2) HB, the bridge site and (c1, c2) HC, the hollow site of H atoms located on the Ti3C2(OH)2 surface
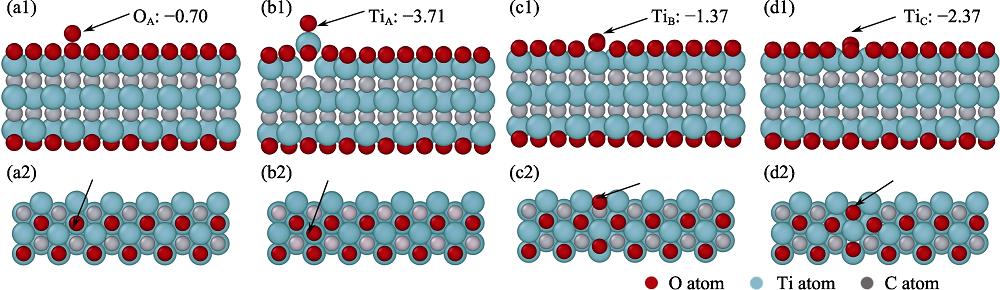
图6 Ti3C2O2纳米片层表面的氧吸附位点正视图和俯视图及其吸附能(eV/O)
Fig. 6 Front and top views of the oxygen adsorption sites on Ti3C2O2 surface and their adsorption energies (a1, a2) OA, the atop of -O functional group, (b1, b2) TiA, (c1, c2) TiB, and (d1, d2) TiC, are respectively the atop, the bridge and the hollow of Ti atoms located on the Ti3C2O2 surface
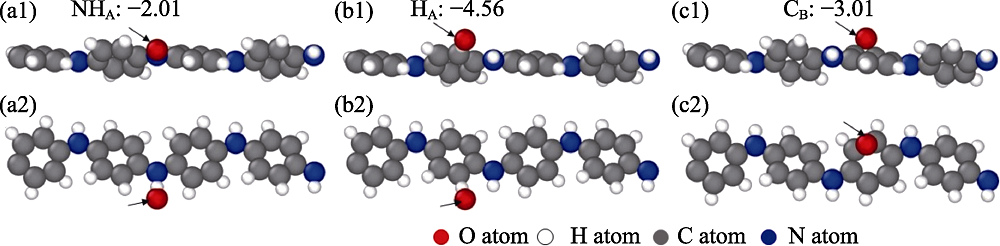
图7 全还原式red-PANI纳米片层表面的氧吸附位点正视图和俯视图及其吸附能(eV/O)
Fig. 7 Front and top views of the oxygen adsorption sites on red-PANI surface and their adsorption energies (a1, a2) NHA, the atop site of -NH, (b1, b2) HA, the atop site of -CH, and (c1, c2) CB, the bridge site of C atoms located on the red-PANI surface
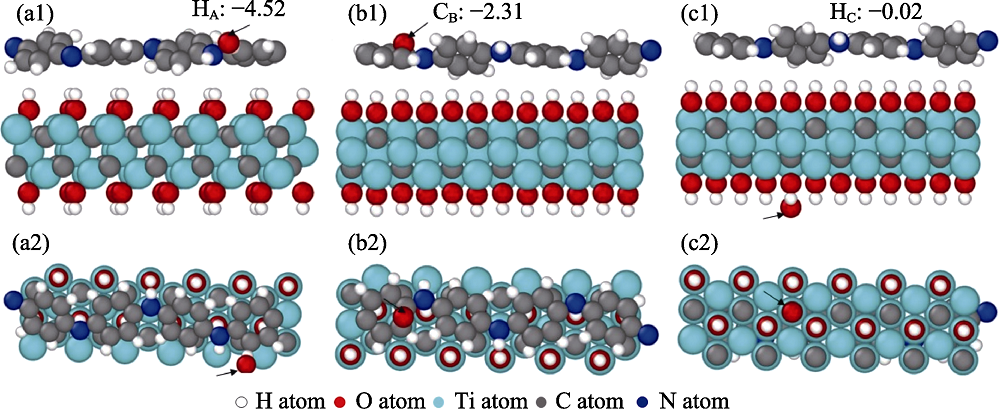
图8 Red-PANI/Ti3C2(OH)2复合材料表面的氧吸附位点正视图和俯视图及其吸附能(eV/O)
Fig. 8 Front and top views of the oxygen adsorption sites on red-PANI/Ti3C2(OH)2 surface and their adsorption energies (a1, a2) HA, the atop site of H atoms, (b1, b2) CB, the bridge site of C atoms located on the red-PANI end surface, and (c1, c2) HC, the hollow site of H atoms located on the Ti3C2(OH)2 end
| Position | TiA/HA/OA | TiB/HB/OB | TiC/HC/OC |
|---|---|---|---|
| @Ti3C2 | 3.10 | 3.08 | 3.09 |
| @Ti3C2(OH)2 | 3.10 | 3.07 | 3.11 |
| @Ti3C2O2 | 3.04 | 3.04 | 3.04 |
表2 Ti3C2、Ti3C2(OH)2和Ti3C2O2表面三类氧吸附位点的晶胞参数a(Å)
Table 2 Lattice parameters a (Å) for three kinds of adsorption sites on surfaces of Ti3C2, Ti3C2(OH)2 and Ti3C2O2
| Position | TiA/HA/OA | TiB/HB/OB | TiC/HC/OC |
|---|---|---|---|
| @Ti3C2 | 3.10 | 3.08 | 3.09 |
| @Ti3C2(OH)2 | 3.10 | 3.07 | 3.11 |
| @Ti3C2O2 | 3.04 | 3.04 | 3.04 |
| System | Atom label | Before | After | Transfer |
|---|---|---|---|---|
| PANI | C1 | -0.01 | -0.02 | -0.01 |
| C2-N | +0.45 | +0.44 | -0.01 | |
| N | -1.22 | -1.23 | -0.01 | |
| H1-N | +0.42 | +0.42 | 0 | |
| H2 | +0.04 | +0.04 | 0 | |
| Ti3C2(OH)2 | Ti1(End) | +1.57 | +1.50 | -0.07 |
| Ti2(Middle) | +1.44 | +1.19 | -0.25 | |
| H | +0.55 | +0.48 | -0.07 | |
| O | -1.23 | -1.15 | +0.08 | |
| C | -1.62 | -1.42 | +0.20 |
表3 Ti3C2(OH)2与PANI复合前后的Bader电荷及复合造成的电荷转移
Table 3 Bader charge of Ti3C2(OH)2 and PANI before and after their combination, together with the electron transfer information
| System | Atom label | Before | After | Transfer |
|---|---|---|---|---|
| PANI | C1 | -0.01 | -0.02 | -0.01 |
| C2-N | +0.45 | +0.44 | -0.01 | |
| N | -1.22 | -1.23 | -0.01 | |
| H1-N | +0.42 | +0.42 | 0 | |
| H2 | +0.04 | +0.04 | 0 | |
| Ti3C2(OH)2 | Ti1(End) | +1.57 | +1.50 | -0.07 |
| Ti2(Middle) | +1.44 | +1.19 | -0.25 | |
| H | +0.55 | +0.48 | -0.07 | |
| O | -1.23 | -1.15 | +0.08 | |
| C | -1.62 | -1.42 | +0.20 |
| [1] |
GOGOTSI Y, HUANG Q. MXenes: two-dimensional building blocks for future materials and devices. ACS Nano, 2021, 15: 5775.
DOI PMID |
| [2] |
XUN K, ZHANG B, WANG Q, et al. Local chemical inhomogeneities in TiZrNb-based refractory high-entropy alloys. J. Mater. Sci. Technol., 2023, 135: 221.
DOI |
| [3] | VAHIDMOHAMMADI A, MOJTABAVI M, CAFFREY N M, et al. Assembling 2D MXenes into highly stable pseudocapacitive electrodes with high power and energy densities. Adv. Mater., 2019, 31: 1806931. |
| [4] | ANASORI B, LUKATSKAYA M R, GOGOTSI Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater., 2017, 2: 16098. |
| [5] |
MURALI G, MODIGUNTA J K R, PARK Y H, et al. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano, 2022, 16: 13370.
DOI PMID |
| [6] | DING L, JIANG R, TANG Z, et al. MXene: nanoengineering and application as electrode materials for supercapacitors. J. Inorg. Mater., 2023, 38(6): 619. |
| [7] | 刘学斌, 林志强, 万艳君, 等. 低反射MXene基电磁屏蔽材料的研究进展. 材料研究与应用, 2023, 17(3): 412. |
| [8] | LIU L, YING G, JIANG Q, et al. Ultra-high-temperature application of MXene: stabilization of 2D Ti3C2Tx for cross-scale strengthening and toughening of 3D TiC. J. Adv. Ceram., 2024, 13(1): 1. |
| [9] | TANG Q, ZHOU Z, SHEN P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X=F, OH) monolayer. J. Am. Chem. Soc., 2012, 134: 16909. |
| [10] | WANG Y, CSANÁDI T, ZHANG H, et al. Enhanced hardness in high-entropy carbides through atomic randomness. Adv. Theory Simul., 2020, 3: 2000111. |
| [11] | ZHOU A, LIU Y, LI S, et al. From structural ceramics to 2D materials with multi-applications: a review on the development from MAX phases to MXenes. J. Adv. Ceram., 2021, 10(6): 1194. |
| [12] | QIU N, HE J, HUANG Q, et al. Tuning the surface stability and Li/Na storage of MXenes by controlling the surface termination coverage. Small, 2024, 10: 2311869. |
| [13] | HABIB T, ZHAO X, SHAH S A, et al.Oxidation stability of Ti3C2Tx MXene nanosheets in solvents and composite films. npj 2D Mater. Appl., 2019, 3: 1. |
| [14] |
CHENG R, WANG J, HU T, et al. Stabilizing MXene suspension with polyhydric alcohols. J. Mater. Sci. Technol., 2023, 165: 219.
DOI |
| [15] | ZHAO X, VASHISTH A, PREHN E, et al. Antioxidants unlock shelf- stable Ti3C2Tx (MXene) nanosheet dispersions. Matter, 2019, 1: 513. |
| [16] | WU X, WANG Z, YU M, et al. Stabilizing the MXenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater., 2017, 29: 1607017. |
| [17] | LI K, WANG X, LI S, et al. An ultrafast conducting polymer@MXene positive electrode with high volumetric capacitance for advanced asymmetric supercapacitors. Small, 2020, 16: 1906851. |
| [18] | WANG S, FENG A, LI X, et al. Pb (II) adsorption process of Fe3O4supported Ti3C2Tx. J. Inorg. Mater., 2023, 38(5): 521. |
| [19] | KONG F, HE X, LIU Q, et al. Improving the electrochemical properties of MXene Ti3C2multilayer for Li-ion batteries by vacuum calcination. Electrochim Acta, 2018, 265: 140. |
| [20] |
MAHLBERG D, SAKONG S, FORSTER-TONIGOLD K, et al. Improved DFT adsorption energies with semiempirical dispersion corrections. J. Chem. Theory Comput., 2019, 15: 3250.
DOI PMID |
| [21] | SKYLARIS C K. A benchmark for materials simulation. Science, 2016, 351(6280): 1394. |
| [22] | SUN M, SHAO P, SUN K, et al. First-principles study on interface of reduced graphene oxide reinforced aluminum matrix composites. J. Inorg. Mater., 2022, 37(6): 651. |
| [23] | KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B, 1999, 59(3): 1758. |
| [24] | BLÖCHL P E. Projector augmented-wave method. Phys. Rev. B, 1994, 50(24): 17953. |
| [25] | PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple. Phys. Rev. Lett., 1996, 77(18): 3865. |
| [26] | GRIMME S, ANTONY J, EHRLICH S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys., 2010, 132(15): 154104. |
| [27] | ZHANG Y, SANVITO S. Atomistic simulations of surface reactions in ultra-high-temperature ceramics: O2, H2O and CO adsorption and dissociation on ZrB2 (0001) surfaces. Appl. Surf. Sci., 2021, 566: 150622. |
| [28] | YU M, TRINKLE D R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys., 2011, 134(6): 064111. |
| [29] | HENKELMAN G, ARNALDSSON A, JÓNSSON H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci., 2006, 36: 354. |
| [30] | KURTOGLU M, NAGUIB M, GOGOTSI Y, et al. First principles study of two-dimensional early transition metal carbides. MRS Commun., 2012, 2: 133. |
| [31] | LI H, LI A, ZHANG D, et al. First-principles study on the structural, electronic, and lithium storage properties of Ti3C2T2 (T = O, F, H, OH) MXene. ACS Omega, 2022, 7: 40578. |
| [1] | 刘会来, 李志豪, 孔德峰, 陈星. 酞菁铁/MXene复合阴极的制备及电芬顿降解磺胺间二甲氧嘧啶[J]. 无机材料学报, 2025, 40(1): 61-69. |
| [2] | 蔡飞燕, 倪德伟, 董绍明. 高熵碳化物超高温陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 591-608. |
| [3] | 刘国昂, 王海龙, 方成, 黄飞龙, 杨欢. B4C含量对(Ti0.25Zr0.25Hf0.25Ta0.25)B2-B4C陶瓷力学性能及抗氧化性能的影响[J]. 无机材料学报, 2024, 39(6): 697-706. |
| [4] | 郑斌, 康凯, 张青, 叶昉, 解静, 贾研, 孙国栋, 成来飞. 前驱体转化陶瓷法制备Ti3SiC2陶瓷及其热稳定性研究[J]. 无机材料学报, 2024, 39(6): 733-740. |
| [5] | 李雷, 程群峰. 高性能MXenes纳米复合材料研究进展[J]. 无机材料学报, 2024, 39(2): 153-161. |
| [6] | 徐向明, Husam N ALSHAREEF. MXetronics—MXene电子学[J]. 无机材料学报, 2024, 39(2): 171-178. |
| [7] | 李腊, 沈国震. 二维MXenes材料在柔性光电探测器中的应用展望[J]. 无机材料学报, 2024, 39(2): 186-194. |
| [8] | 巴坤, 王建禄, 韩美康. MXene的红外特性及其应用研究展望[J]. 无机材料学报, 2024, 39(2): 162-170. |
| [9] | 尹建宇, 刘逆霜, 高义华. MXene在压力传感中的研究进展[J]. 无机材料学报, 2024, 39(2): 179-185. |
| [10] | 邓顺桂, 张传芳. 多功能MXene油墨:面向印刷能源及电子器件的新视角[J]. 无机材料学报, 2024, 39(2): 195-203. |
| [11] | 陈泽, 支春义. MXene在锌离子电池中的应用: 研究进展与展望[J]. 无机材料学报, 2024, 39(2): 204-214. |
| [12] | 万胡杰, 肖旭. MXenes及其复合物的太赫兹电磁屏蔽与吸收[J]. 无机材料学报, 2024, 39(2): 129-144. |
| [13] | 费玲, 雷蕾, 汪德高. 二维MXene材料在新型薄膜太阳能电池技术中的研究进展[J]. 无机材料学报, 2024, 39(2): 215-224. |
| [14] | 吴晓维, 张涵, 曾彪, 明辰, 孙宜阳. 杂化泛函HSE和PBE0计算CsPbI3缺陷性质的比较研究[J]. 无机材料学报, 2023, 38(9): 1110-1116. |
| [15] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||